Summary
The present study utilized event-related fMRI to address the role of the human perirhinal cortex (PRC), and its interactions with the hippocampus, in memory consolidation. Participants encoded object-based and scene-based associations and then restudied them either after a ‘long’ or ‘short’ delay during which consolidation could occur. We found that BOLD activation in left PRC and hippocampal-PRC functional connectivity were significantly enhanced during the restudy of the long vs. short delay word-object pairs. Second, hippocampal-PRC connectivity during restudy of the long delay word-object pairs predicted the subsequent reduction in associative forgetting. By contrast, hippocampal-PRC connectivity did not predict subsequent resistance to forgetting for the short delay or novel associations. Together, these results provide the first evidence for perirhinal-hippocampal interactions in the selective consolidation of object-based associative memories and provide support for the notion that, during early stages of consolidation, memories become more distributed across brain regions.
Keywords: perirhinal cortex, memory consolidation, hippocampus, functional connectivity, episodic memory, medial temporal lobe, object memory
Introduction
It is widely accepted that there are at least three stages in the life of a memory: encoding, retrieval and consolidation. There has been a wealth of cognitive neuroscience research in the last decade focused on revealing the mechanisms by which the medial temporal lobe (MTL) creates, or encodes, a lasting trace of our experience so that we can later retrieve it. A number of recent reviews describe our current knowledge with respect to the roles of distinct MTL subregions in memory encoding and retrieval (Davachi, 2006; Eichenbaum et al., 2007; Diana et al., 2007; Squire et al., 2007). In sum, there is growing evidence from both animal and human research that the perirhinal cortex is important in the encoding of individual items or objects from an experience while the hippocampus is important for linking distinct item representations in memory (Davachi et al., 2003; Staresina and Davachi, 2009; Ranganath et al., 2004; Tubridy and Davachi, 2011; Brown and Aggleton, 2001). Further, the perirhinal cortex also appears to contribute to some forms of associative encoding. Recent fMRI work has shown that BOLD activation in the perirhinal cortex is related to item encoding as well as the associative encoding of item features, but not extra-item episodic details (Staresina and Davachi, 2008; Staresina et al., 2011). However, little is known about how MTL structures interact to support memory consolidation.
Systems-level memory consolidation is typically conceptualized as the process by which initially hippocampal-dependent memories become less reliant on the hippocampus and become more widely supported by cortical regions. This shift, or distribution of the memory trace, is thought to provide resistance to local damage and confer a resistance to forgetting (see Wixted, 2004). One emerging mechanism hypothesized to support memory consolidation is hippocampal-mediated replay or reactivation. Replay has been defined as the reactivation of brain activity characteristic of a prior experience during post-encoding time periods (Buzsaki, 1989; Stickgold et al., 2001; Marr, 1971; see also Kali and Dayan, 2004). Thus far, evidence in support of this proposal has emerged primarily from animal electrophysiological studies demonstrating that hippocampal neural firing patterns associated with maze running and learning are subsequently replayed during sleep and awake rest (e.g., Skaggs and McNaughton, 1996; Qin et al., 1997; Wilson and McNaughton, 1994; Ji and Wilson, 2007; Karlsson and Frank, 2009). Furthermore, disruption of neural signatures of replay (i.e. sharp-wave ripples) has been shown to impair learning, providing a causal link between memory consolidation and neural replay (Ego-Stengel and Wilson, 2010; Jadhav et al., 2012). Thus, processes contributing to memory consolidation appear to be present as early as immediately following an experience as well as in post-experience bouts of sleep.
In humans, while many factors have been known to contribute to long-term memory, perhaps the most reliable means to enhance memory is distributing study over time. Specifically, studying an item with a long inter-study interval typically conveys longer lasting memory than studying an item with a shorter inter-study interval (see for review Cepeda et al. 2006). While this ‘spacing effect’ is robust and can be seen across very small intervals (i.e. on the order of seconds) as well as with much longer intervals (e.g., 24 hours), the mechanisms that contribute to these effects remain largely unknown. It is intriguing to consider that distributing learning across a longer inter-study interval would allow more time for off-line consolidation processes, such as replay, to occur. As an initial test of this hypothesis in a prior behavioral experiment, we showed that distributing encoding over a 24-hour period, as compared to a 20 minute spacing, led to a reduced forgetting rate over a subsequent 24-hour period (Litman and Davachi, 2008). Importantly, immediate memory was not affected, implying that distributing learning over a 24-hour period may primarily enhance memory durability via consolidation mechanisms.
According to the model of replay-mediated memory consolidation, cortical regions that are most important in the initial encoding of an experience should continue to interact with the hippocampus in the service of memory consolidation. MTL cortical regions, such as the perirhinal cortex, are closely situated to the hippocampus and thus, may be the most relevant and important structures in the early stages of consolidation. In the past decade, evidence consistent with the idea that the perirhinal cortex is involved in the consolidation of object recognition memory in the rodent has begun to emerge. A handful of studies have demonstrated that inhibition of perirhinal function via the administration of a protein synthesis inhibitor, lidocaine, or NMDA and muscarinic receptor antagonists closely following object encoding results in impairments in long-term memory, supporting the idea that the perirhinal cortex participates in memory consolidation after the initial encoding period (e.g., see Winters and Bussey, 2005a; 2005b; Balderas et al., 2008; Warburton et al., 2003). Furthermore, these post-encoding inhibitory effects have not been reported when more spatially-based memory tasks were employed (see e.g., Ramos, 2008; Larkin et al., 2008). These findings are consistent with a domain-selective role for the perirhinal cortex in memory consolidation, and while they speak to the necessity of that brain region in the durability of recently encoded object-based memories, they do not address what systems-level mechanisms are involved in memory stabilization (but see Paz et al., 2007). In the current experiment, we set out, first, to examine whether memories that have had more time to consolidate are associated with enhanced hippocampal-cortical connectivity, providing some support for the proposal that consolidation of a memory involves the distribution of the representation of that memory across brain regions.
Thus far, a few studies have reported changes in hippocampal-cortical and cortico-cortical connectivity as the time over which a memory could consolidate prior to retrieval increased (Takashima et al., 2009; Gais et al., 2007), but, to our knowledge, none has linked the identified brain changes with a behavioral measure of consolidation. In other words, in order to more closely link changes in functional connectivity to memory consolidation, per se, our second aim was to examine whether the resulting connectivity differences by study-restudy delay predicted subsequent forgetting, a behavioral hallmark of memory consolidation. To this end, we adopted the distributed learning paradigm (see Figure 1) as means to modulate the duration of delay before re-studying the same stimulus pairs. We reasoned that if a longer delay period allowed for more offline consolidation, those changes may be evident during re-study. Importantly, using the same timing parameters, Litman and Davachi (2008) have previously shown that a longer delay before re-study reduces subsequent associative forgetting (over the next day). In the current experiment, participants first studied an intermixed list of word-scene and word-object pairs (long delay: LD). Twenty-four hours later they returned to the lab and studied another intermixed set of novel stimulus pairs (short delay: SD). Immediately after this study session was completed, participants were scanned while all previously studied pairs (LD,SD) were restudied intermixed with a final set of novel word-scene and word-object pairs (single session set: SS). Following scanning, an associative memory task was administered using half of all studied words and new words. Memory for the remaining pairs was tested 24 hours later.
Figure 1.
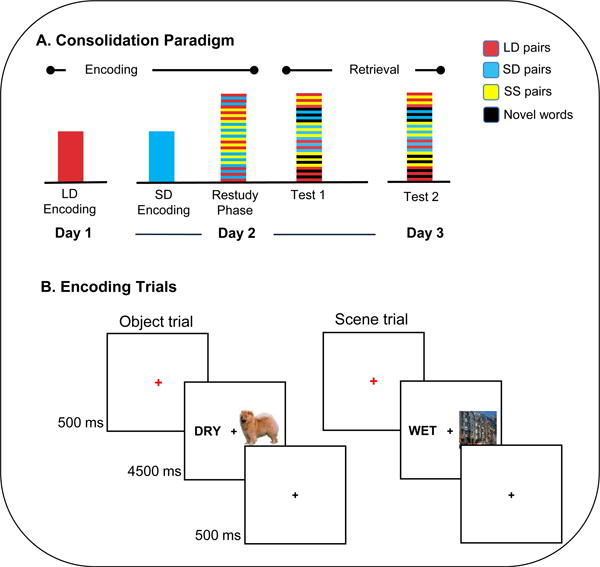
Overview of experimental design. (A) Sequence of sessions in each day. On day 1, subjects studied long delay (LD) word-object and word-scene pairs for the first time. On day 2, subjects performed the initial encoding on short delay (SD) pairs, and then completed the restudy phase while functional data were acquired. During this restudy phase, subjects restudied all LD and SD pairs, as well as a novel set of single session (SS) pairs, in an interleaved fashion. Immediately after the restudy phase, a localizer task was performed, followed by a memory test, including half of all previously studied LD, SD, and SS pairs, as well as novel words. Another memory test was administered 24 hours later. (B) During the encoding and restudy phases, trials began with a 500ms red fixation, followed by a word-image pair for 4500ms, and a black fixation for 500ms. Two types of images were used: objects, and scenes. For each pair the subject was to form an association between the displayed word and image and rate the quality of their association on a three-point scale.
Results
Behavioral Results
Item recognition performance for each stimulus category and repetition condition is shown in Table 1. Mean correct rejection rates were .75 and .69 (with standard deviations of .03 and .04) for the immediate and 24-hour tests, respectively. Associative memory performance on each test was indexed as the proportion of correct responses for trials of that type. As in Litman and Davachi (2008), we opted to test memory for half of the pairs on each test rather than all pairs twice in order to avoid contamination of 24-hour memory test performance by an additional learning opportunity that an additional memory test affords. Thus, we use the term forgetting to describe differences in performance between our two tests across different trials. For this forgetting measure, because poor performance on the initial test limited how much information each subject could have potentially forgotten across test days, we adjusted forgetting rates for initial test performance. This forgetting measure was based on an associate memory accuracy index where we corrected for source false alarms by subtracting the proportion of trials of a given condition that were given an incorrect source response from the proportion of trials afforded a correct source response for that condition. Thus, our index of associative forgetting was the following: (associative memory accuracy on test 1 - associative memory accuracy on test 2) / (associative memory accuracy on test 1). Greenhouse-Geisser corrected degrees of freedom are reported for repeated measures ANOVAs where appropriate.
Table 1. Item recognition memory performance for studied items.
| Condition | Object | Scene | ||
|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 2 | |
| LD | .73 (.02) | .64 (.03) | .76 (.03) | .69 (.03) |
| SD | .71 (.03) | .63 (.03) | .78 (.03) | .67 (.03) |
| SS | .55 (.03) | .47 (.03) | .60 (.03) | .50 (.04) |
Notes: Standard deviations are indicated in parentheses.
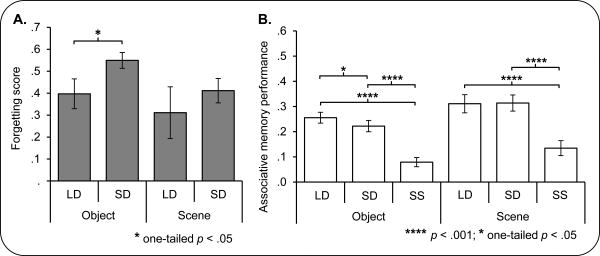
As expected, associative memory performance decreased across tests, F(1, 23) = 160.6, p < .001. Analysis of associative recognition performance on each test separately revealed main effects of condition (LD, SD, SS) for both objects and scenes (immediate test: for object trials, F(2, 45.7) = 58.8, p < .001, for scene trials, F(1.9, 42.8) = 32.9, p < .001; 24-hour test: for object trials, F(2, 45.7) = 63.2, p < .001, for scene trials, F(1.9, 44.1) = 32.7, p < .001). These effects manifest as better associative recognition for both the LD and SD trials compared to SS trials for both object and scene pairs (for the immediate test, LD vs. SS objects: F(1, 23) = 81.4, p < .001, SD vs. SS objects: F(1, 23) = 98.9, p < .001, LD vs. SS scenes: F(1, 23) = 54.5, p < .001, SD vs. SS scenes: F(1, 23) = 44.9, p < .001; for the 24-hour test, LD vs. SS objects: F(1, 23) = 108.7, p < .001, SD vs. SS objects: F(1, 23) = 80.5, p < .001, LD vs. SS scenes: F(1, 23) = 40.3, p < .001, SD vs. SS scenes: F(1, 23) = 54.7, p < .001). These results were not surprising given that both LD and SD trials were studied twice while the SS trials were only studied once. While no differences in associative recognition between object and scene trials were identified on the immediate test, F(1, 23) = 3.2, p > .08, on the 24-hour test, scene trials were associated with better associative recognition performance than object trials, F(1, 23) = 10.3, p < .005. See Figure 2 for 24-hour associative recognition performance, and Supplemental Figure S1 for immediate associative recognition performance.
Figure 2.
A: Forgetting for each condition and stimulus category. Significantly reduced forgetting (worse associative memory performance on the 24-hour than the immediate test; See Results) was evident in the long delay (LD) condition compared to the short delay (SD) condition for word-object (OBJECT) pairs. The same effect was not seen for word-scene (SCENE) trials. B: 24-hour associative recognition by condition and stimulus category. Poorer associative memory performance was seen for SD than LD object trials. SS trials in both object and scene conditions were associated with the poorest performance. Asterisks indicate the significance of effects. Errors bars denote standard error of the mean. See also Figure S1.
Consistent with our predictions, based on the findings of Litman and Davachi (2008), LD object pairs were associated with better associative memory than SD object pairs, t(23) = 1.9, p < .05 on the 24hour test. Crucially, LD object pairs were also associated with significantly reduced forgetting over the two test days compared to the SD object pairs, t(23) = 2.0, p < .05 (see Figure 2), consistent with the notion that reactivation after a longer intervening interval was associated with greater consolidation. Interestingly, we did not see the parallel effect for scene trials. Specifically, there was no significant difference between the LD and SD scene conditions in associative memory performance on the 24-hour test, t(23) = .1, one-tailed p > .4, nor was there a difference in forgetting of scene trials between the LD and SD conditions, t(23) = .8, one-tailed p > .2. Thus, because we did not see a behavioral consolidation effect for the scene stimuli, we will primarily focus the reported fMRI analyses on object pair trials.
Importantly, analyses of behavioral responses during the scanned reactivation phase did not reveal any differences in task performance (assayed by the number of ‘Poor’, ‘Moderate’, ‘Well’ responses made) between the LD and SD conditions for objects or scenes, each F(1,23) < 4, p > .05. Analyses of test response times (RT) revealed a main effect of restudy delay on the immediate test, F(1.4, 31.6) = 9.3, p < .005, but no differences on the 24-hour test, F(1.3, 29.6) < 2, p > .1. The effect manifest as slower associative RTs for the novel SS trials compared to both LD and SD trials.
FMRI Results
The first aim of the fMRI analyses was to identify changes in MTL brain activation and connectivity as a function of the restudy delay. To this end, we examined both overall BOLD activation in ROIs (see Figure 3A for a depiction of ROI locations) and connectivity between seed regions using a beta series correlation (BSC) approach (Rissman et al., 2004). We performed these analyses pairwise between a task-derived left hippocampal ROI and left and right perirhinal object-sensitive ROIs, as well as between the left hippocampal ROI and a left parahippocampal place area (PPA) scene-sensitive ROI for comparison. These latter ROIs were created around the peaks of object > scene and scene > object localizer effects in the medial temporal cortex (see Methods for additional details on ROI selection).
Figure 3.
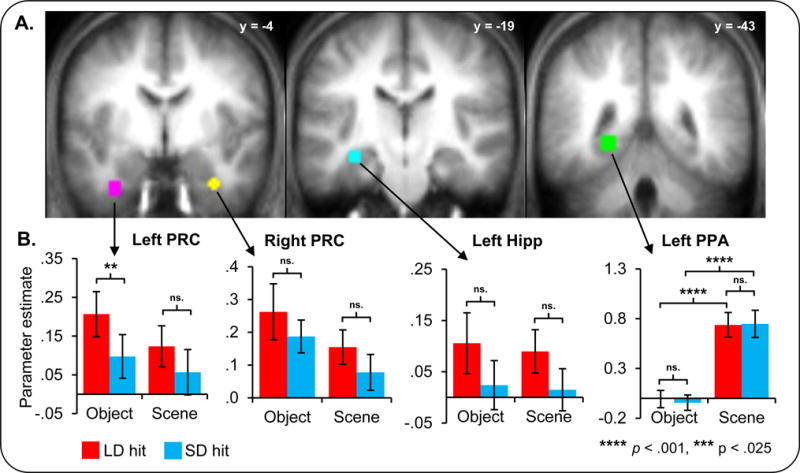
BOLD activation changes with consolidation. A: ROI spheres are displayed on the mean anatomical image. The ROI colors are as follows: pink = left perirhinal (PRC), yellow = right perirhinal, cyan = left hippocampus, and green = left parahippocampal place area (PPA). The y coordinate of each section is displayed. B: Average parameter estimates from each ROI for subsequently remembered trials (hits) are shown. Red bars correspond to hit trials in the long delay (LD trials) and blue bars correspond to the short delay (SD trials). Greater activity in the left PRC was associated with the restudy of long than short delay word-object pairs (LD > SD). Asterisks indicate the significance of effects. Errors bars denote standard error of the mean.
Activity within each ROI
BOLD activation for trials later correctly recognized as having been previously paired with a member of a given category (‘hit’ trials) in the left perirhinal cortex (LPRC) was modulated by restudy delay, exhibiting greater activity for LD compared to SD object hits, F(1,17)=7.17, p < .025 (see Figure 3B). By contrast, BOLD activation in the left hippocampal (Lhipp), right perirhinal (RPRC), and left parahippocampal (LPPA) ROIs failed to exhibit differences by restudy delay, F(1,23)=3.48, p > .7, F(1,20)=1.60, p > .2, and F(1,23)=.62, p > .4, respectively.
Connectivity: Beta Series Correlations
Using Fisher transformed correlations of activity between the seed regions (see Methods), we found that Lhipp-LPRC correlations were significantly greater during the restudy of LD object hits than SD object hits, F(1,17) = 7.27, p < .025. In support of the domain specificity of the effect, Lhipp-LPRC correlations did not differ by consolidation interval for later remembered scene trials, F(1,17) = .03, p > .8, and the correlations exhibited a significant interaction between stimulus type and restudy delay, F(1, 17) = 5.56, p < .05. Interestingly, this pattern of findings was restricted to left PRC cortex, as no difference by restudy delay was detected for the Lhipp-RPRC pair. By contrast, correlations of activity in the Lhipp-LPPA ROI pair differed for later remembered object trials by restudy delay, but these effects took the form of greater correlations for SD than LD object hit trials, F(1,23) = 4.76, p < .05. No such effects were apparent for scene trials nor was there an interaction between trial type and restudy delay, F(1,23) = .13, p > .7, and F(1, 23) = 1.03, p > .3, respectively. Thus, the only regions to show enhanced connectivity related to the longer delay interval were the Lhipp and LPRC (see Figure 4).
Figure 4.
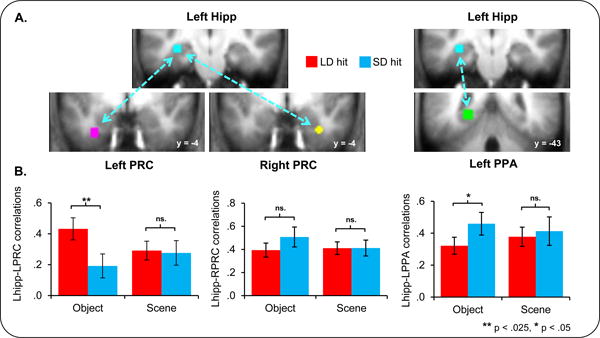
Connectivity selectively changes with consolidation A: Hippocampal, perirhinal, and parahippocampal seeds are displayed. B: Significantly enhanced connectivity was seen between the hippocampal and left perirhinal ROIs for LD word-object pairs. Fisher transformed hippocampal beta series correlations (see Methods) for subsequently remembered word-object (OBJECT) and word-scene (SCENE) pairs are shown for each of the left perirhinal (PRC), right perirhinal, and left parahippocampal place area (PPA) ROIs. Asterisks indicate the significance of effects. Errors bars denote standard error of the mean.
The question arises whether the LD vs. SD object hit Lhipp-LPRC connectivity difference is specific to those trials where the associate was retrieved successfully. To address this issue, we conducted a secondary analysis utilizing object ‘item only hits’, trials upon which the test cue was successfully recognized and either a) the associate was classified incorrectly as a member of the other class or a b) the participant was uncertain as to the identity of the associate. This analysis revealed significantly greater Lhipp-LPRC connectivity for LD object hits than LD object item only hits, F(1, 17) = 8.11, p < .05. No significant differences were apparent between LD and SD object item only hits (F(1, 17) = 0, p > .9), nor for SD object trials according to subsequent memory (F(1, 17) = .62, p > .4). These results are depicted in Figure 5.
Figure 5.
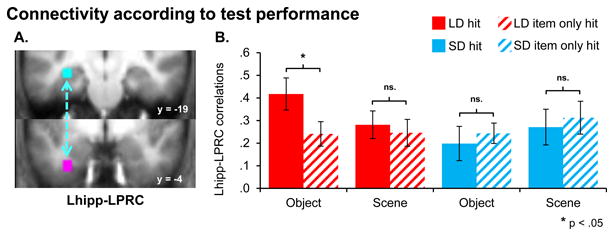
Connectivity is greater for subsequently remembered pairs. A: Left hippocampal and perirhinal seeds are displayed. B: Significantly enhanced connectivity was seen between the left hippocampal and perirhinal ROIs for LD word-object pairs that were subsequently remembered in conjunction with a correct paired associate response (‘hits’) than those that were later not recognized with a correct paired associate response (‘item only hits’). Fisher transformed hippocampal-perirhinal beta series correlations (see Methods) for subsequently remembered and associatively forgotten pairs are shown for LD and SD object and scene pairs. Asterisks indicate the significance of effects. Errors bars denote standard error of the mean.
Relationships Between Connectivity and Behavior
In order to more directly test whether the observed enhancement in Lhipp-LPRC correlations is related to memory consolidation per se, we next asked to what extent connectivity between regions predicted memory longevity. Specifically because memory consolidation is thought to relate to the durability of memories, we asked whether connectivity related to our behavioral measure of forgetting across time. We found that, across subjects, the magnitude of Lhipp-LPRC correlations for the LD object hit trials negatively correlated with forgetting (see Figure 6), r(16) = -.58, p < .025. Specifically, the greater the connectivity, the less forgetting was seen across the two subsequent memory tests. By contrast, connectivity did not predict forgetting for the SD object hit trials, r(16) = .22, p > .3. These relationships differed significantly from one another, Fisher's Z= -2.35, p < .025. Furthermore, no other region tested showed correlations with the hippocampus that significantly predicted associative forgetting for later remembered LD object trials (Lhipp-RPRC and Lhipp-LPPA ROI pairs failed to exhibit significant predictive power of LD object hit beta series correlations on LD object forgetting, r(19) = -.22 p > .3 and r(22) = -.30 p > .1, respectively; see Figure S2). Thus, hippocampal-left perirhinal connectivity was related to reduced forgetting specifically for the long delay trials, providing strong support for a role of this connectivity in ongoing memory consolidation.
Figure 6.
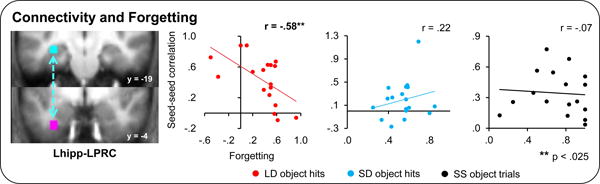
Hippocampal-perirhinal connectivity predicts subsequent forgetting for LD object hits only. Across-participant correlations between hippocampal-perirhinal connectivity for subsequently remembered pairs of a given type and forgetting for that pair type are shown. Correlation (r) values, calculated between forgetting and the Fisher transformed beta series correlation values, are displayed. Asterisks indicate the significance of effects. Errors bars denote standard error of the mean. See also Figure S2.
While the relationship between connectivity in the left hippocampal and left perirhinal cortices with associative forgetting is consistent with the proposal that this connectivity is a measure of the extent to which paired stimuli are being consolidated, it is possible that the relationship was observed for some other reason. One possibility is that LD trials might have been perceived as more novel than SD trials given that they were previously encountered longer ago and, thus, could have undergone more forgetting prior to restudy. In order to evaluate whether this explanation could account for the Lhipp-LPRC findings, we examined connectivity between these same regions for the completely novel object trials of the SS object condition. Unlike the prior analysis, however, insufficient subsequent hit SS object trials were available to enable this analysis to be conducted on SS subsequent hit object trials alone. Therefore, this and subsequent analyses utilizing SS object data collapsed data from all SS object trials, regardless of subsequent memory status. Inconsistent with the novelty explanation, SS object trial connectivity did not predict forgetting across subjects for the Lhipp-LPRC seed pair, r(16) = -.07, p > .6 (nor for any other pair tested; See Figure 6, Figure S2, and Supplemental Materials), nor was SS object trial connectivity greater than that for LD object trials, F(1, 17) = .45, p > .5. However, as shown in in Figure 7, BOLD activation in the hippocampal ROI, on its own, does predict subsequent forgetting in the SS condition which (1) is consistent with published work linking hippocampal processing of new associates with successful memory formation and (2) demonstrates the power to detect such effects in the current data set. Thus, taken together, it is unlikely that the differences seen between the LD and SD conditions are solely driven by a perceived novelty/encoding response to the LD pairs.
Figure 7.
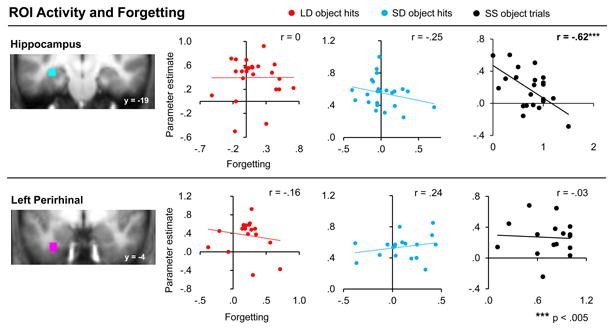
Overall BOLD activation in the hippocampus and left perirhinal cortex does not predict forgetting for subsequently remembered LD or SD object pairs. Across-participant correlations between BOLD activation in the left hippocampal and left perirhinal ROIs (displayed separately for LD object hits, SD object hits, and SS object trials) and forgetting for that trial type are displayed. Correlation (r) values, calculated between forgetting and BOLD activity estimates are displayed. Asterisks indicate significance of effects. Errors bars denote standard error of the mean. Note that hippocampal activation does predict forgetting, but only for the novel pairs. See also Figure S3.
We additionally evaluated the possibility that our across-subjects BSC-behavior correlation results for the LD object hit trials might have emerged via independent predictive relationships between BOLD signal in each ROI and behavior. To this end, we examined whether the parameter estimates derived from each of the ROIs correlated with forgetting for each consolidation interval. Critically, this relationship was not observed for LD object or SD object hit trials. BOLD signal in the Lhipp and the LPRC ROIs for LD object hits did not predict subsequent forgetting, r(22) = 0, p > .9 and r(16)=-.16, p > .5, respectively (see Figure 7). The LPPA and RPRC ROIs likewise failed to demonstrate a significant relationship between BOLD signal and forgetting for LD object hits (see Figure S3). Additionally, no relationship was identified in any ROI for SD object hits, each p > .15. When the analogous activity- behavior correlation analysis was repeated utilizing the SS object trial data, a significant relationship between activity and forgetting was identified in the Lhipp ROI, r(21) = -.62, p < .005. No other ROIs demonstrated a link between activity and forgetting for SS object trials. Note that these analyses were conducted on all SS object trials, rather only SS object hits given a lack of sufficient (9+) trials in over half of our participants. Figure 7 (and Figure S3) depicts the results of these analyses. Taken together, these findings do not support the idea that consolidation-related increases in connectivity predicting subsequent forgetting emerged as a result of a simple relationship between BOLD activity within each ROI and forgetting.
Discussion
The present study demonstrates that enhanced connectivity between the left perirhinal cortex and hippocampus is associated with a behavioral marker of the consolidation of object-based associative memories. These results extend prior human and animal findings showing that the perirhinal cortex in particular plays an integral role in the encoding of object-based memory representations (see e.g., Staresina et al., 2011; Winters and Bussey, 2005b) and is necessary for their consolidation (see e.g., Winters and Bussey, 2005b). Our results provide the first evidence that interactions between the human perirhinal cortex and hippocampus might be related to the consolidation of object-based associative memories. Crucially, we found, first, that hippocampal-LPRC connectivity was enhanced following a longer restudy delay and, second, that the magnitude of connectivity across subjects predicted subsequent forgetting only for the more- but not less-consolidated later remembered object pairs. The findings cannot be interpreted as resulting merely from greater perceived novelty of LD object hit pairs at restudy, as no relationship between forgetting and connectivity was identified for the entirely novel SS object pairs. These results build on recent findings demonstrating that hippocampal-cortical interactions during rest following encoding predict later associative memory performance (e.g., Tambini et al., 2010) by showing that interactions between hippocampus and cortical regions are modulated by the length of the inter-study interval and can be measured during the restudy of previously encoded information. Furthermore, our results suggest that, at least in the early stages of consolidation, connectivity measures are a better predictor of subsequent memory than overall BOLD activation in any one brain region.
In the present study, we chose to utilize a relatively short delay between the initial encoding and restudy of paired associates specifically because we sought to examine brain activity during an interval over which we think these mnemonic representations are still undergoing consolidation. The current findings are consistent with current models of medial temporal lobe function that proposed a domain-specific role for perirhinal cortex in supporting object-based memories (Davachi 2006, Eichenbaum et al., 2007). Specifically, connectivity between the hippocampus and PPA, unlike that between the hippocampus and left perirhinal cortex, did not show (1) any significant differences in connectivity related to restudy delay for the object hit trials nor (2) a predictive relationship between measures of connectivity and subsequent forgetting for long delay word-object hits. Thus, it is not the case that general correlated fluctuations in activity over the entire MTL contribute to the longevity of object-based memories in the present study, but rather, selective interactions between left perirhinal cortex and left hippocampus are enhanced after a longer delay interval and contributed to the subsequent resistance to forgetting for word-object pairs. Whether the same type of relationships between restudy delay, correlated fluctuations in activity, and behavior will be observed between the hippocampus and PPA for scene-based associations, however, remains to be determined. Further investigations of the specificity of consolidation-related interactions between the hippocampus and MTL regions which are selectively engaged in the encoding of different classes of stimuli are necessary.
Despite the fact that consolidation is generally conceived of as occurring over months or even years (see Squire and Alvarez, 1995), the present results are convergent with prior findings that the changes accompanying associative memory consolidation begin to take place very soon after the original learning episode (Takashima et al., 2006, 2009; Gais et al., 2007; Tambini et al., 2010; van Kesteren et al., 2010). These prior studies have focused primarily on examining both BOLD activation changes in specific brain regions and connectivity changes between brain regions, during the retrieval of older versus newer memories. However, there are discrepancies in the published reports. Some papers report reduced hippocampal activation with consolidation (Takashima et al., 2006; 2009; Milton et al., 2011) whereas others report enhanced hippocampal activation (Gais et al., 2007; Lewis et al., 2011) or no difference (Payne and Kensinger, 2011). Only a few have examined changes in connectivity and these results are also somewhat inconsistent, citing enhanced hippocampal-cortical connectivity (Gais et al., 2007), reduced hippocampal-cortical connectivity (Takashima et al., 2009), and enhanced cortico-cortical connectivity (Takashima et al., 2009; Payne and Kensinger, 2011; Lewis et al., 2011). Thus, these prior human brain-based approaches to identifying the changes associated with memory consolidation are not presenting a unified picture, as of yet. However, one of the reasons why the literature may be producing seemingly discrepant findings is that the reported effects have not been linked directly to a behavioral measure that characterizes consolidation. In other words, the effects seen in any one study could be related to memory strengthening or they could be related to a whole host of other differences present when retrieving an older versus newer memory. Thus, in the current experiment, we assessed whether changes in the interactions between hippocampus and other MTL regions were associated with or related to subsequent resistance to forgetting. Using this approach, our findings indicated that by 24 hours following associative encoding, interactions between the hippocampus and the left perirhinal cortex were increased in conjunction with the restudy of those associations. More importantly, these interactions predicted resistance to forgetting. This latter finding is of particular importance as it provides the first report of a relationship specifically between inter-regional interactions in brain activity and forgetting in humans. Future studies which examine how interactions between the hippocampus and other MTL regions change with time and relate to forgetting will be essential to developing a more complete understanding of the timescale at which these interactions play a role in consolidation. It will additionally be of particular interest to determine whether, following a longer consolidation interval, the perirhinal cortex might itself demonstrate increased interactions with other cortical regions that were involved in the encoding of object-based memories.
We think it is important to consider some potential limitations of the current results as well. It is possible that our main result of greater connectivity for the long- versus short-delay pairs may be related to differential contextual processing during those trials. Specifically, the long-delay pairs were initially encoded on the prior day and thus, during restudy there may be more contextual encoding compared to SD trials that were initially studied on the same day as restudy. First we want to note that, behaviorally, a contextual encoding account should theoretically lead to differences in memory immediately as well as in the long-term. But in our data, the manipulation only led to differential memory effects on the long-term memory test, 24 hours later (see Supplemental Figure S1 and Figure 2B). This pattern of behavioral memory stabilization, one that emerges with time, has been long attributed to consolidation. That said, it is still possible that differences in contextual encoding may be related to our functional connectivity measures. One way to address this concern with our current data is to consider the single-session (SS), or novel, trials which, by definition, do not have any prior (experimental) context associated with them since they are being presented for the first time. Thus, if novel contextual encoding or novelty more broadly was driving the hippocampal-perirhinal connectivity differences between the LD and SD trials, one would predict connectivity to be the greatest in the novel SS condition. However, this was not the case (see Results). Furthermore, connectivity measures during SS trial encoding were not related to our behavioral index of forgetting. Thus, novel contextual encoding is unlikely to be able to explain the pattern of results seen here. Alternatively, it is also possible that the probability of retrieving prior context is higher for LD than SD trials. Taking this a step farther, it is also possible that the reinstatement of the prior context could enhance the memorability of those items compared to the SD pairs whose repetition may engender less overall item and contextual processing. Again, however, if this were the case, we might expect contextual retrieval to be positively related to immediate measures of memory, but this was not the case (see above). Thus, we think the BOLD-behavior correlations observed here are most consistent with a consolidation account. However, the intimate relationship between the role of context encoding, retrieval and memory consolidation will benefit greatly from future work designed to distinguish between offline reactivation (Tambini et al., 2010; Rudoy et al., 2009; Antony et al., 2012; Oudiette et al., 2013; Oudiette and Paller, 2013) associated with memory consolidation and the more online directed reactivation characteristic of retrieval. In fact, recent work has even suggested that neural measures of replay in rodents may be a mechanism for directed retrieval (see Carr et al., 2012) further raising questions about how these mechanisms might be distinct and what they have in common.
In conclusion, the present findings add to our current knowledge about how interactions between the hippocampus and other MTL regions might underlie associative memory consolidation. Specifically, our results provide the first evidence in humans of consolidation-related modulations of connectivity between the hippocampus and left perirhinal cortex. These modulations were elicited in a stimulus-selective fashion, being apparent only for word-object pairs and not word-scene pairs. Finally, across subjects, connectivity between these ROIs was associated with resistance to forgetting. Reactivation has been identified as mechanism for memory consolidation whether it occurs during sleep (for review, see Born and Wilhelm, 2012), during awake rest (see e.g., Tambini et al., 2010; Karlsson and Frank, 2009) or during direct task performance (Wimber et al., 2012; see also Peigneux et al., 2006). One important area of future work will be to compare and contrast reactivation during these different time periods and to better determine their respective roles in memory strengthening, updating and integration.
Methods
Subjects
Thirty-four individuals enrolled in the fMRI experiment. Four participants failed to complete all sessions of the experiment. One subject was excluded due to scanner noise, one for excessive motion, and one subject failed to perform the encoding task as instructed. An additional 3 subjects were excluded on the basis of failing to contribute sufficient (9+) trials to each of the conditions of interest (subsequent associative hits collapsed across both tests for LD object, LD scene, SD object, and SD scene and SS trials). Of the remaining 24 subjects, fifteen were female, and all were right-handed, 18-29 years of age (median = 21), native English speakers with no history of psychological or neurological illness. Informed consent was obtained from each participant in accordance with the NYU IRB approval for the study. Participants were compensated for their participation.
Stimuli
Scene stimuli were selected from a subset of 727 images of outdoor scenes (derived from an online database at http://cvcl.mit.edu/database.htm; Oliva and Torralba, 2001). Object stimuli were selected from a subset of 934 images previously used by Brady et al. (2008; MIT Massive Memory set). Scrambled object stimuli were created by dividing each object image into a 50 by 50 grid and randomly reassigning these squares to locations within the grid. Word stimuli were selected from a pool of 506 adjectives downloaded from the MRC psycholinguistics database (available at http://www.psych.rl.ac.uk/). All word stimuli were presented in black 36 point Helvetica font, in all caps, on a white background. For each subject, 120 words were randomly selected from the word pool to be used in each study condition (LD, SD, SS). One hundred and twenty additional words were also randomly selected for each subject to be used as new items on the two memory tests (60 per test). For each subject and condition, half of the words were randomly paired with scene images and the other half were randomly paired with object images. Each study word was paired with a single image. Each of the LD and SD study sessions were composed of 120 trials, 60 per stimulus category. These encoding sessions each contained 2 breaks during which participants were instructed to relax for a minute or two and press a key to continue when ready. The scanned restudy session was composed of these previously studied 240 stimulus pairs plus an additional 120 single session (SS) stimulus pairs. For each participant, half of the words studied in each study condition and stimulus category were pseudo-randomly selected for use in each memory test. Thus, for each test, 180 studied and 60 non-studied words were presented.
Object, scene and scrambled object images for use in the localizer were randomly selected, for each subject, from the remaining unemployed images from each pool. The localizer session was composed of 15 mini-blocks of each condition plus an additional 18 mini-blocks of fixation only. During each of the object, scene, and scrambled object mini-blocks, 18 stimuli of a given type were presented, with two stimuli repeating immediately following their initial presentation. The order of each image block containing one scene, one object and one scrambled object mini-block, were counterbalanced across subjects. Following the presentation of each image block, a fixation only mini-block was presented. The localizer session was divided into three scans, each containing 6 fixation only mini-blocks and 5 image blocks. These scans directly followed the restudy phase. In the scanner, all stimuli were presented via projector to a screen positioned at the back of the scanner bore which was visible by way of a mirror attached to the head coil.
Procedure
Encoding trials were composed of a central red fixation (500ms) followed by the presentation of a word-image pair for 4500ms, followed by a central black fixation for 500ms. For the remaining 8.5 seconds in each trial, participants completed an active baseline task (see Stark and Squire, 2001). In this task, participants made odd/even judgments on a series of centrally presented single digit numbers ranging from 1-9 that were randomly generated on a subject-specific basis. Each number was presented for a maximum of 2 seconds or until the subject made a response. Once a response was registered, or 2 seconds elapsed, a black fixation cross was presented for 250ms prior to the appearance of the next digit. The final 1-3 seconds of each trial were composed of a black fixation cross, depending on the timing of the subject's prior responses. At encoding regardless of trial type, participants were instructed to attempt to form an association between the word and image presented on the screen and to rate how well they could accomplish this task using a 3-way button press. The right index finger was used to indicate ‘Poor’, the middle finger to indicate ‘Moderate’ and the ring finger to indicate ‘Well’. This task was completed on all trials in each encoding block as well as on all trials in the restudy phase. For the odd even task, participants were instructed to use the right index finger to indicate ‘Odd’ and the middle finger to indicate ‘Even’. The keyboard was used to record responses in all tasks performed outside the MRI. In the MRI, an MRI-compatible button box was used to record responses. Finger-response mappings were held constant regardless of whether the task was performed inside or outside of the MRI.
In the image blocks of the localizer task, individual images were presented for 500ms with an inter-stimulus interval of 300ms. In each image mini-block, 20 trials of a given category were presented in a row. The fixation only mini-blocks each lasted 16 seconds each. Participants were instructed to keep their eyes open and respond with a key under the index finger anytime an image repeated immediately following its initial presentation.
At test, all stimuli were presented centrally. Test trials were composed of a 500ms red fixation cross followed by a word for 12s or until a response was registered. A final 200ms black fixation cross followed the word presentation. Participants were instructed to evaluate their memory for the cue word and respond using a 4-way response. The response options were: Old with scene, old with object, old unsure of pairing, and new. Participants were instructed to only use the old pair-specific responses when they were able to recollect the associated image. Participants were instructed to use the ‘New’ response anytime they were uncertain as to whether a word as studied or not studied. The ‘Old unsure of pairing’ response was to be used when the test word was recognized as having been studied but the studied image was not recollected.
Prior to starting each session, participants received written and verbal task instructions. On day 1, participants performed the encoding task on LD pairs. Approximately 24 hours later, they returned to the lab and completed the encoding task for SD pairs. Following a short break, participants were positioned in the MRI for the restudy phase. During this session, participants studied, in a pseudo-randomly intermixed list, the previously encoded LD and SD pairs, as well as a new set (SS) of 60 word-scene and 60 word-object pairs that they had not previously studied (single session condition). The restudy phase was broken into 6 blocks, each including 60 trials. Following this restudy session, participants engaged in a simple one-back task for which they received instructions prior to beginning the first session on day 2. The fMRI data from this one-back task were later used in the creation of object- and scene-sensitive ROIs (the localizer task). Before exiting the scanner, an anatomical image of each subject's brain was obtained. Subjects were then given a short break, and returned to the lab to complete an immediate memory test on half of all the items from the LD, SD and SS lists. Instructions were given prior to beginning the first test session. Approximately 24 hours after the MRI session, participants returned to lab to complete a final memory test on the remaining untested studied words and new words. Studied pairs were divided into two test lists as in Litman and Davachi (2008). This was done to avoid contamination of 24-hour memory test performance by the additional learning opportunity afforded by an extra test session on the same items.
FMRI Data Acquisition
High-resolution T1-weighted anatomical images and blood oxygenation level-dependent (BOLD), T2*-weighted echoplanar functional images (TR = 2s, TE = 30ms, FOV = 192mm, flip angle 70º) were acquired using a 3T Siemens Allegra MRI system with a whole head coil. Each volume comprised 36 slices oriented parallel to the AC-PC line (thickness 3mm, .6mm inter-slice gap, 3mm3 voxels) acquired in an interleaved sequence. The first six volumes of each session were discarded to allow equilibration of tissue magnetization. Four hundred and twenty six volumes were acquired during each restudy phase scan and 172 volumes were acquired during each localizer scan.
FMRI Data Analysis
Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK), run under Matlab R2010a (The Mathworks Inc., USA) was used for fMRI data analysis. Functional imaging time-series were subjected to slice timing correction, reorientation, realignment to the first volume of each session, and co-registration with the anatomical image. These time-series were then concatenated across runs for the reactivation and localizer runs separately. The anatomical data were spatially normalized to the standard T1 template (based on the Montreal Neurological Institute (MNI) reference brain; Cocosco et al., 1997), and these normalization parameters were applied to the functional volumes. The resulting functional volumes were then smoothed with an 8mm FWHM Gaussian kernel.
Analyses of restudy phase data were performed using a General Linear Model (GLM) in which a 4s boxcar was convolved with the canonical hemodynamic response function (HRF) and its temporal and dispersion derivatives for each trial to model the BOLD response (Friston et al., 1998). For each restudy block, 10 event-types (subsequent associative/category hits (‘hits’) for LD object, LD scene, SD object, SD scene, and SS trials (collapsed across study pair category); and subsequent item only hits (‘item only hits’) for LD object, LD scene, SD object, SD scene, and SS trials (collapsed across study pair category) were modeled. Hit trials were defined as those restudy phase trials for which associative recognition was later successful on either memory test. Likewise, subsequent item only hit trials were defined as those trials for which associative memory was unsuccessful but item recognition was successful on either memory test. Hit trials were utilized in analyses evaluating the relationships between brain activity, connectivity, and behavior in order to ensure that the relationships revealed actually relate to activity or connectivity associated with subsequent successful associative memory retrieval rather than due to some difference in the ratio of subsequently remembered to forgotten trials in a given condition. While the previously described model was utilized in ROI identification, a second model was generated in which SS trials were segregated according to study pair category (objects, scenes). For this model, we were unable to further segregate trials according to subsequent memory status given that few subjects contributed sufficient (9+) SS object hit trials to enable their inclusion in the analysis as such. Thus, for this model, all SS object trials were collapsed into a single event type, as were all SS scene trials, separately.
The average number of trials in each of the LD object, LD scene, SD object, and SD scene hit conditions was 21, 20, 23, and 24, with minimum-maximum ranges of 11-36, 11-39, 10-43, and 9-41, respectively. Four of the 24 subjects analyzed did not have sufficient SS hit trials so their data were not used in ROI specification involving the SS hit condition. The localizer blocks were also modeled using a GLM but here a 16 second boxcar was convolved with the canonical HRF and its temporal and dispersion derivatives to model the BOLD response. Three event types were modeled for the localizer blocks (scene, object, and scrambled object mini-blocks).
For each block and task, each model also included as covariates the across-scan mean and six regressors representing motion-related variance (three for rigid-body translation and three for rotation). For each voxel, the image time-series was high-pass filtered to 1/128 Hz and scaled to create a grand mean of 100 across voxels and scans. An AR(1) model was used to estimate and correct for non-sphericity of the error covariance (Friston et al., 2002). The GLM was used to obtain parameter estimates representing the activity elicited by the events of interest. A statistical threshold of p < 0.001, uncorrected, with an extent threshold of 5 contiguous voxels was employed for principal unidirectional contrasts.
In addition to performing standard event-related modeling of the data, we performed beta series correlation (BSC) analyses in which each trial was modeled as a separate event of interest (see Rissman et al., 2004). For these analyses, the beta series associated with each trial type for a set of regions of interest (ROIs) were extracted and sorted by study condition, stimulus category, and response type. Note that for this analysis, the following set of event types were employed (as in the second GLM described above): LD object hits, LD object item only hits, SD object hits, SD object item only hits, LD scene hits, LD scene item only hits, SD scene hits, SD scene item only hits, SS object trials regardless of test response, and SS scene trials regardless of test response. After calculating the correlations between activity in ROI seed pairs individually for each subject and for each of the conditions across the time-series, these correlation values were subjected to Fisher transformation prior to statistical analysis. ANOVAs were employed to evaluate the degree to which correlations between responses in the different ROIs varied by condition and stimulus type. Fisher's test was utilized to detect whether relationships between connectivity (as indexed via BSCs) and forgetting differed by consolidation interval. Individual subject ROI data were excluded from analyses when over 20% of the voxels in a given ROI failed to contribute data. Data from one subject were excluded from all correlational analyses utilizing SS object trial data as this participant, unlike all others, failed to exhibit a decrease in memory performance between the two tests for this trial type. BSC analyses were performed pairwise between a task-derived hippocampal ROI and left and right perirhinal object-sensitive ROIs, as well as between the left hippocampal ROI and a left parahippocampal scene-sensitive ROI for comparison (see below for ROI information).
Region of Interest Generation
Regions of interest (ROIs) were selected for use in the beta series correlation analyses from both the main task data as well as from the localizer data. To create a stimulus-general hippocampal ROI, we inclusively masked the SS hit > baseline contrast from the first GLM (see above) with an anatomical hippocampal mask (from the AAL toolbox in SPM), and created the ROI from a 5mm radius sphere centered on the peak, including only those voxels that were not excluded by the anatomical mask. The peak of this stimulus-general ROI was in the left hippocampus (center at MNI coordinate 33, -19, -11). To identify our stimulus-specific ROIs, we utilized the localizer data. To create object-sensitive ROIs, we used the object > scene localizer contrast. Three scene localizer ROIs, created from the scene > object localizer contrast, were also created but only the outcome of analyses utilizing the left PPA seed (center at -24, -43, -2) are reported. The right PPA (center at 21, -34, -5) and right retrosplenial cortex seeds (center at -21, -52, 19) demonstrated qualitatively similar results to those of the left PPA and are available from the authors by request.
Given the extensive activation of the medial temporal lobe in the localizer contrasts (> 500 voxels), both peaks and sub-peaks of activity within our regions of interest were utilized as central points in the generation of ROI spheres (each with a 5mm radius; See Supplemental Materials for the peak and sub-peak coordinates of effects identified in these contrasts). The seeds were generated such that only active voxels within each sphere for a given contrast were included in the ROI. In the event that any voxels were shared between two generated ROIs within a stimulus type, shared voxels were removed from each of the relevant seeds. From the object localizer contrast, we report the outcome of analyses involving two seeds, one in left perirhinal cortex (center at -33, -4, -32) and the other in right perirhinal cortex (center at 33, -7, -29). An additional seed was created around a left hippocampal peak (center at -33, -19, -14) from the object > scene contrast, but given that this seed overlapped with the stimulus-general seed, and results arising from its use were roughly identical to those when the stimulus-general seed was employed, we do not report the outcome of general analyses utilizing this seed. See Figure 3A for a depiction of the reported ROIs on the mean anatomical image. Importantly, it should be noted that all ROIs were created from independent data from the conditions that were utilized in the main beta series correlation analyses (see Kriegeskorte et al., 2009). Additionally, given that the ROIs were not created with respect to behavioral performance in the conditions for which the analyses were conducted (LD object, SD object, LD scene, and SD scene trials), the correlational analysis with behavioral performance reported in the Results section is also not subject to a non-independence issue.
Supplementary Material
Acknowledgments
This work was funded by NIMH RO1 –MH074692 and Dart Neuroscience to L.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–6. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Burmudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–24. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Wilhelm I. System consolidation of memory during sleep. Psychological Research. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–70. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75:700–13. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychological Bulletin. 2006;132:354–80. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- Cocosco C, Kollokian V, Kwan RS, Evans A. Brainweb: Online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:S425. [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–62. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher PC, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: Applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M, Rauchs G, Schabus M, Sterpenich V, Vandewalle G, Maquet P, Peigneux P. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–83. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–8. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Káli S, Dayan P. Off-line replay maintains declarative memories in a model of hippocampal-neocortical interactions. Nat Neurosci. 2004;7:286–94. doi: 10.1038/nn1202. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–8. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin AE, Fahey B, Gobbo O, Callaghan CK, Cahill E, O'Mara SM, Kelly AM. Blockade of NMDA receptors pre-training, but not post-training, impairs object displacement learning in the rat. Brain Res. 2008;1199:126–32. doi: 10.1016/j.brainres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Cairney S, Manning L, Critchley HD. The impact of overnight consolidation upon memory for emotional and neutral encoding contexts. Neuropsychologia. 2011;29:2619–29. doi: 10.1016/j.neuropsychologia.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Davachi L. Distributed learning enhances relational memory consolidation. Learn Mem. 2008;15:711–6. doi: 10.1101/lm.1132008. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Milton F, Muhlert N, Butler CR, Smith A, Benattayallah A, Zeman AZ. An fMRI study of long-term everyday memory using SenseCam. Memory. 2011;19:733–44. doi: 10.1080/09658211.2011.552185. [DOI] [PubMed] [Google Scholar]
- Oliva A, Torralba A. Modeling the shape of the scene: A holistic representation of the spatial envelope. International Journal of Computer Vision. 2001;42:145–75. [Google Scholar]
- Oudiette D, Antony JW, Creery JD, Paller KA. The role of memory reactivation during wakefulness and sleep in determining which memories endure. J Neurosci. 2013;33:6672–8. doi: 10.1523/JNEUROSCI.5497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci. 2013;17:142–9. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. Sleep leads to changes in the emotional memory trace: evidence from FMRI. J Cogn Neurosci. 2011;23:1285–97. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Learning-related facilitation of rhinal interactions by medial prefrontal inputs. J Neurosci. 2007;27:6542–51. doi: 10.1523/JNEUROSCI.1077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Phil Trans R Soc Lond B. 1997;352:1525–33. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JM. Perirhinal cortex lesions produce retrograde amnesia for spatial information in rats: consolidation or retrieval? Learn Mem. 2008;15:587–96. doi: 10.1101/lm.1036308. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivation them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–3. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–77. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 2008;20:1478–89. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron. 2009;63:267–76. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31:8739–47. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–7. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernández G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–93. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernández G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–90. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex. 2011;21:272–80. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren MT, Fernández G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A. 2010;107:7550–5. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholingeric neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;28:987–96. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wimber M, Maaβ A, Staudigl T, Richardson-Klavehn A, Hanslmayr S. Rapid memory reactivation revealed by oscillatory entrainment. Curr Biol. 2012;22:1482–6. doi: 10.1016/j.cub.2012.05.054. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005a;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005b;25:4243–51. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–69. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.