Abstract
Objective
Endothelial cells (ECs) can undergo an endothelial-mesenchymal transition (EndMT) with tissue fibrosis. Wnt- and Msx2-regulated signals participate in arteriosclerotic fibrosis and calcification. We studied the impact of Wnt7, Msx2, and Dkk1 – a Wnt7 antagonist -- on EndMT in primary aortic endothelial cells (AoECs).
Approach and Results
Transduction of AoECs with vectors expressing Dkk1 suppressed EC differentiation and induced a mineralizing myofibroblast phenotype. Dkk1 suppressed claudin 5, PECAM, cadherin 5 (Cdh5), Tie1 and Tie2. Dkk1 converted the cuboidal cell monolayer into a spindle-shaped multilayer and inhibited EC cord formation. Myofibroblast and osteogenic markers –SM22, type I collagen, Osx, Runx2, alkaline phosphatase -- were upregulated by Dkk1 via activin-like kinase / Smad pathways. Dkk1 increased fibrotic mineralization of AoECs cultured under osteogenic conditions – the opposite of mesenchymal cell responses. Msx2 and Wnt7b maintained morphology and upregulated markers of differentiated ECs. Deleting EC Wnt7b with the Cdh5-Cre transgene in Wnt7b(fl/fl);LDLR−/− mice upregulated aortic osteogenic genes (Osx, Sox9, Runx2, Msx2) and nuclear pSmad1/5, and increased collagen and calcium accumulation.
Conclusions
Dkk1 enhances EndMT in AoECs, while Wnt7b and Msx2 signals preserve EC phenotype. EC responses to Dkk1, Wnt7b, and Msx2 are the opposite of mesenchymal responses, coupling EC phenotypic stability with osteofibrogenic predilection during arteriosclerosis.
INTRODUCTION
With advanced age, longstanding hypertension, and certain dysmetabolic states, the arterial vasculature becomes stiff, losing the elastic compliance necessary to ensure smooth distal tissue perfusion1, 2. This arteriosclerotic stiffening arises from atherosclerotic plaque burden, mural thickening and fibrosis, medial calcification, elastin fragmentation, non-enzymatic matrix glycation, and endothelial dysfunction1. Inflammatory cytokine and redox signals elicited by metabolic stressors (dyslipidemia, hyperglycemia and hyperphosphatemia), innate immune responses, neuroendocrine cues, and disturbed blood flow represent key pathophysiological stimuli1, 2. Multiple labs have now identified bone morphogenetic proteins (BMPs) and Wnts – polypeptides that play critical roles during bone morphogenesis – as rate-limiting components of the pro-fibrotic and pro-calcific injury responses that induce arteriosclerotic disease3. In our studies of the LDLR−/− mouse fed high fat diets (HFD), we identified that osteogenic Msx-Wnt signals in vascular myofibroblasts were activated in response to the diabetes, dyslipidemia, and obesity arising with HFD challenge4. Vascular myofibroblast expression of the osteoblast transcription factor Msx2 and Wnt/β-catenin activation was shown to be upregulated by TNF-dependent signals that support aortic calcification responses in LDLR−/− mice5. Wnts capable of activating pro-calcific responses downstream of Msx2 in adventitial myofibroblasts, vascular smooth muscle (VSMC) and C3H10T1/2 cells were identified as Wnt3a, Wnt7b, and Wnt7a4–7.
However, during development, Msx28 and Wnt7 family members9, 10are also expressed in endothelial cells (ECs). ECs participate in valve calcification in part by providing osteoprogenitors via the endothelial –mesenchymal transition (EndMT)11. During the EndMT process, ECs down-regulate homotypic cell-cell interactions that stabilize EC monolayer function and phenotype, with concomitant upregulation of proliferative and synthetic myofibroblast program -- including the deposition of type I collagen3. An EndMT process also contributes to the pathogenesis of myocardial fibrosis with ischemia12. Furthermore, in the ectopic muscle ossification of fibrodysplasia ossificans progressiva, constitutive activin-like kinase (ALK)-2 activity drives EndMT to generate osteoprogenitors13. Thus, EndMT has emerged as an important process in the pathobiology of fibrotic and calcific tissue injury responses3, 14. Of note, Msx genes15 and Wnt/β-catenin signaling16 support EndMT during cardiac development, modulated by mechanical strain17.
We wished to better understand the role of endothelial Wnt/β-catenin signaling and arteriosclerosis. Therefore, we studied the actions of Wnt7, Dkk1, and Msx2 in primary cultures of bovine aortic ECs (AoECs). We find that Msx2 and Wnt7 family members stabilize the AoEC phenotype whereas Dkk1 promotes an EndMT in cultured bovine AoECs. Via an ALK/Smad-dependent mechanism, Dkk1 increased whilst Msx2 and Wnt7b decreased fibrotic mineralization of AoECs cultured under osteogenic conditions. Furthermore, targeted knockout of EC Wnt7b in vivo increased aortic fibrosis. Thus, AoEC arteriosclerotic responses to cell-autonomous Msx2, Wnt7b, and Dkk1 are the opposite of those elaborated by mesenchymal cells, providing a mechanism that may couple angiogenesis with osteofibrogenic predilection during tissue injury.
METHODS
Materials and Methods are provided in the online-only Supplement.
RESULTS
Dkk1 inhibits epithelial cobblestone morphology and capillary-like network formation in primary bovine AoEC cultures
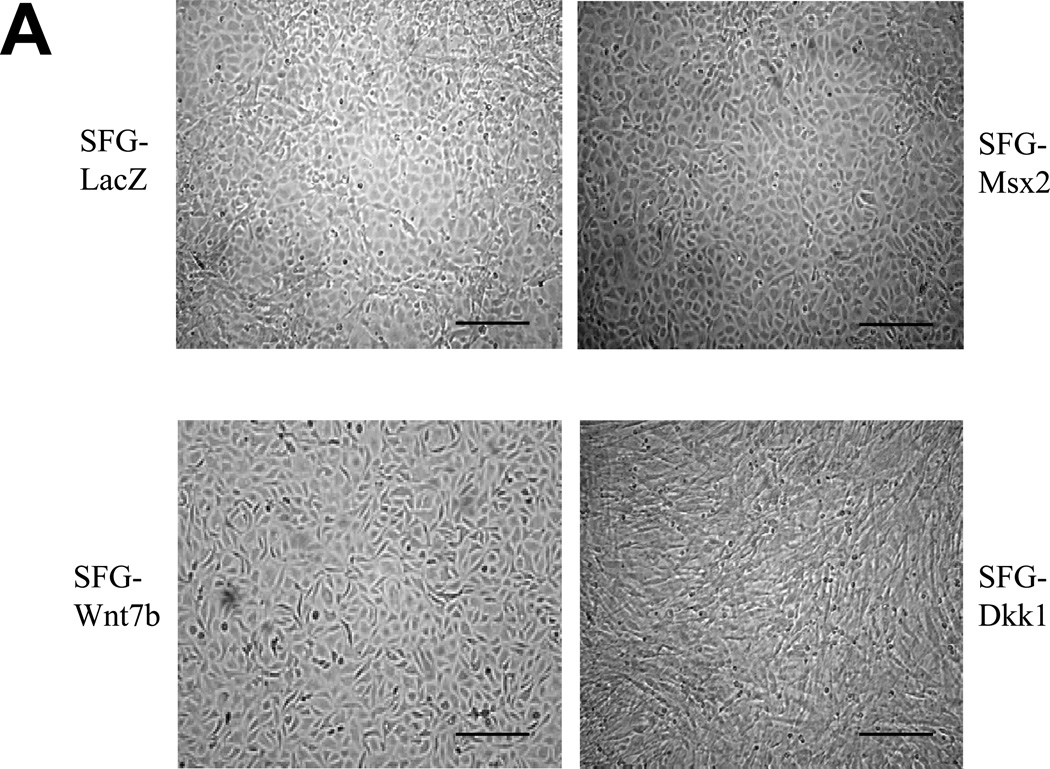
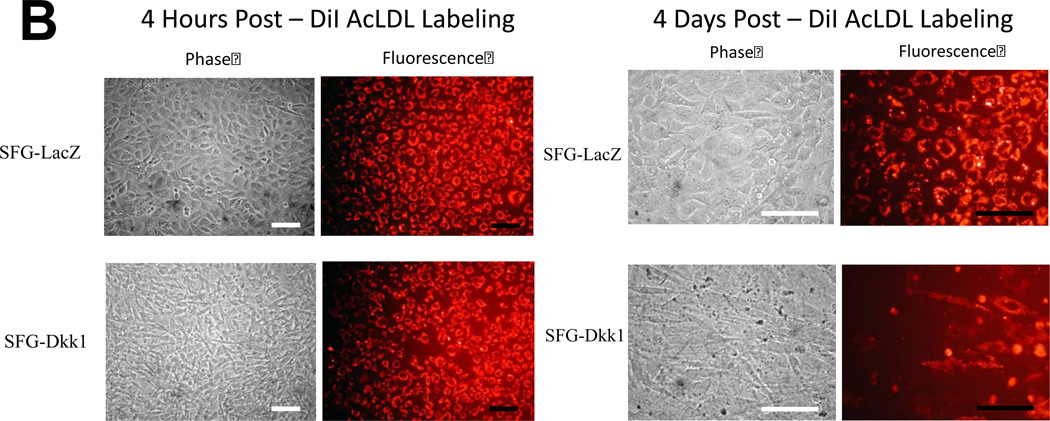
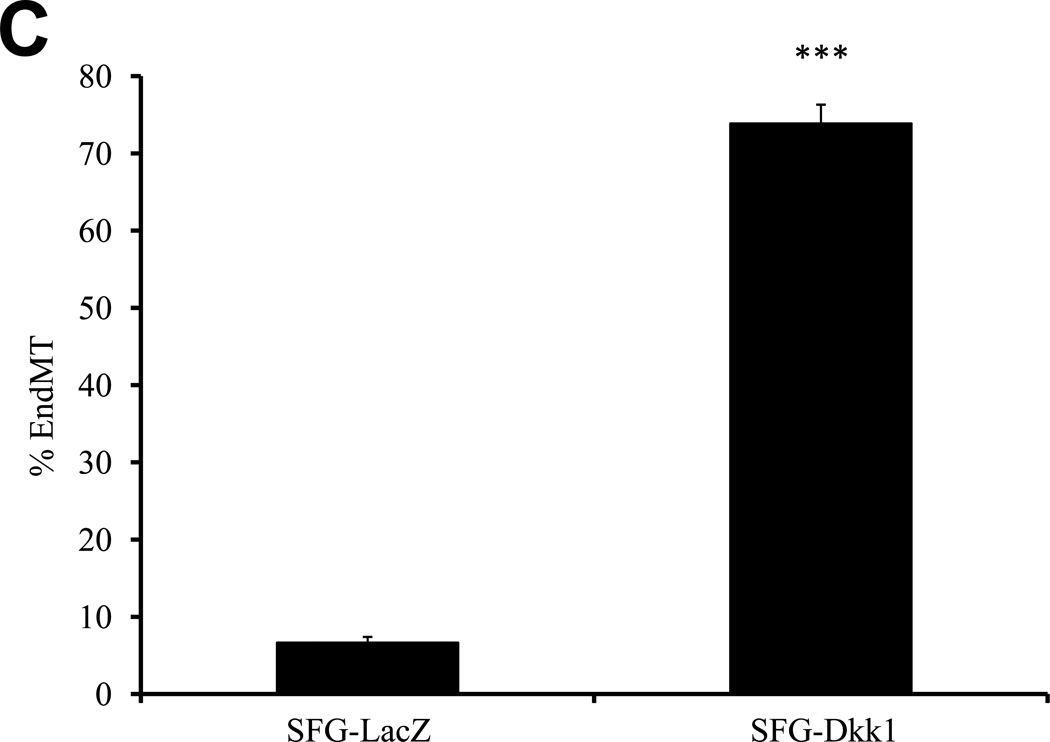
We previously identified that activation of osteogenic Msx-Wnt signaling in arterial myofibroblasts promotes medial VSMC β-catenin transactivation and calcium accumulation in diabetic arteriosclerosis4, 18. This cascade can be phenocopied by mesenchymal expression of Msx2, a transcriptional stimulus for myofibroblast Wnt7b, Wnt7a, and Wnt3a expression and inhibitor of Dkk1 transcription4, 7. We wished to understand the role for Msx-Wnt signaling in endothelial cells, a vascular cell type that also elaborates Msx28, Dkk19, and Wnt7b19,9expression. Therefore, we studied the actions of retroviral SFG-Msx2 transduction on primary AoECs. When grown to high cell densities, AoECs transduced with the control virus SFG-LacZ adopt the cobblestone epithelial morphology characteristic of ECs (Figure 1A, upper left). This organized epithelial morphology appeared more pronounced in cells transduced with SFG-Msx2 (Figure 1A, upper right) and preserved with SFG-Wnt7b (Figure 1A, lower left). By contrast, cells transduced with SFG-Dkk1, expressing the potent Wnt-LRP5/6 receptor antagonist Dkk120, lost epithelial features and adopted a markedly spindle-shaped morphology characteristic of the myofibroblast (Figure 1A, lower right panel). This change was associated with the up-regulation of cellular phospho-β-catenin and down-regulation of dephospho-β-catenin as expected with Dkk1-mediated inhibition of canonical Wnt signaling (Supplement Figure S-I). To demonstrate that the spindle-shaped cells were derived from epithelioid ECs, AoECs were tagged with fluorescent acetylated-LDL (DiI AcLDL), a ligand for the scavenger receptor expressed on ECs but not myofibroblasts21. As shown in Figure 1B, DiI AcLDL-labeled only the epithelioid cells in SFG-LacZ - and SFG-Dkk1 - transduced cultures when imaged following a 4 hour labeling “pulse” (left panels). The spindle-shaped cells that were already present in the SFG-Dkk1 transduced AoEC cultures were not labeled by DiI AcLDL fluorescent dye immediately after pulse (Figure 1B, Left lower panels). Of note, only 3.9% of AoEC primary cells stain for VSMC alpha-actin (online Supplement Figure S-II), in excellent agreement with the observation that 97% +/− 1.9% of cells in primary AoEC cultures were labeled with DiI AcLDL (N = 369 cells scored in 6 separate wells, data not shown). Thus, in primary AoEC culture, approximately 4% of cells were of non-endothelial phenotype. Following a 4 day “chase,” achieved by incubation in the absence of DiI probe, the initial DiI AcLDL labeling of SFG-LacZ control AoEC cultures was retained only in cuboidal epithelial cells (Figure 1B, right upper panel); however, in SFG-Dkk1 cultures the DiI AcLDL label was mostly observed in spindle or oval-shaped cells (Figure 1B, right lower panel), confirming derivation of myofibroblastic cells from AoECs. The percentage of DiI-positive cells possessing a spindle or oval shaped morphology 4 days after labeling increased 10-fold with constitutive Dkk1 expression, ranging from 7% of cells in SFG-LacZ control AoEC cultures to just over 70% of the cell population in SFG-Dkk1 cultures (Figure 1C).
Figure 1. Dkk1 inhibits EC epithelial cobblestone morphology and capillary–like network formation in primary bovine AoEC cultures.
Panel A, as compared to SFG-LacZ controls (upper left), SFG-Dkk1 transduced AoEC cultures (lower right) exhibit spindle-shaped morphology and perturbed cobblestoning. SFG-Wnt7b and SFG-Msx2 transduced cultures more closely resemble SFG-LacZ controls (scale bars 100µm). Panel B, pulse-chase labeling of endothelial cell cultures indicate that AoECs labeled with DiI AcLDL for 4 h (left panel, 100×) adopt a spindle- or oval-shape in SFG-Dkk1 transduced cells after a 4 day chase (right panel, 400×). Note that the spindle-shaped cells, which were already present in the SFG-Dkk1 transduced EC culture at the beginning of pulse, were not labeled by the DiI AcLDL fluorescent dye during the 4 h pulse (lower left panels). (scale bars = 100µm).
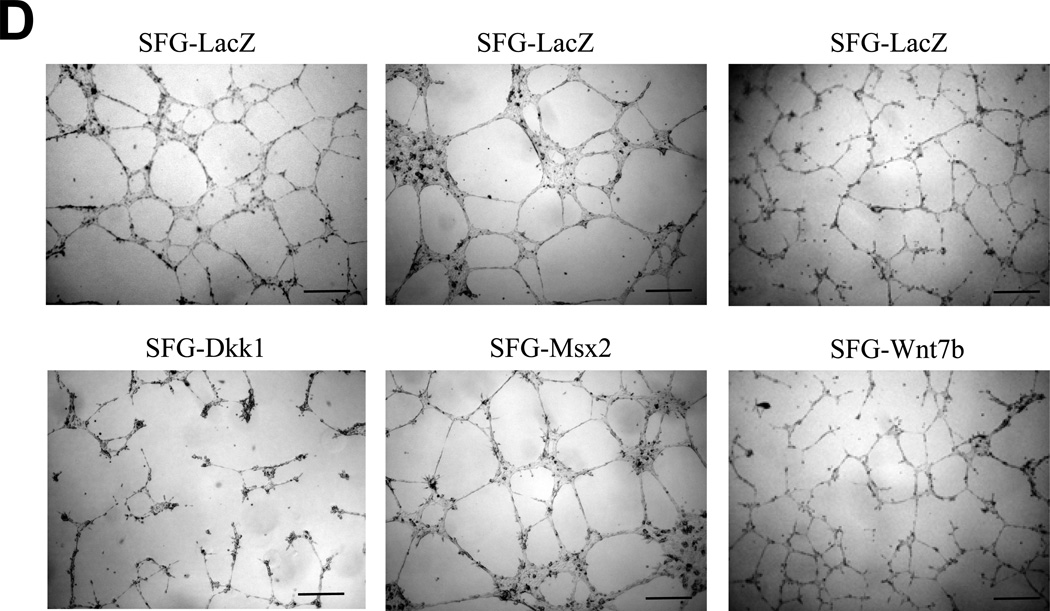
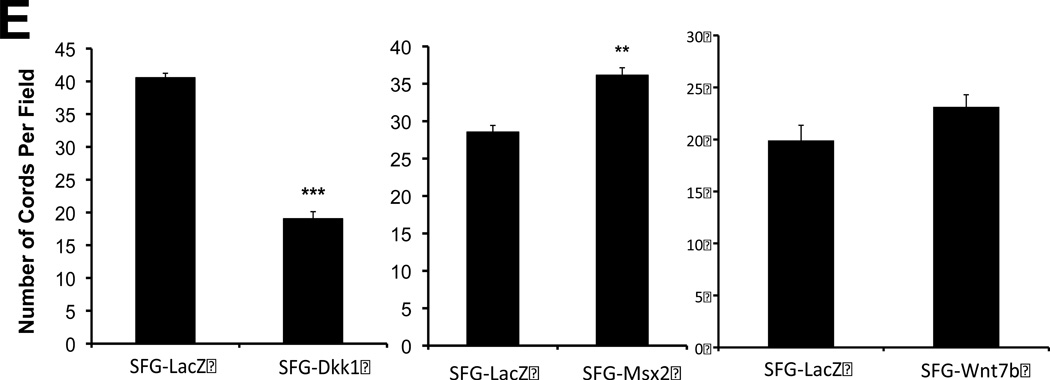
Panel C, SFG-Dkk1 increases the percentage of spindle- and oval-shaped cells derived from labeled cuboidal AoEC. Cells were labeled with DiI AcLDL for 4 hours. Four days later, the number of labeled spindle- and oval-shaped cells and the total number of labeled cells were quantified. %EndMT is defined as (number of DiI AcLDL-labeled spindle- and oval-shaped cells/total number of labeled cells) X 100. SFG-LacZ: n=37 fields assessed; SFG-Dkk1: n=56 fields assessed. ***, p<0.001 vs. SFG-LacZ. Panel D, as compared to SFG-LacZ controls, AoECs transduced with SFG-Dkk1 exhibit reduced capillary-like network formation on Matrigel while SFG-Msx2 and SFG-Wnt7b maintain this activity. Shown are representative photomicrographs taken at 40× magnification (scale bars 200µm). Panel E, SFG-Dkk1 significantly decreases capillary–like network formation by AoECs cultured on Matrigel. Unlike SFG-Dkk1, SFG-Msx2 and SFG-Wnt7b network formation. ***, p<0.001 vs. SFG-LacZ; **, p<0.01 vs. SFG-LacZ. The cords formed were counted in at least 6 fields per well and there were 3 wells for each cell line.
We wished to further confirm the inhibitory actions of Dkk1 on EC morphology and function. Therefore, we examined the effects of Msx2 and Wnt7b vs. Dkk1 expression on capillary-like network formation by bovine AoECs cultured in Matrigel, a permissive basement membrane-based extracellular matrix for vascular network assembly22. Expression of Dkk1 significantly reduced endothelial cord morphogenesis (Figure 1D, left panels). In contrast, expression of Msx2 and Wnt7b retained endothelial cord formation (Figure 1D, middle and right panels). Quantitative data obtained from digital images demonstrated a >50% reduction in capillary-like network density per high power field in Dkk1 cells (Figure 1E, left panel). By contrast, Msx2 under these same conditions increased network density by 27% (Figure 1E, middle panel). Wnt7b expression induced a non-significant trend (p= 0.08) for increased network density as well (Figure 1E, right panel). Thus the Wnt receptor antagonist Dkk1 inhibits AoEC epithelial morphology and endothelial cord network assembly in vitro, exerting actions opposite to those of activated Msx-Wnt signaling.
Dkk1 signaling inhibits the expression of EC differentiation markers and promotes the endothelial-mesenchymal transition in primary bovine AoEC cultures
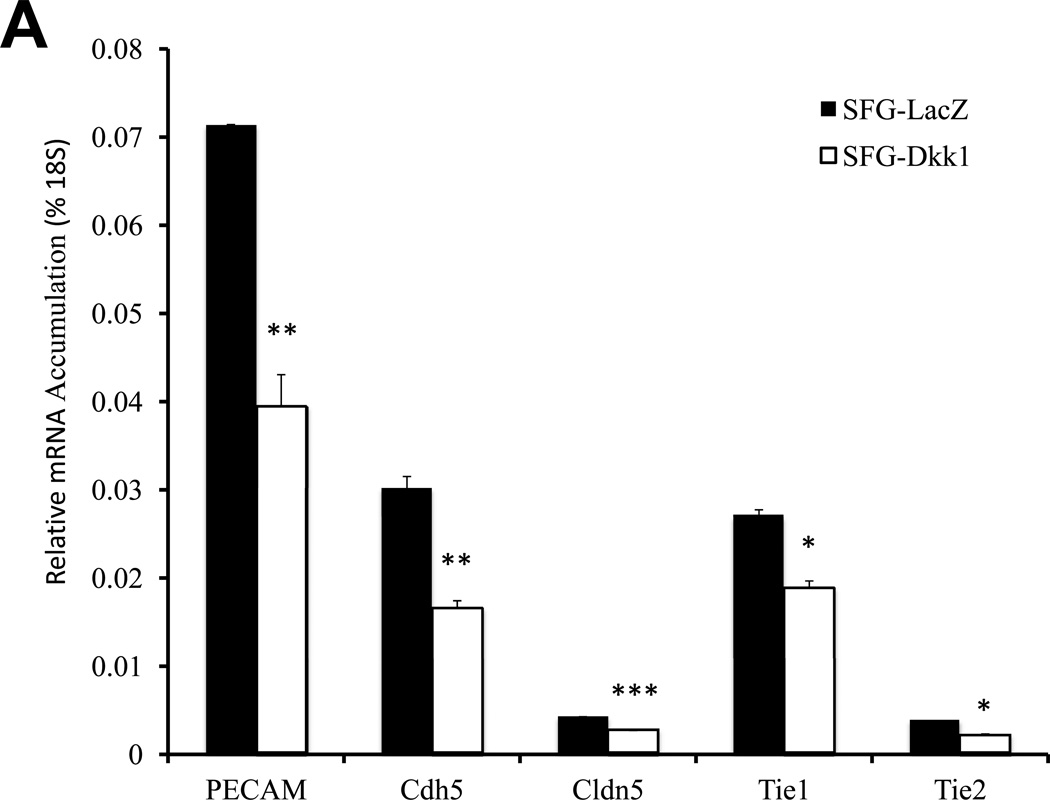
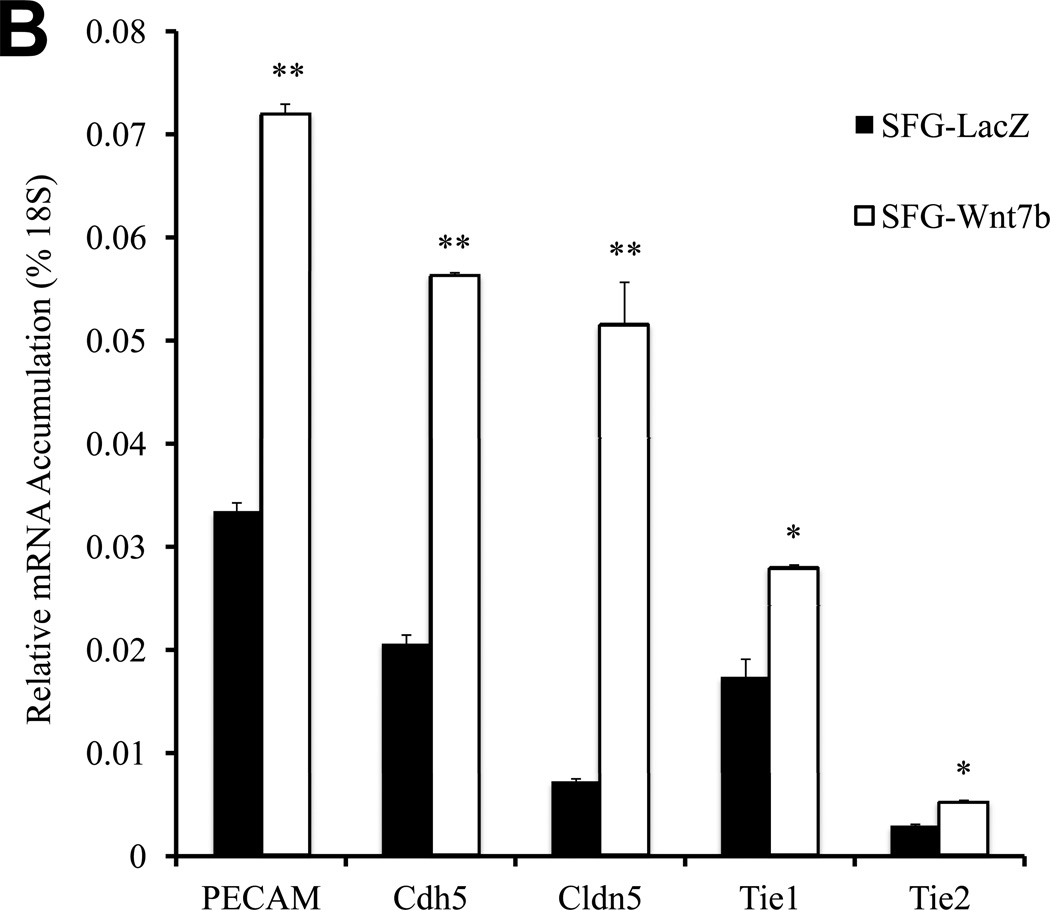
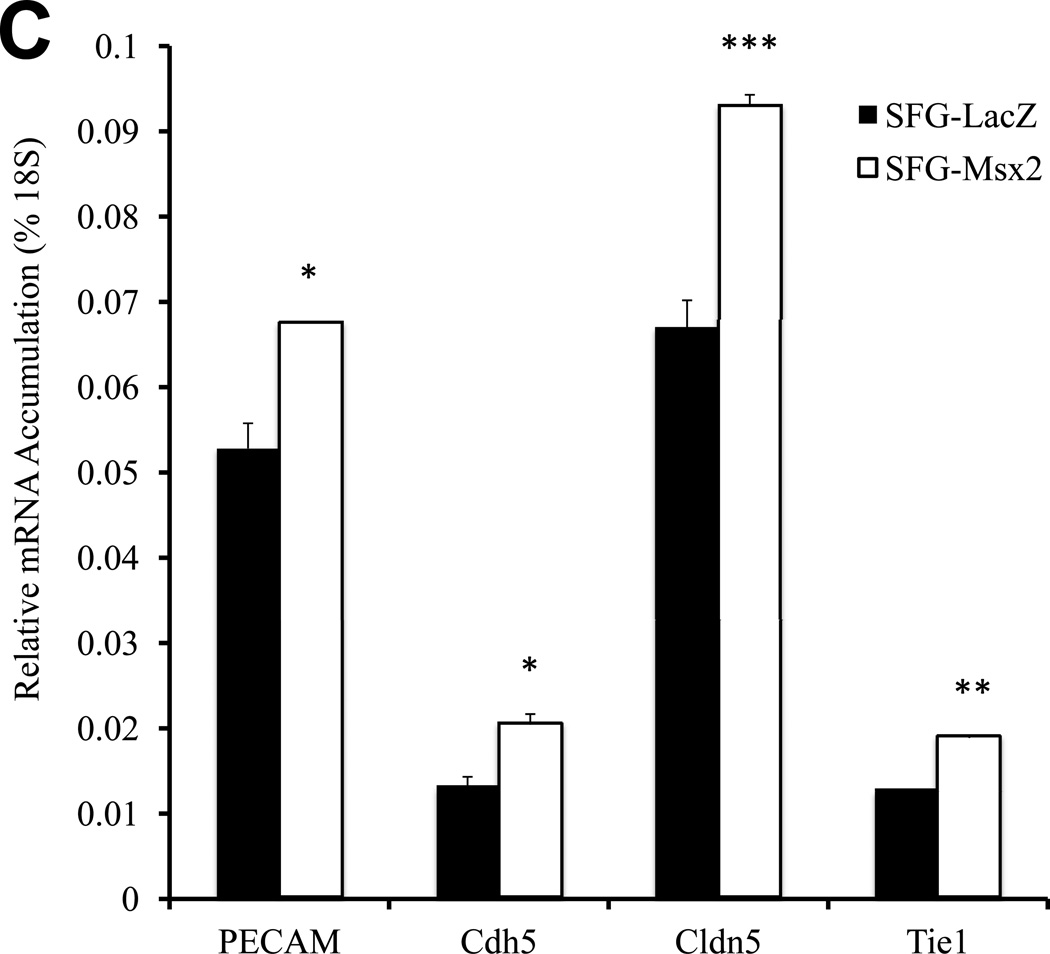
Epithelial morphology of EC in culture is dependent upon cell adhesion molecules such as platelet endothelial cell adhesion molecule (PECAM a.k.a. CD31) that drive and demarcate EC differentiation23. Vascular endothelial cadherin (VECAD a.k.a. Cdh5)24, PECAM25, and claudin 5 (Cldn5)26 are 3 EC phenotypic markers that maintain epithelioid morphology and endothelial functional integrity. Thus, we examined the impact of Dkk1, Msx2 and Wnt7b on the expression of these EC markers. Consistent with the perturbation in epithelial morphology and capillary tube formation, SFG-Dkk1 significantly down-regulated Cdh5, PECAM, and Cldn5 in bovine AoEC cultures (Figure 2A; all p < 0.05 vs. SFG-LacZ control). The mRNA for Tie1 (an EC- and early hematopoietic cell- restricted receptor tyrosine kinase27) was also down-regulated by 30% (Figure 2A; all p < 0.05). Tie2 mRNA was also reduced by 45% (p=0.02; Figure 2A). By contrast, SFG-Wnt7b (Figure 2B) -- and to a lesser extent SFG-Msx2 (Figure 2C) -- significantly augmented expression of EC markers in cultured AoECs. Of note, SFG-Wnt7a elicited responses similar to those observed with SFG-Wnt7b in transduced AoECs (data not shown). Thus, Dkk1 inhibits and Msx2-Wnt7 signaling promotes EC differentiation programs and capillary-like network morphogenesis in AoECs.
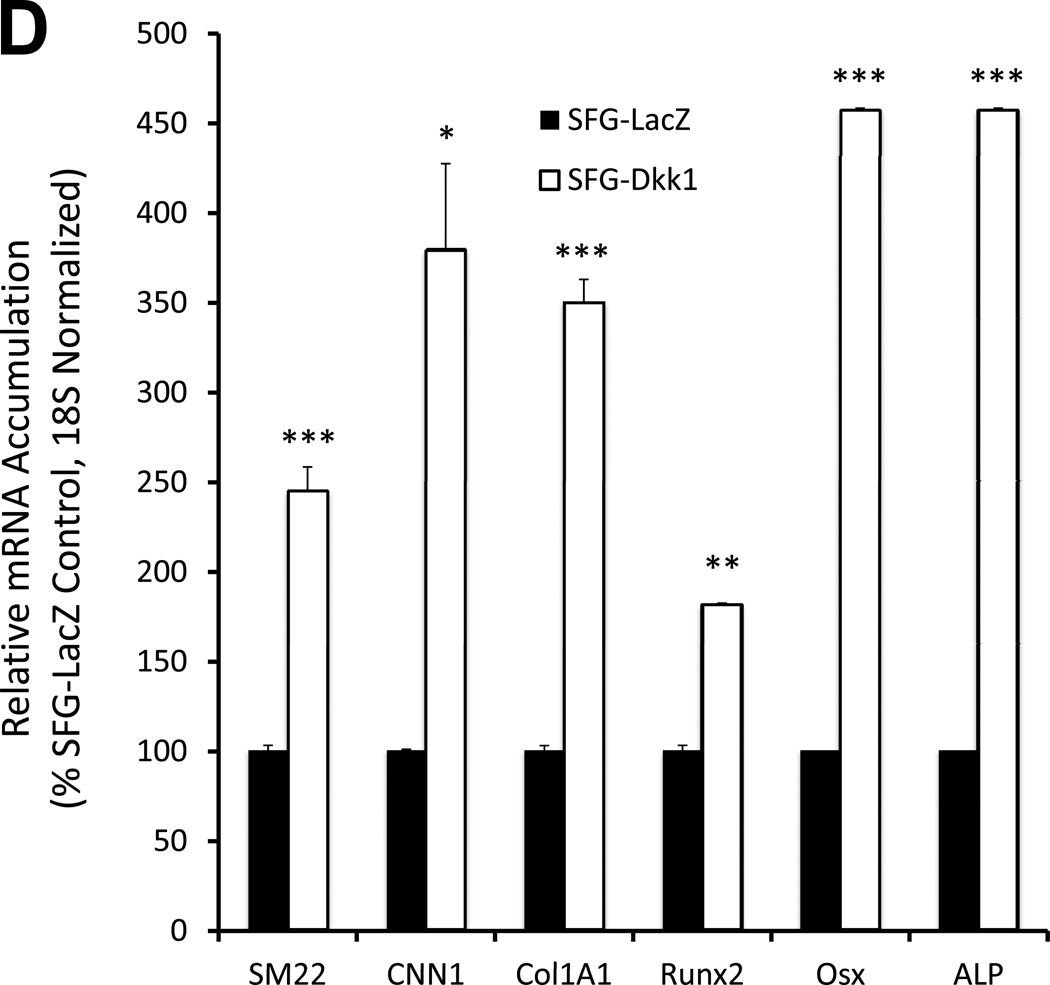
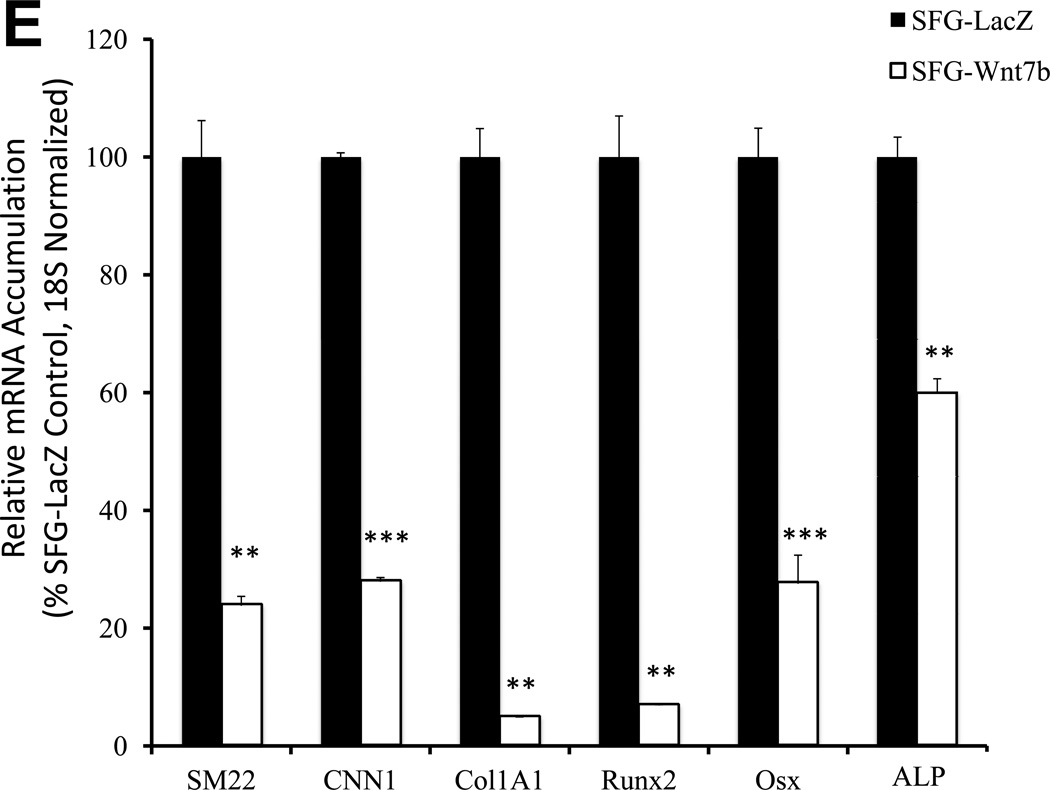
Figure 2. Dkk1 signaling inhibits the expression of EC differentiation markers and promotes the endothelial-mesenchymal transition in primary bovine AoEC cultures.
Panel A, SFG-Dkk1 down-regulates expression of EC differentiation markers. RNA was harvested for analysis following 5 days in culture. Panel B, by contrast, SFG-Wnt7b promotes expression of the EC phenotype. Panel C, SFG-Msx2 also upregulates EC markers. Panel D, SFG-Dkk1 concomitantly upregulates VSMC and osteofibrogenic gene expression. Panel E, SFG-Wnt7b suppresses VSMC and osteofibrogenic gene expression in aortic endothelial cells. *, p < 0.05 vs. SFG-LacZ; **, p < 0.01 vs. SFG-LacZ; ***, p < 0.001 vs. SFG-LacZ. All experiments were performed in triplicate and repeated at least twice.
The down-regulation of EC differentiation markers and epithelial morphology with concomitant adoption of spindle-shaped morphology suggested that SFG-Dkk1 was promoting an endothelial-mesenchymal transition (EndMT) -- a post-natal vascular injury response recently identified as contributing to tissue fibrosis, ectopic ossification and calcification in several disease contexts12, 13. To confirm this notion, we assessed the expression of SM22 (transgelin) and CNN1 (calponin) -- mesenchymal cell markers expressed by myofibroblasts28 and vascular smooth muscle cells29 – and the Col1A1 transcript characteristic of wound myofibroblasts30 and differentiating osteoblasts31. Consistent with an EndMT, SFG-Dkk1 upregulated SM22, CNN1, and Col1A1 in AoECs (Figure 2D). Furthermore, SFG-Dkk1 concomitantly upregulated the expression of Runx2, Osx, and ALP (alkaline phosphatase) -- phenotypic markers characteristic of mineralizing osteogenic cells (Figure 2D). Conversely, SFG-Wnt7b suppressed myofibroblast and osteogenic gene expression in AoECs (Figures 2E). These genomic responses to Wnt7b and Dkk1 are the opposite of responses elicited in C3H10T1/2 mesenchymal cells4, 7. Thus, Dkk1 promotes while Wnt7b signaling inhibits gene expression changes characteristic of the EndMT in bovine AoEC cultures.
Dkk1 promotes and Wnt7b inhibits osteogenic calcium deposition directed by AoECs cultured under mineralizing conditions
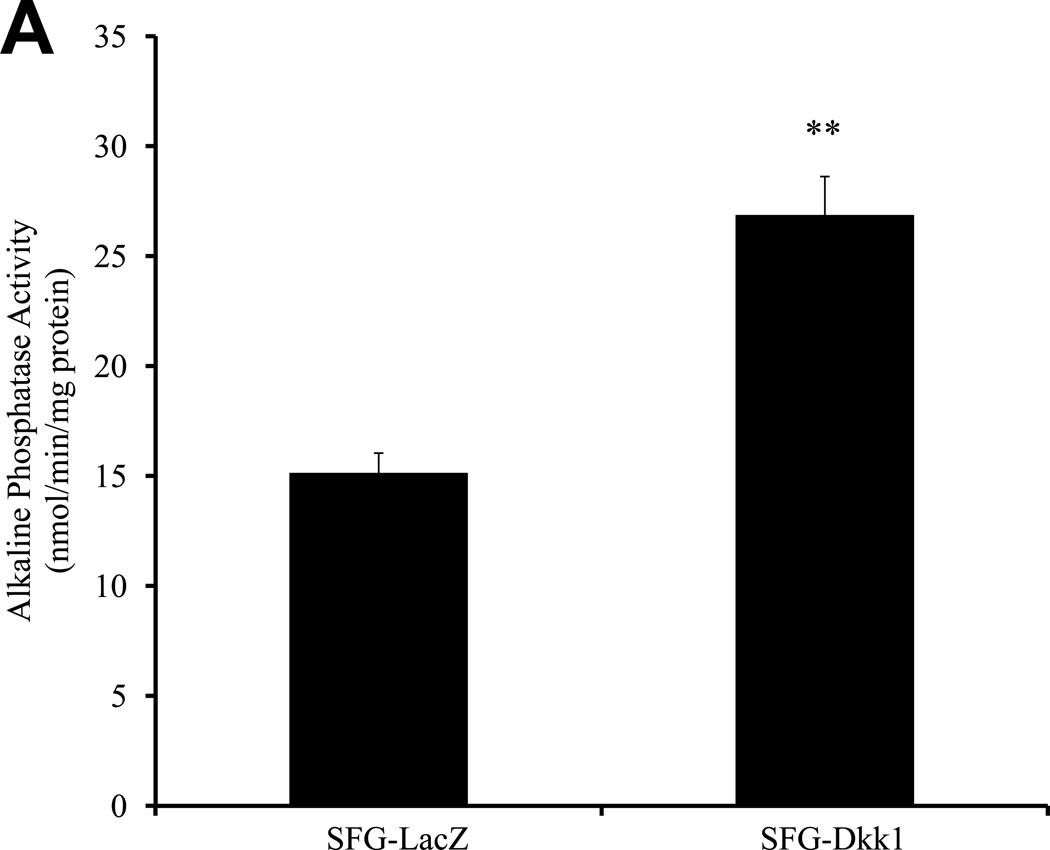
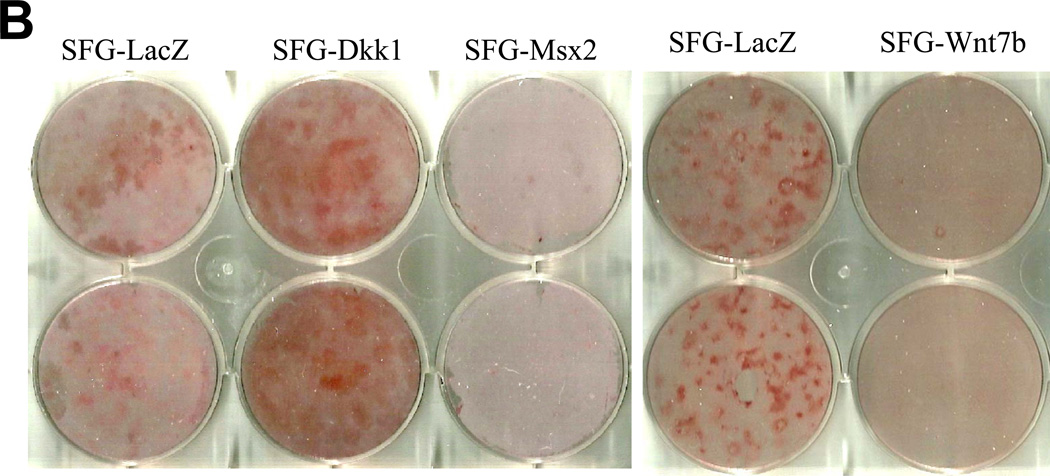
The changes observed strongly suggested that Dkk1 promotes, whilst Msx2 and Wnt7b inhibit, the EndMT in AoECs. To provide additional evidence supporting this notion, we examined the impact of SFG-Dkk1, SFG-Msx2, and SFGWnt7b on osteogenic calcium accumulation in AoECs cultured under conditions that support osteogenic mineralization. As shown in Figure 3A, SFG-Dkk1 upregulated ALP enzyme activity in bovine AoECs -- an enzyme absolutely required for osteogenic mineralization32. Moreover, matrix calcium deposition as assessed by Alizarin Red S staining33 was significantly enhanced by SFG-Dkk1 (Figure 3B, 3C). By contrast, SFG-Wnt7b and SFG-Msx2 suppressed AoEC mineralization (Figure 3B, 3C). Following 11 days of culture, collagen protein accumulation was consistently greater in SFG-Dkk1 vs. SFG-LacZ cultures (29.3 +/− 1.0 ug collagen/cm2 culture vs. 20.5 +/− 0.5 ug/cm2; n = 3, p < 0.01, seeding density 10,000 cells/cm2). Of note, the pro-calcific actions of Dkk1 and anti-calcific actions of Msx2-Wnt7 signaling in AoECs are the opposite of the responses elicited in adventitial myofibroblasts4, 18, 3T3-L1 cells34, and C3H10T1/2 cells7 – i.e., cells already committed to the mesenchymal lineage. Thus, endothelial Dkk1 promotes and Wnt7b inhibits osteofibrogenic differentiation of cultured bovine AoECs.
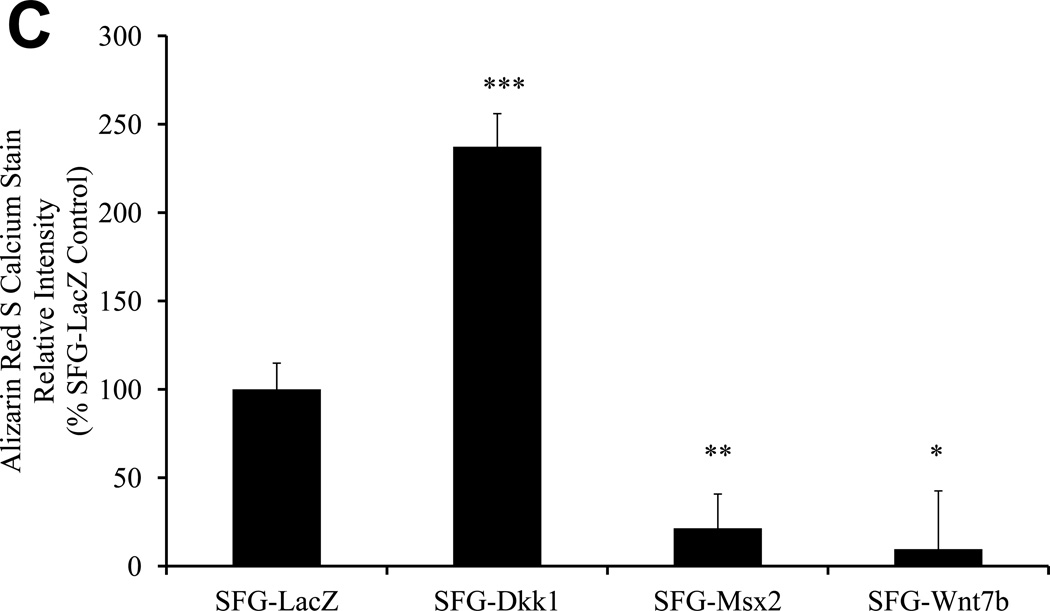
Figure 3. Dkk1 promotes and Wnt7b inhibits osteogenic calcium deposition directed by bovine AoECs.
Panel A, SFG-Dkk1 upregulates alkaline phosphatase enzyme activity in AoECs, a key component of osteogenic mineralization. **, p<0.01 vs. SFG-LacZ. Panel B, Alizarin red S staining reveals SFG-Dkk1 upregulates while SFG-Msx2 and SFG-Wnt7b suppress calcification of bovine AoECs cultured under mineralization conditions. This is the opposite of the response elicited in mesenchymal cells (ref. 7). Panel C, digital quantification of Alizarin Red S staining demonstrates significant and reciprocal regulation of calcification in bovine AoECs transduced with Dkk1, Msx2, and Wnt7b expression vectors. ANOVA p < 0.0001; *, p < 0.05 vs. SFG-LacZ; **, p < 0.01 vs. SFG-LacZ; ***, p < 0.001 vs. SFG-LacZ control. All experiments were performed in triplicate and repeated at least twice.
Dkk1 promotes Smad activation, Smad-dependent transcription, and ALK-mediated signaling in bovine AoECs
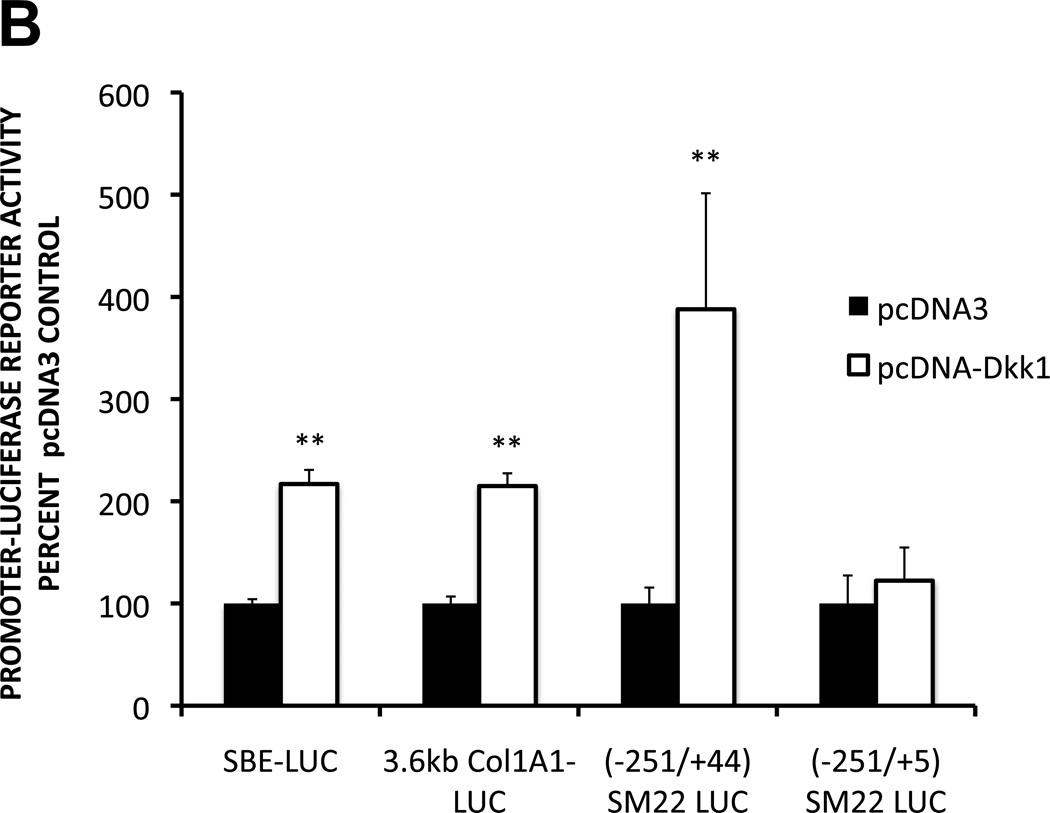
Smad transcription factor pathway activation is a consistent molecular mediator of both EndMT35 and osteogenic mineralization36, 37, signaling downstream of ALK receptor activation. We posited that the responses induced by SFG-Dkk1 transduction of AoECs might be dependent upon these same Smad-dependent signaling pathways. To test this notion, we examined the expression and nuclear accumulation of phospho-Smad1/5 (pSmad1/5) as an index of osteogenic pathway activation36. As shown in Figures 4A, bovine AoECs transduced with SFG-Dkk1 exhibited between 1.5- to 3.1 -fold higher levels of pSmad1/5 as compared to SFG-LacZ controls. Conversely, bovine AoECs transduced with SFG-Wnt7b accumulated levels of pSmad1/5 that were reduced by 40% (Figure 4A). Moreover, cells transduced with SFG-Dkk1 consistently yielded greater extent and relative intensity of nuclear pSmad1/5 staining (Supplement Figures S-IIIA and S-IIIB). Similar responses were noted for pSmad3 in both nuclear and cytoplasmic compartments (Supplement Figure S-IIIC, and data not shown). Furthermore, co-transfection of a pcDNA3-Dkk1 expression vector upregulated the activity of SBE-LUC (Smad binding element promoter –luciferase reporter) in AoECs, as well as the Smad-dependent COL1A16 and SM2238 promoter-LUC reporters (Figure 4B). The SM22 promoter encodes a GTCTG Smad binding element in exon I that is required for VSMC expression in vivo39. Deletion of this exonic Smad response element -- as occurs by 3’ promoter truncation of SM22[−215/+44] to SM22[−215/+5] -- abrogated induction by pcDNA3-Dkk1 co-transfection (Figure 4B), providing additional evidence for the importance of Smad signaling in Dkk1 control of AoEC gene expression.
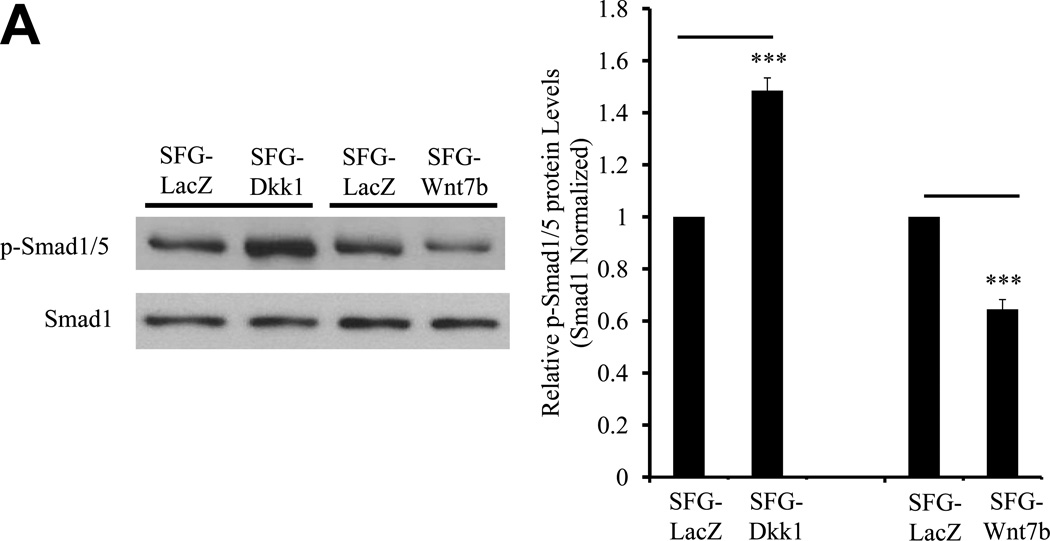
Figure 4. Dkk1 promotes Smad activation, Smad-dependent transcription, and ALK-mediated signaling in bovine AoECs.
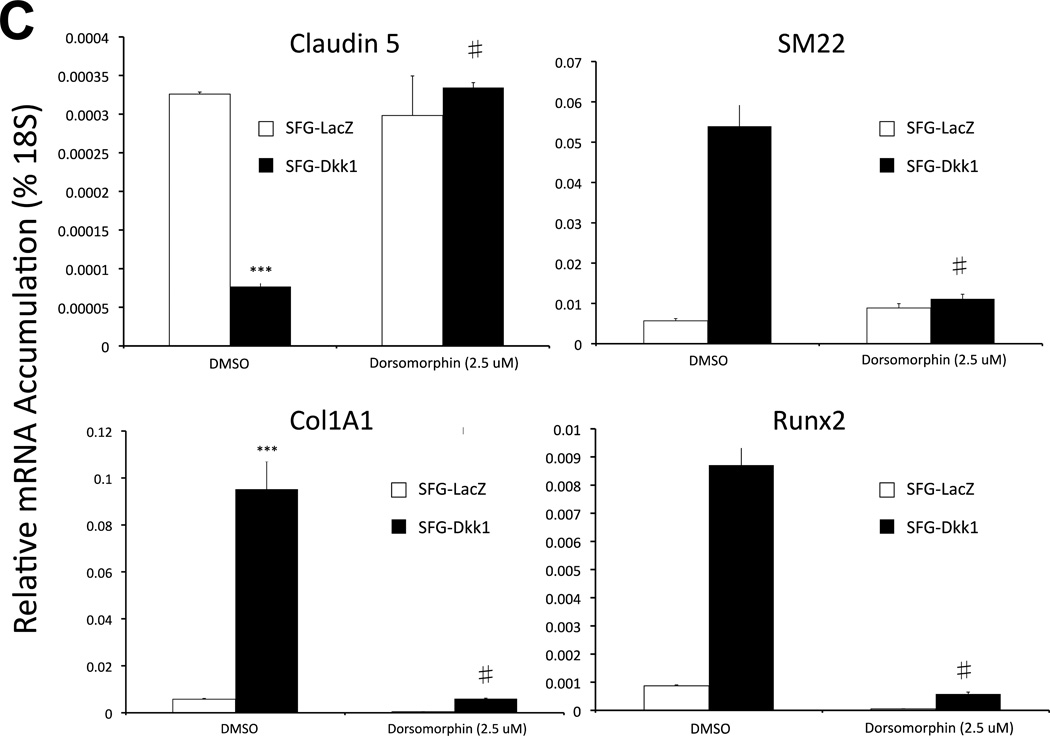
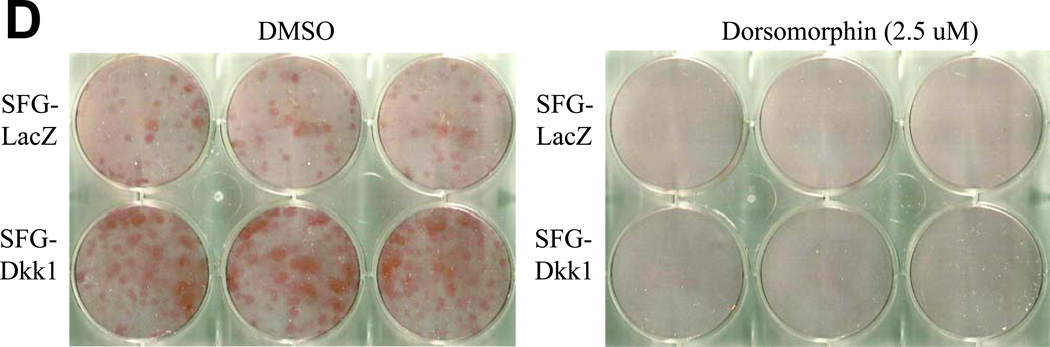
Panel A, SFG-Dkk1 upregulates the accumulation of Smad 1/5 Ser phosphorylation, an index of activated ALK/Smad signaling. By contrast, SFG-Wnt7b suppresses Smad 1/5 phosphorylation in AoEC cultures. Left: representative Western blot analysis; Right: Results of digital image analysis of relative pSmad band intensity; n = 6 / group. ***, p<0.001 vs. respective SFG-LacZ. Panel B, co-transfection of the expression plasmid pDNA-Dkk1 upregulates Smad-dependent promoter-luciferase reporters. (−251/+5) SM22LUC, lacking an exonic Smad binding element, is not regulated.**, p < 0.01 vs. pcDNA3 control. All transfections were performed in quadruplicate. Panel C, dorsomorphin, an inhibitor of multiple ALK Smad kinases, inhibits Dkk1 actions on AoEC and osteofibrogenic markers. ***, p < 0.001 vs. SFG-LacZ control. #, p < 0.001 vs. DMSO-treated SFG-Dkk1 transduced cells. Panel D, Alizarin Red S staining reveals that dorsomorphin inhibits basal and Dkk1-stimulated osteogenic calcium deposition of AoECs cultured under mineralizing conditions. All experiments were performed in triplicate and repeated at least twice.
To further confirm the functional contributions of Smad signaling, we examined the impact of dorsomorphin, an inhibitor of ALK receptors mediating Smad1/5 phosphorylation and activation40. As shown in Figure 4C, treatment of AoECs with dorsomorphin abrogated Dkk1 mediated induction of Col1A1, SM22, and Runx2 – and prevented Dkk1 suppression of claudin 5. Dorsomorphin also inhibited basal and Dkk1-enhanced mineralization of AoEC cultures (Figure 4D). Thus, Dkk1 exhibits “cross-talk” with ALK/Smad-dependent pathways previously identified to support EndMT and osteogenic mineralization. ALK/Smad-signaling is necessary for osteofibrogenic signaling responses elicited in Dkk1 in AoECs.
Conditional deletion of Wnt7b in aortic endothelial cells increases aortic collagen accumulation in LDLR−/− mice fed high fat diabetogenic diets
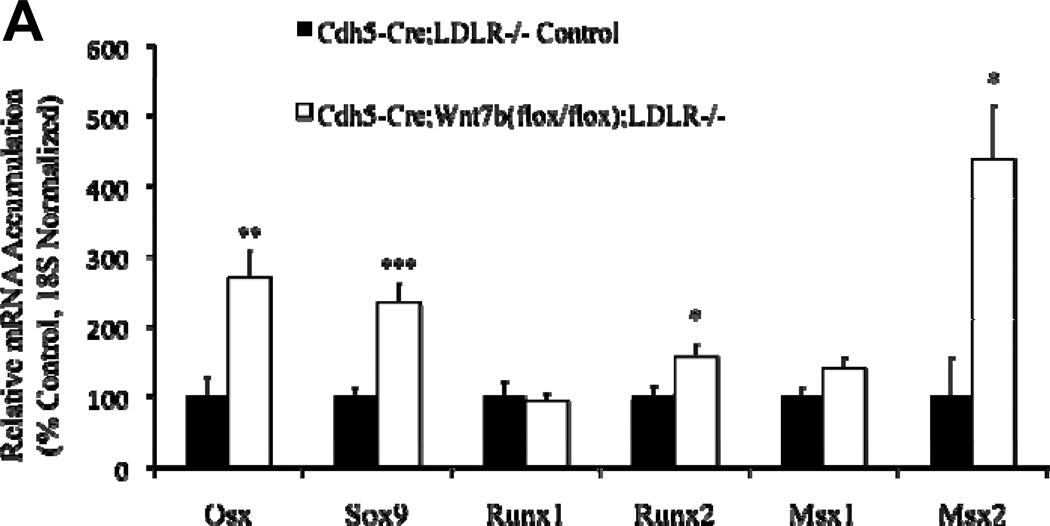
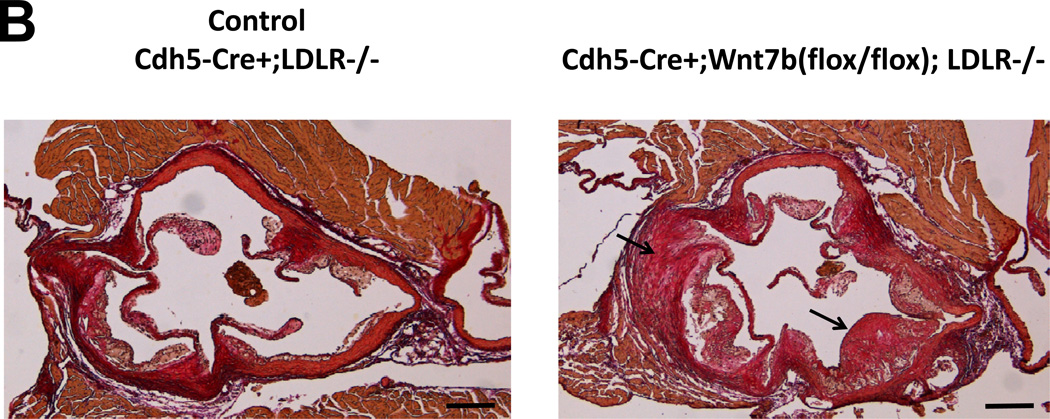
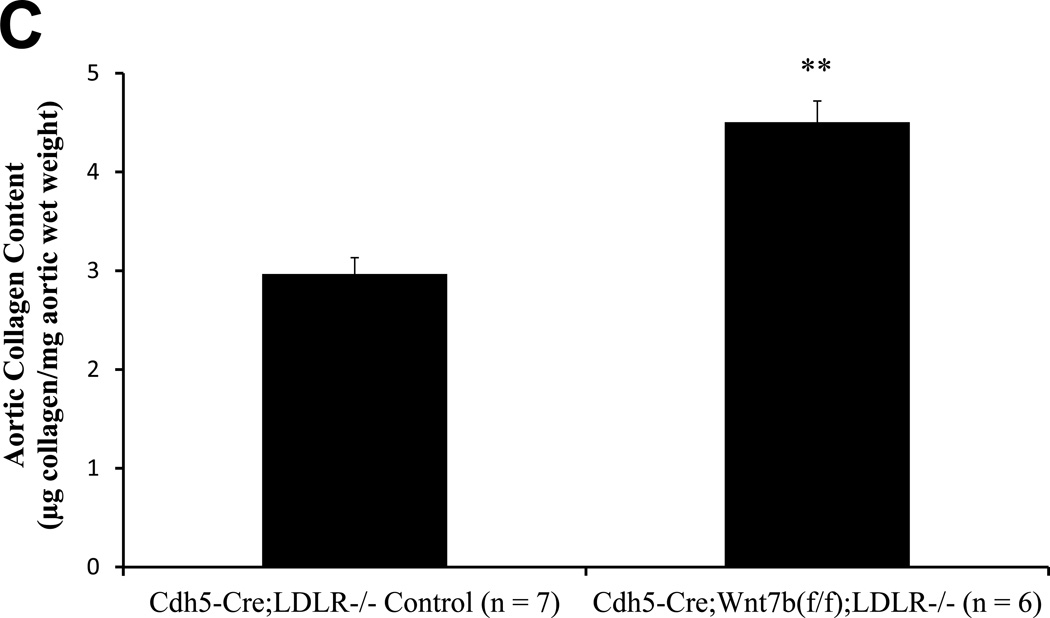
By immunohistochemistry, Wnt7b is detected in the endothelium of adult aortic and aortic valve endothelial cells10(Supplement Figure S-IVA and S-IVB), consistent with prior reports9,19. SFG-Wnt7b “super-transduction” of bovine AoECs previously transduced with SFG-Dkk1 effectively reverses Col1A1 induction by Dkk1 and restores claudin 5 expression (Supplement Figure S-V). This confirmed that Dkk1 and Wnt7b reciprocally regulate EC physiology, with Wnt7b maintaining EC function and mitigating sclerotic responses in vitro. We wished to test this notion in vivo. Therefore, using existing murine reagents we generated Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice in which the Cdh5 promoter is used to direct EC Cre transgene expression41 and thus delete the floxed Wnt7b gene42 in ECs. As compared to Cdh5-Cre;LDLR−/− controls, Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice exhibited greater levels of aortic osteogenic transcription programs (Figure 5A). Moreover, after 3 months of high fat diet challenge, Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice exhibited histological evidence of larger, more fibrotic atherosclerotic lesions (Figure 5B). Biochemical analysis of aortic collagen content by Sircol assay confirmed the histological data; Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− possessed 52% greater levels of aortic collagen content (Figure 5C; p = 0.003). Moreover, in this same cohort Alizarin Red S staining for calcium deposition revealed increased intensity in the aortas of Cdh5Cre;Wnt7b(fl/fl);LDLR−/− mice (Supplement Figure S-VI). Of note, in an independent experimental cohort, biochemical analysis confirmed increased aortic calcium accumulation in Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice vs. controls (0.24 +/− 0.02 ug calcium / mg aortic dry weight vs. 0.19 +/− 0.01 ug/mg; p = 0.03; n = 10 and 12 per group, respectively) following 6 weeks of HFD. Immunohistochemistry demonstrated increased intensity and extent of nuclear pSmad1/5 in aortas of Cdh5Cre;Wnt7b(fl/fl);LDLR−/− mice vs. controls (Supplement Figure S-VIIA–C; 1.5 fold, p<0.0001, Mann-Whitney U test). No significant differences existed between the two groups in fasting serum glucose, cholesterol or triglyceride levels (Supplement Figure S-VIII). Immunohistochemistry verified reduction in endothelial Wnt7b in Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice vs. Cdh-Cre;LDLR−/− controls. Because the Wnt7 antibody used detects both Wnt7a and Wnt7b, the lack of significant Wnt7 staining after Wnt7b deletion suggested that Wnt7b was the major Wnt7 protein expressed by ECs at this stage (Supplement Figure S-IX). Thus, as observed in vitro with primary bovine AoECs, endothelial Wnt7b serves to limit expression of vascular osteogenic gene program and mitigate the arteriosclerotic injury response in vivo.
Figure 5. Conditional deletion of Wnt7b in aortic endothelial cells increases aortic osteogenic gene expression, collagen accumulation, and calcification in LDLR−/− mice.
Panel A, Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice express significantly greater levels of osteogenic transcription factor mRNAs in aortic tissue vs. controls. N = 6 – 7 per genotype. *, p < 0.05 vs. control; **, p < 0.01 vs. Control; ***, p < 0.001 vs. Control. Panel B, picrosirius red histochemistry for collagen deposition indicates greater fibrosis in Cdh5-Cre:Wnt7b(fl/fl);LDLR−/− mice (arrows) following 12 weeks of high fat diet (HFD). (scale bars 200µm). Panel C, biochemical measurement of aortic collagen by Sircol assay demonstrates significantly increased collagen deposition in Cdh5-Cre:Wnt7b(fl/fl);LDLR−/− mice vs. Cdh5-Cre;LDLR−/− controls following 12 weeks of HFD. Calcium deposition is also increased in Cdh5-Cre:Wnt7b(fl/fl);LDLR−/− mice (see text). **, p<0.01 vs. Control.
DISCUSSION
The spectrum of bioactivities recognized as being conveyed by Wnt signaling in vascular development and disease is rapidly expanding43, 44. Early data demonstrated placental capillary defects in Wnt2 knockout mice that cause embryonic lethality43, 44. Subsequent reports emphasized the expression, signaling, and biological consequences of Wnt/β-catenin and Wnt/Ca++ signaling in the developing vasculature43, 44. Both ECs and VSMCs express several Wnt ligands including Wnt7b9. Wnt7b, Wnt7a, and β-catenin signaling maintain the blood-brain barrier and vascular integrity of the developing central nervous system19, 44. With respect to postnatal vascular disease, Rajamannan’s group45 and our own4 identified canonical Wnt signaling as being an important component of aortic valve and vascular calcification. Recently, LRP5 signaling and downstream claudin 5 actions have been shown to control retinal EC proliferation in response to Wnt3a and Wnt7a46, relevant to the retinopathies of prematurity and diabetes.
The roles for the Dickkopf (Dkk) family of Wnt/LRP5/6 antagonists in vascular biology have only begun to be studied. Mice lacking both Dkk1 and Dkk2 die in utero with ventricular septal defects and hypertrophic failure arising from abnormal epicardial cell fating and myocardial proliferation47. Isolated deficiency in either Dkk1 or Dkk2 does not cause an overt myocardial phenotype, indicating some functional redundancy47. However, Dkk2 can differ markedly from Dkk1 in other contexts. Dkk2 enhances angiogenesis whilst Dkk1 suppresses angiogenesis via differential modulation of EC LRP6/Cdc42 signals48. The precise structural features conveying differential vascular responses of Dkk2 vs. Dkk1 have yet to be elucidated. Of note, Mercola identified that the N-terminal domain of Dkk1 promotes cardiogenesis independent of canonical Wnt signaling49. Structurally, Dkk1 and Dkk2 diverge most significantly in the N-terminal domain, and it is the conserved C-terminal CYS2 domain that binds LRP5 and LRP650. Thus, the unique activities elaborated by Dkk1 and Dkk2 likely relate in part to the N-terminal structural divergence. Nevertheless, the CYS2 domain of Dkk2 can activate rather than inhibit LRP6 signaling in certain cellular contexts,50 indicating that C-terminal domains also encode unique paralog functions. Circulating Dkk1 levels are elevated in patients with clinically significant atherosclerosis, and Dkk1 elicits an inflammatory endothelial response conveyed in part via inhibition of Wnt/β-catenin signaling51.
Our studies of Msx2-Wnt signaling and Dkk1 in cardiovascular calcification initially emphasized responses in adventitial myofibroblasts – the site of most robust Msx2 gene expression in diseased vessels4. In this mesenchymal cell background, others and we have shown that Msx2-Wnt signaling promotes early VSMC38, 52 and osteofibrogenic4, 7, 34, 53differentiation in part via canonical Wnt pathways inhibited by Dkk1. We now identify that EC responses are the opposite of those elicited in mesenchymal cells4, 7, 18. Dkk1 upregulates myogenic, myofibrogenic and osteochondrogenic programs in ECs – programs that are suppressed by Wnt7b in ECs. In vivo, the arteriosclerotic consequences of reduced endothelial Wnt agonism / Dkk1 antagonism were apparent; Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice exhibited more significant aortic fibrosis, collagen accumulation, and calcium deposition following challenge with atherogenic high fat diets. Based upon these data and the recent literature, a working model begins to emerge (Figure 6). Wnt7b helps to stabilizes the aortic EC phenotype and maintains endothelial integrity, consistent with activity first identified in CNS vasculature19. By inhibiting EC Wnt7b signals, Dkk1 provokes EC phenotypic modulation and increases the pool of mesenchymal progenitors in the VSMC lineage available for tissue repair. In this way, platelet-derived Dkk151 may synergize with other growth factors during the early phases of arterial injury. However, within the mesenchymal lineage, Msx-Wnt signaling supports the early myogenic and osteogenic differentiation programs – actions also opposed by Dkk1. Under such a model, the balance of Wnt7/Dkk1 “tone” modulates vascular cell access to EC, VSMC, and osteogenic fates. While providing a mechanistic advantage for responses to traumatic arterial wound healing, long-term endothelial Dkk1 exposure may worsen arterial fibrosis, calcification, and vascular stiffening in diabetic vascular disease. This latter notion has yet to be tested, but Dkk1 has been strongly implicated in the pathobiology of renal fibrosis in a preclinical model of diabetic nephropathy54.
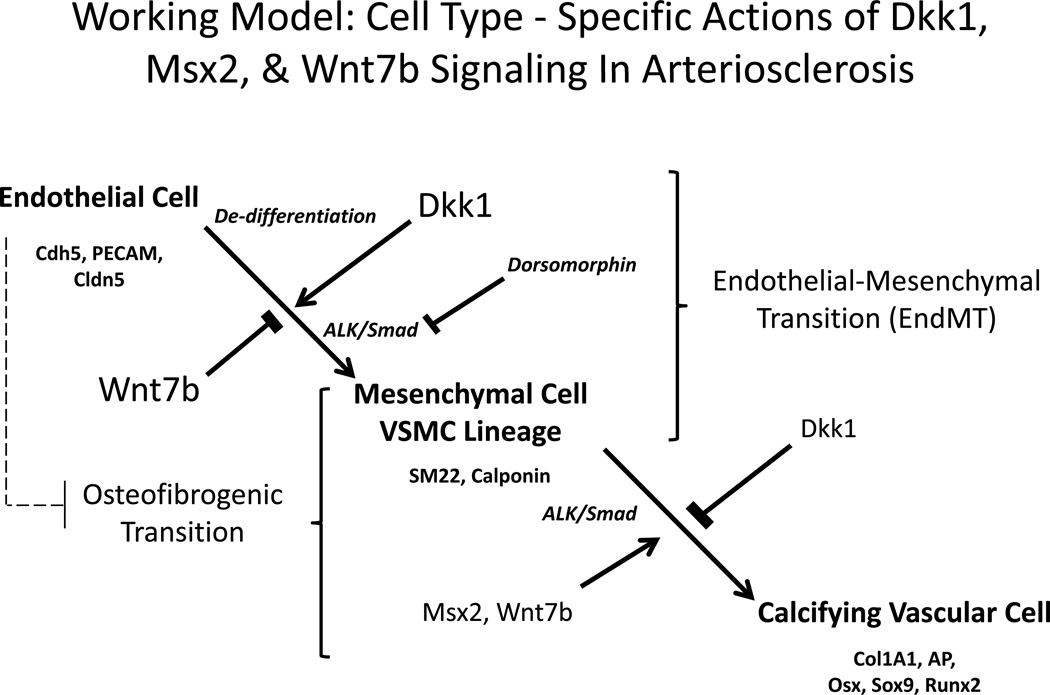
Figure 6. Working model of the cell type – specific actions of Dkk1, Msx2, and Wnt7b signaling in arteriosclerosis.
Dkk1, arising from platelets or cytokine –stimulated vascular cells can impair the autocrine actions of EC Wnt7b that stabilize the EC phenotype. This promotes EndMT, thus increasing the mesenchymal cell pool that contributes to vascular fibrosis. Committed mesenchymal progenitors, by contrast, elaborate the calcifying vascular cell phenotype in response to Msx-Wnt signaling, e.g. an osteofibrogenic transition. Juxtacrine EC cues may also regulate this latter process (dashed line). ALK/Smad signals are required for both mesenchymal and calcifying vascular programs. See Discussion.
There are, of course, limitations to our study. We identified that Dkk1 augments and Wnt7b inhibits ALK/Smad signaling to modulate EC phenotype; however, we have not yet identified how these ligands for multiple heterodimeric receptor complexes – e.g., LRP4/5/6, Frizzled and Kremen families -- control ALK/Smad signaling55. LRPs differentially heterodimerize with co-receptors dependent upon the ligand56, and we speculate that Dkk1 may alter the production or localization of LRP-ALK receptor signaling complexes. Of note, the genomic programs elaborated by EC are determined by epigenomic regulators57. It will be interesting to assess whether differential EC vs. VSMC chromatin modifications determine responses to Wnt7b and Dkk158. Moreover, we have not yet examined how EC-mediated juxtacrine control59 of previously committed VSMCs is altered as ECs undergo phenotypic change with Wnt7b deficiency. Changes in EC-mesenchymal cell “cross-talk” in the absence of EC Wnt7b may also contribute to the total myofibroblast numbers and phenotype in vivo (Figure 6). Furthermore, vascular cell types other than ECs and VSMCs9 express Wnt7b60. We have noted non-endothelial Wnt7b – expressing cells in aortas and valves of Cdh5-Cre;Wnt7b(fl/fl);LDLR−/− mice (supplement Figure S-IVA), and are currently determining the lineages of these cells. Nevertheless, our results extend emerging data that indicate roles for Dkk1 and Wnt in vascular pathobiology. Sophisticated strategies that selectively target EC Dkk1 actions may help limit aortic fibrosis and reduce calcium accrual – and thus improve arterial compliance and lower extremity perfusion in diabetic vascular disease.
Supplementary Material
Significance.
With advancing age, longstanding hypertension, and diabetes, the arterial vasculature becomes stiff, losing elastic compliance necessary for smooth distal tissue perfusion. This arteriosclerotic stiffening arises from atherosclerotic plaque burden, mural thickening and fibrosis, medial calcification, elastin fragmentation, non-enzymatic matrix glycation, and endothelial dysfunction. Msx-Wnt signaling induces fibrosis and calcification in myofibroblasts (mesenchymal cells) and is inhibited by Dkk1. However, endothelial cells (ECs) can contribute to fibrosis via the endothelial-mesenchymal transition (EndMT), and roles for canonical Wnt signaling, Msx2, and Dkk1 in EndMT remain unclear. We show that Dkk1 enhances EndMT in aortic ECs, while Wnt7b and Msx2 preserve EC phenotype. Msx2-, Wnt7b- and Dkk1- regulated arteriosclerotic responses in ECs are opposite of those elicited in mesenchymal cells. These cell type-specific responses provide a mechanism that couples angiogenesis with osteofibrogenic predilection during tissue injury. Sophisticated strategies that selectively target vascular cell type - specific Wnt signaling may help limit arteriosclerotic disease.
Acknowledgments
Funding Sources – Supported by NIH grants HL81138, HL69229, and HL88651 to D.A.T., the Barnes-Jewish Hospital Foundation, and the Sanford-Burnham Medical Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures – D.A.T. serves as a consultant for Eli Lilly and for Merck & Co.
LITERATURE CITED
- 1.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 2.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–282. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283:20505–20522. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goupille O, Saint Cloment C, Lopes M, Montarras D, Robert B. Msx1 and Msx2 are expressed in sub-populations of vascular smooth muscle cells. Dev Dyn. 2008;237:2187–2194. doi: 10.1002/dvdy.21619. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 10.Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wylie-Sears J, Aikawa E, Levine RA, Yang JH, Bischoff J. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YH, Ishii M, Sucov HM, Maxson RE., Jr Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium. BMC Dev Biol. 2008;8:75. doi: 10.1186/1471-213X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 17.Balachandran K, Alford PW, Wylie-Sears J, Goss JA, Grosberg A, Bischoff J, Aikawa E, Levine RA, Parker KK. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc Natl Acad Sci U S A. 2011;108:19943–19948. doi: 10.1073/pnas.1106954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 19.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 20.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netland PA, Zetter BR, Via DP, Voyta JC. In situ labelling of vascular endothelium with fluorescent acetylated low density lipoprotein. Histochem J. 1985;17:1309–1320. doi: 10.1007/BF01002528. [DOI] [PubMed] [Google Scholar]
- 22.Ponce ML. In vitro matrigel angiogenesis assays. Methods Mol Med. 2001;46:205–209. doi: 10.1385/1-59259-143-4:205. [DOI] [PubMed] [Google Scholar]
- 23.Sheibani N, Frazier WA. Down-regulation of platelet endothelial cell adhesion molecule-1 results in thrombospondin-1 expression and concerted regulation of endothelial cell phenotype. Mol Biol Cell. 1998;9:701–713. doi: 10.1091/mbc.9.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burek M, Arias-Loza PA, Roewer N, Forster CY. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler Thromb Vasc Biol. 2010;30:298–304. doi: 10.1161/ATVBAHA.109.197582. [DOI] [PubMed] [Google Scholar]
- 27.Woo KV, Qu X, Babaev VR, Linton MF, Guzman RJ, Fazio S, Baldwin HS. Tie1 attenuation reduces murine atherosclerosis in a dose-dependent and shear stress-specific manner. J Clin Invest. 2011;121:1624–1635. doi: 10.1172/JCI42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molleken C, Sitek B, Henkel C, Poschmann G, Sipos B, Wiese S, Warscheid B, Broelsch C, Reiser M, Friedman SL, Tornoe I, Schlosser A, Kloppel G, Schmiegel W, Meyer HE, Holmskov U, Stuhler K. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology. 2009;49:1257–1266. doi: 10.1002/hep.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prosdocimo DA, Wyler SC, Romani AM, O'Neill WC, Dubyak GR. Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: synergistic modulation by cyclic AMP and hyperphosphatemia. Am J Physiol Cell Physiol. 2010;298:C702–C713. doi: 10.1152/ajpcell.00419.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos N, Poulalhon N, Farge D, Madelaine I, Mauviel A, Verrecchia F. In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther. 2007;321:583–589. doi: 10.1124/jpet.106.117051. [DOI] [PubMed] [Google Scholar]
- 31.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969;17:110–124. doi: 10.1177/17.2.110. [DOI] [PubMed] [Google Scholar]
- 34.Qadir AS, Lee HL, Baek KH, Park HJ, Woo KM, Ryoo HM, Baek JH. Msx2 is required for TNF-alpha-induced canonical Wnt signaling in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2011;408:399–404. doi: 10.1016/j.bbrc.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M, Okubo N, Chosa N, Hasegawa T, Ibi M, Kamo M, Kyakumoto S, Ishisaki A. TGF-beta-Operated Growth Inhibition and Translineage Commitment into Smooth Muscle Cells of Periodontal Ligament-Derived Endothelial Progenitor Cells through Smad- and p38 MAPK-Dependent Signals. Int J Biol Sci. 2012;8:1062–1074. doi: 10.7150/ijbs.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- 37.Kim BG, Lee JH, Yasuda J, Ryoo HM, Cho JY. Phospho-Smad1 modulation by nedd4 E3 ligase in BMP/TGF-beta signaling. J Bone Miner Res. 2011;26:1411–1424. doi: 10.1002/jbmr.348. [DOI] [PubMed] [Google Scholar]
- 38.Shafer SL, Towler DA. Transcriptional regulation of SM22alpha by Wnt3a: convergence with TGFbeta(1)/Smad signaling at a novel regulatory element. J Mol Cell Cardiol. 2009;46:621–635. doi: 10.1016/j.yjmcc.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu P, Ritchie RP, Fu Z, Cao D, Cumming J, Miano JM, Wang DZ, Li HJ, Li L. Myocardin enhances Smad3-mediated transforming growth factor-beta1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22alpha transcription in vivo. Circ Res. 2005;97:983–991. doi: 10.1161/01.RES.0000190604.90049.71. [DOI] [PubMed] [Google Scholar]
- 40.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 42.Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ, Yu J, Zhou Q, McMahon AP, Melton DA. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 44.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 45.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124:1871–1881. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips MD, Mukhopadhyay M, Poscablo C, Westphal H. Dkk1 and Dkk2 regulate epicardial specification during mouse heart development. Int J Cardiol. 2011;150:186–192. doi: 10.1016/j.ijcard.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, Shim S, Chai JH, Koo BK, Hong HJ, Yun CO, Choi C, Kim YM, Hwang KC, Kwon YG. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121:1882–1893. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korol O, Gupta RW, Mercola M. A novel activity of the Dickkopf-1 amino terminal domain promotes axial and heart development independently of canonical Wnt inhibition. Dev Biol. 2008;324:131–138. doi: 10.1016/j.ydbio.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22:6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 52.Brunelli S, Tagliafico E, De Angelis FG, Tonlorenzi R, Baesso S, Ferrari S, Niinobe M, Yoshikawa K, Schwartz RJ, Bozzoni I, Cossu G. Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circ Res. 2004;94:1571–1578. doi: 10.1161/01.RES.0000132747.12860.10. [DOI] [PubMed] [Google Scholar]
- 53.Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun. 2010;391:1087–1092. doi: 10.1016/j.bbrc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH, Wang FS. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol. 2010;21:124–135. doi: 10.1681/ASN.2008101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cselenyi CS, Lee E. Context-dependent activation or inhibition of Wnt-beta-catenin signaling by Kremen. Sci Signal. 2008;1:pe10. doi: 10.1126/stke.18pe10. [DOI] [PubMed] [Google Scholar]
- 57.Matouk CC, Marsden PA. Epigenetic regulation of vascular endothelial gene expression. Circ Res. 2008;102:873–887. doi: 10.1161/CIRCRESAHA.107.171025. [DOI] [PubMed] [Google Scholar]
- 58.Stenmark KR, Frid MG, Yeager M, Li M, Riddle S, McKinsey T, El Kasmi KC. Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change. Pulm Circ. 2012;2:3–14. doi: 10.4103/2045-8932.94817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway. Circ Res. 2008;103:289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.