Abstract
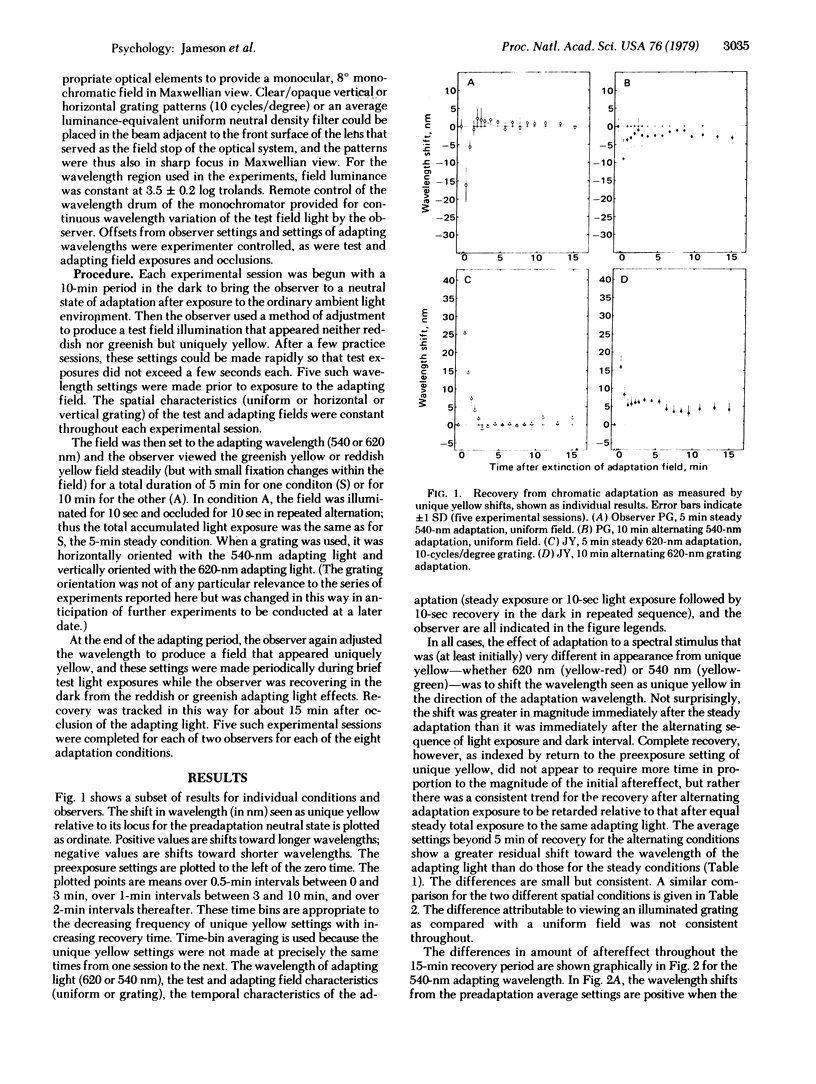
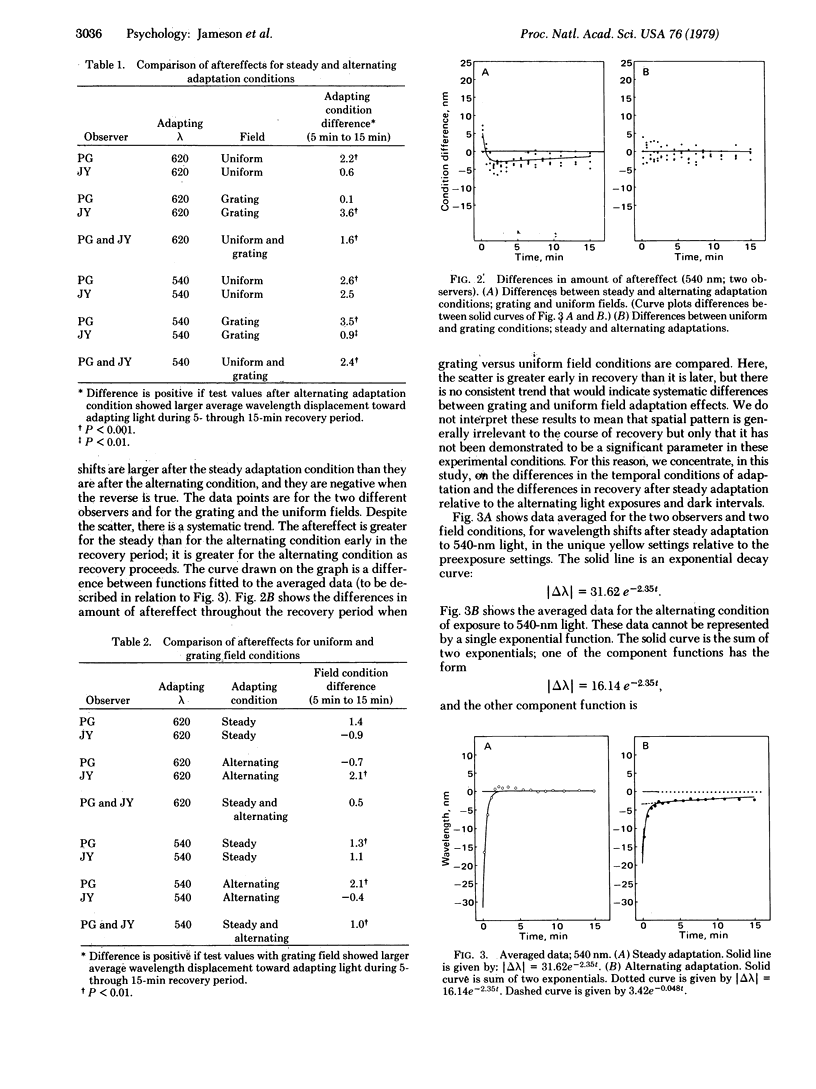
The time course of recovery from chromatic adaptation in human vision was tracked by determining the wavelength of light that appears uniquely yellow (neither red nor green) both before and after exposure to yellowish green and yellowish red adapting lights. Recovery is complete within 5 min after steady light exposure. After exposure to the alternating repeated sequence 10-sec light/10-sec dark, the initial magnitude of the aftereffect is reduced but recovery is retarded. The results are interpreted in terms of two processes located at different levels in the hierarchical organization of the visual system. One is a change in the balance of cone receptor sensitivities; the second is a shift in the equilibrium baseline between opposite-signed responses of the red/green channel at the opponent-process neural level. The baseline-shift mechanism is effective in the condition in which repeated input signals originating at the receptors are of sufficient strength to activate the system effectively. Hence, this process is revealed in the alternating adaptation condition when the receptors undergo partial recovery after each light exposure, but receptor adaptation during continued steady light exposure effectively protects the subsequent neural systems from continued strong activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH E., WALD G. The participation of different types of cones in human light and dark adaptation. Am J Ophthalmol. 1955 Feb;39(2 Pt 2):24–40. doi: 10.1016/0002-9394(55)90006-4. [DOI] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Selective chromatic adaptation in primate photoreceptors. Vision Res. 1972 May;12(5):855–874. doi: 10.1016/0042-6989(72)90011-9. [DOI] [PubMed] [Google Scholar]
- JAMESON D., HURVICH L. M. Opponent chromatic induction: experimental evaluation and theoretical account. J Opt Soc Am. 1961 Jan;51:46–53. doi: 10.1364/josa.51.000046. [DOI] [PubMed] [Google Scholar]
- JAMESON D., HURVICH L. M. Perceived color and its dependence on focal, surrounding, and preceding stimulus variables. J Opt Soc Am. 1959 Sep;49:890–898. doi: 10.1364/josa.49.000890. [DOI] [PubMed] [Google Scholar]
- Jameson D., Hurvich L. M. Theory of brightness and color contrast in human vision. Vision Res. 1964 May;4(1):135–154. doi: 10.1016/0042-6989(64)90037-9. [DOI] [PubMed] [Google Scholar]
- Larimer J., Krantz D. H., Cicerone C. M. Opponent process additivity. II. Yellow/blue equilibria and nonlinear models. Vision Res. 1975 Jun;15(6):723–731. doi: 10.1016/0042-6989(75)90291-6. [DOI] [PubMed] [Google Scholar]
- Larimer J. Opponent-process additivity--I: red-green equilibria. Vision Res. 1974 Nov;14(11):1127–1140. doi: 10.1016/0042-6989(74)90209-0. [DOI] [PubMed] [Google Scholar]
- McCollough C. Color Adaptation of Edge-Detectors in the Human Visual System. Science. 1965 Sep 3;149(3688):1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- Romeskie M. Chromatic opponent-response functions of anomalous trichromats. Vision Res. 1978;18(11):1521–1532. doi: 10.1016/0042-6989(78)90007-x. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., SMITH P. H. Iodopsin. J Gen Physiol. 1955 May 20;38(5):623–681. doi: 10.1085/jgp.38.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J. S., Wooten B. R. Opponent chromatic mechanisms: relation to photopigments and hue naming. J Opt Soc Am. 1979 Mar;69(3):422–434. doi: 10.1364/josa.69.000422. [DOI] [PubMed] [Google Scholar]


