Abstract
Tumor necrosis factor α (TNF-α) elicits its biological activities through activation of TNF receptor 1 (TNFR1, also known as p55) and TNFR2 (also known as p75). The activities of both receptors are required for the TNF-α–induced proinflammatory response. The adaptor protein TNFR-associated factor 2 (TRAF2) is critical for either p55- or p75-mediated activation of nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling, as well as for target gene expression. Here, we identified nonmuscle myosin II (myosin) as a binding partner of p75. TNF-α–dependent signaling by p75 and induction of target gene expression persisted for substantially longer in cells deficient in myosin regulatory light chain (MRLC, a component of myosin) than in cells replete in myosin. In resting endothelial cells, myosin was bound constitutively to the intracellular region of p75, a region that overlaps with the TRAF2-binding domain, and TNF-α caused the rapid dissociation of myosin from p75. At early time points after exposure to TNF-α, p75 activated Rho-associated kinase 1 (ROCK1). Inhibition of ROCK1 activity blocked TNF-α–dependent phosphorylation of MRLC and the dissociation of myosin from p75. ROCK1-dependent release of myosin was necessary for the TNF-α–dependent recruitment of TRAF2 to p75 and for p75-specific activation of NF-κB and MAPK signaling. Thus, our findings have revealed a previously uncharacterized, noncanonical regulatory function of myosin in cytokine signaling.
Introduction
TNF-α receptors (TNFRs) TNFR1 (also known as p55) and TNFR2 (also known as p75) activate both common and distinct signaling pathways; For example, p55, but not p75, activates caspases (1). Conversely, Etk (also known as Bmx)-mediated transactivation of vascular endothelial growth factor receptor 2 (VEGFR2) and subsequent pro-angiogenic signaling is mediated exclusively by p75 (2). Members of the TNFR family do not possess intrinsic catalytic activity to induce intracellular signal transduction; instead, they depend on cytosolic adaptor proteins for signaling (3). Both p55 and p75 are capable of independently activating the transcription factors nuclear factor κB (NF-κB) and activating protein 1 (AP-1) (4, 5), which are necessary for inducing the expression of TNF-α target genes as part of the proinflammatory response in endothelial cells (6). The mechanism of p55 signaling is well-characterized and involves the orchestrated recruitment of adaptor proteins to its cytosolic death domain upon stimulation with TNF-α (3, 7). One such adaptor protein is TNFR-associated death domain protein (TRADD). The binding of TRADD to p55 stimulates the recruitment of another adaptor protein, TNFR - associated factor 2 (TRAF2). Although the intracellular region of p75 does not share common domains with p55, TRAF2 directly binds to the cytosolic tail of p75 (8). In TNF-α-stimulated cells, TRAF2 binds to p75 as a homodimer or as a heterodimer with TRAF1 and mediates the activation of NF-κB and mitogen-activated protein kinase (MAPK) signaling and the expression of target genes (9-11). Two independent studies provided evidence of a second TRAF2-binding site in the C-terminus of the p75 cytosolic tail (T2bs-C) (12, 13). Although a physical association between p75 and TRAF2 is well-established, the underlying molecular mechanism involved in the TNF-α-induced recruitment of TRAF2 to p75 is unknown.
Rho-associated kinases (ROCKs) participate in TNF-α-mediated inflammatory responses (14, 15). Members of the family of Rho guanosine triphosphatases (GTPases), which are the activators of ROCKs, mediate NF-κB activation in cells stimulated with growth factors and cytokines, including TNF-α (16). The two isoforms of ROCK, ROCK1 and ROCK2 share 65% overall identity in their amino acid sequences and 92% identity in their kinase domains (17). In experiments with haplo-insufficient ROCK-1 mice, Noma et al. demonstrated the participation of this kinase in inducing the expression of genes encoding endothelial-leukocyte adhesion molecules and factors involved in leukocyte recruitment and neointima formation in a murine vascular injury model (18). ROCK activity is thought to be upstream of TNF-α-mediated activation of MAPK and NF-κB; however, the mechanism by which ROCKs activate such signaling molecules has not been elucidated. Here, we identified ROCK1-mediated phosphorylation of nonmuscle myosin II as a critical step in the recruitment of TRAF2 to p75, as well as in p75-specific activation of MAPK and NF-κB and the expression of genes mediating the pro-inflammatory response.
Results
Nonmuscle myosin II is a p75-associated protein
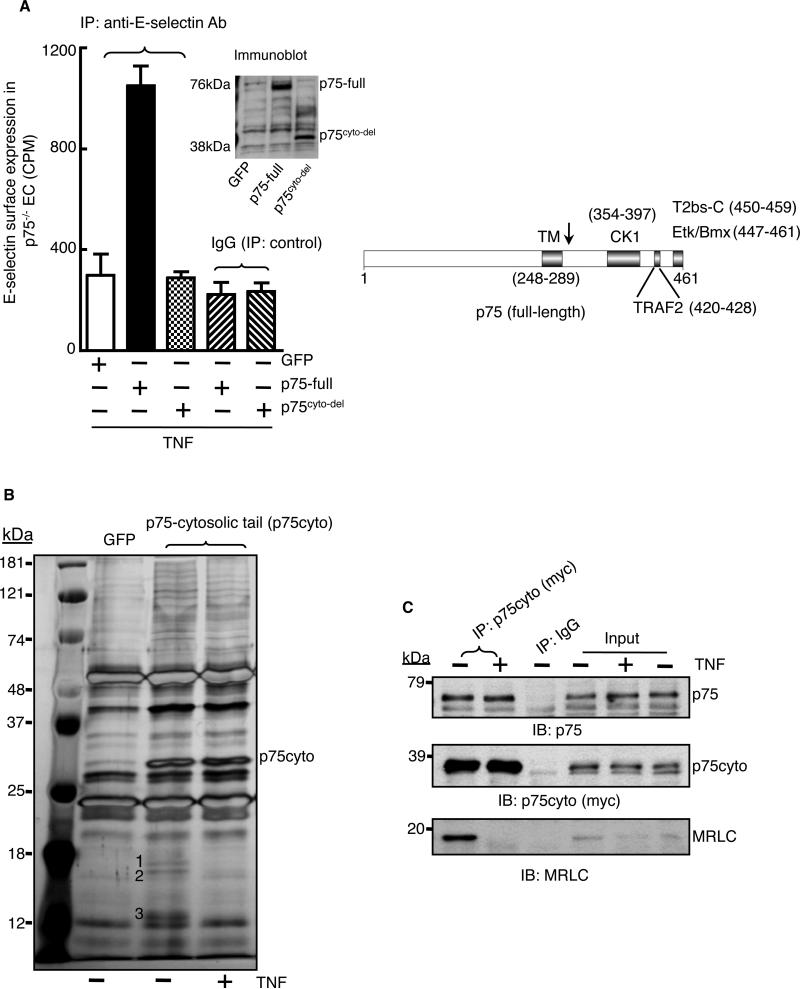
We showed previously that endothelial cells isolated from p75-null mice are nonresponsive to TNF-α in inducing the expression of genes encoding endothelial-leukocyte adhesion molecules, including E-selectin (19). Here, we demonstrated the rescue of TNF-α-dependent cell-surface expression of E-selectin in p75-null endothelial cells with full-length, recombinant p75 (Fig. 1A). In the same cells, a mutant form of p75 from which the cytosolic tail was deleted (p75cyto-del) failed to mediate a TNF-α-dependent increase in E-selectin abundance at the cell surface, suggesting the involvement of a p75-dependent intracellular signaling pathway in TNF-α-mediated gene induction.
Fig. 1. Nonmuscle myosin II is a p75-associated protein.
(A) Full-length p75 (p75-full) or a mutant p75 protein from which the cytosolic tail was deleted (p75cyto-del) were expressed in endothelial cells isolated from p75-null mice. Cells were treated with TNF-α (2 ng/ml) for 5 hours, and the cell-surface abundance of E-selectin was measured by immunoprecipitation (IP) with a biotin-conjugated anti-E-selectin antibody followed by 125I-labeled streptavidin. GFP expressing cells served as a negative control. Experiments were performed in triplicate with independent endothelial cell isolates and data are expressed as means ± SD. The right panel shows a scheme of the known protein-binding regions in p75 (13, 59, 60). The arrow denotes the point of the truncation in the p75cyto-del mutant protein. (B) The Myc-tagged cytosolic tail of p75 (p75cyto) was immunoprecipitated from control or TNF-α-stimulated (2 ng/ml, 5 min) human endothelial cells with anti-myc antibody, and immunoprecipitates were size-fractionated by SDS-PAGE and then silver-stained. Bands labeled 1 to 3 were excised and analyzed by mass spectrometry and identified as MRLC (bands 1 and 2) and MELC (band 3). (C) The p75cyto protein from untreated or TNF-α-treated human endothelial cells was immuoprecipated with an anti-myc antibody, samples were size-fractionated by SDS-PAGE, and examined by Western blotting (IB) with antibodies specific for p75 and MRLC. Inputs: 5% of the total lysates. Data in (C) are representative of three independent experiments.
To define p75-specific signaling pathways, we performed coimmunoprecipitation studies to identify previously uncharacterized TNF-α-responsive signaling intermediates that could interact with the intracellular region of p75. We expressed a myc-tagged cytosolic tail fragment of p75 (p75cyto) in endothelial cells and immunoprecipitated the recombinant p75cyto protein from TNF-α-stimulated or unstimulated cells with an anti-myc antibody. The immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining. Three proteins with molecular weights of ~18, ~16, and ~13 kD coimmunoprecipitated with p75cyto from extracts of untreated cells, but not from extracts of TNF-α-treated cells (Fig. 1B). Mass spectrometric analysis identified the ~18 and ~16 kD proteins as myosin regulatory light chain (MRLC) and the ~13 kD protein as myosin essential light chain (MELC) of the nonmuscle myosin II family. Peptide sequences identified by the mass spectrometric analyses of corresponded to ~29 and ~41% of the sequences of MRLC and MELC, respectively (fig. S1). We coimmunoprecipitated endogenous p75 with p75cyto from untreated and TNF-α-treated cells, whereas myosin was coimmunoprecipitated with p75cyto only from untreated cells (Fig. 1C). It is possible that TNF-α signaling caused the release of myosin from both p75-bound and unbound recombinant cytosolic tail.
The myosin II holoenzyme consists of two molecules of myosin heavy chain (MHC) and one molecule each of MRLC and MELC associated with each MHC at the neck region (20). To investigate potential interactions between endogenous p75 and myosin, we immunoprecipitated p75 from endothelial cells. We found that both MRLC and MHC coimmunoprecipitated with p75 from untreated cells, but not from TNF-α-treated cells (Fig. 2A). Sequential immuproprecipitation of myosin from the cell lysate showed that in resting cells, p75 predominantly associated with myosin (fig. S2A). In contrast, substantial myosin remained in the lysate even after p75 was depleted from the lysates with a specific immunoprecipitating antibody (fig. S2B), suggesting that only a small fraction of the total cellular myosin associated with p75 in resting cells.
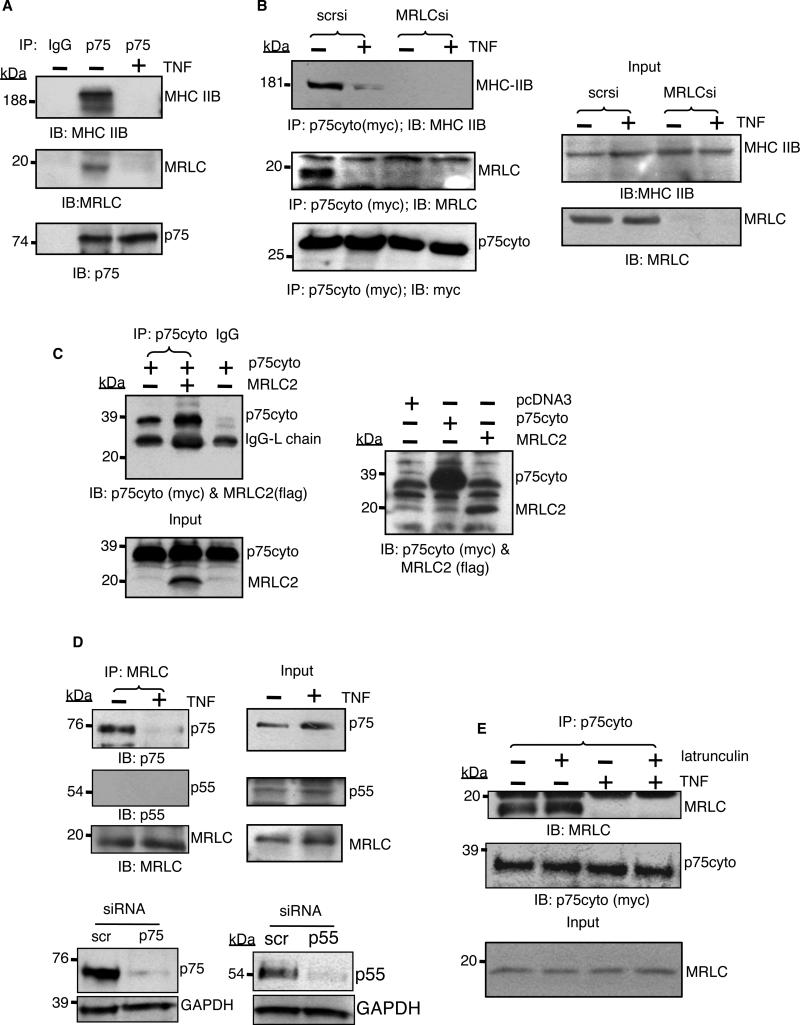
Fig. 2. Myosin physically interacts with p75.
(A) Endogenous p75 was immunoprecipitated from untreated cells or cells that were treated with TNF-α (2 ng/ml) for 5 min, and samples were analyzed by Western blotting for coimmunoprecipitated MHC IIB or MRLC with specific antibodies. (B) The p75cyto protein was immunoprecipitated from MRLC-depleted cells exposed to vehicle or TNF-α (2 ng/ml) for 5 min, and samples were analyzed by Western blotting for MHC IIB. scr, scrambled. Right panel shows the Western blotting analysis of the respective inputs (5% of the total lysate). (C) Myc-tagged p75cyto and FLAG-tagged MRLC2 were generated by in vitro translation with a TNT coupled transcription/translation system in independent tubes. The TNT reactions were mixed in a 1:1 ratio. The p75cyto protein was immunoprecipitated from the mixture with an anti-Myc antibody, samples were size-fractionated and then analyzed by Western blotting for MRLC2 and p75cyto simultaneously with anti-Myc and anti-FLAG antibodies. Right panel shows the Western blotting analysis of p75cyto and MRLC2 in the TNT reactions. (D) Endogenous myosin was immunoprecipitated from human endothelial cells with an MRLC-specific antibody and samples were analyzed by Western blotting to detect the indicated proteins. Bottom panel shows Western blotting analysis of antibody specificity in samples from which either p75 or p55 had been depleted with specific siRNA. (E) Myc-tagged p75cyto was immunoprecipitated from untreated or TNF-α-treated human endothelial cells that had previously been untreated or were treated with 5 mM latrunculin A for 30 min. Samples were then analyzed by Western blotting for coimmunoprecipitated MRLC. Inputs: 5% of the total lysates. Data are representative blots of three independent experiments.
Because both the heavy and light chains of myosin coimmunoprecipitated with p75, we reasoned that the preassembled myosin molecule, rather than individual subunits, was bound to p75. In agreement with this hypothesis, we did not detect any physical association between p75 and MHC in MRLC-depleted cells (Fig. 2B), and in vitro transcribed and translated MRLC failed to bind to the p75 cytosolic tail (Fig. 2C). We found that p55 did not coimmunoprecipitate with MRLC, consistent with the absence of structural motifs from the cytosolic domain of p55 that are similar to those of p75 (Fig. 2D). We did not detect actin in our immune complex. Furthermore, latrunculin, an inhibitor that prevents actin polymerization, did not block the physical interaction between p75cyto and myosin, nor did it affect the TNF-α-mediated disruption of this interaction (Fig. 2E). Together, these data lead us to conclude that p75 binds to myosin independently of actin.
The p75-mediated activation of ROCK disrupts the interaction between p75 and myosin in TNF-α-stimulated cells
Phosphorylation of MRLC at Thr18 and Ser19 stimulates myosin II motor activity and filament assembly (21). TNF-α stimulates the phosphorylation of MRLC at Thr18 and Ser19 predominantly through the activity of myosin light chain kinase (MLCK) or ROCK (22). Therefore, we determined whether phosphorylation of MRLC was required for TNF-α-induced dissociation of p75 from myosin. We pretreated endothelial cells with ML-7, an MLCK inhibitor, or Y27632, a ROCK inhibitor, before we stimulated the cells with TNF-α, immunoprecipitated myosin from the cell lysate with an MRLC-specific antibody, and looked for coimmunoprecipitated p75. As expected, we found that p75 coimmunoprecipitated with myosin (Fig. 3A). The ROCK inhibitor did not prevent the p75-myosin interaction in resting cells, but it blocked the TNF-α-mediated release of myosin from p75. ML-7 had no effect on the interaction between p75 and myosin. Often, we observed two bands corresponding to p75 in human endothelial cells, which was most likely a result of differential posttranslational modifications of the protein.
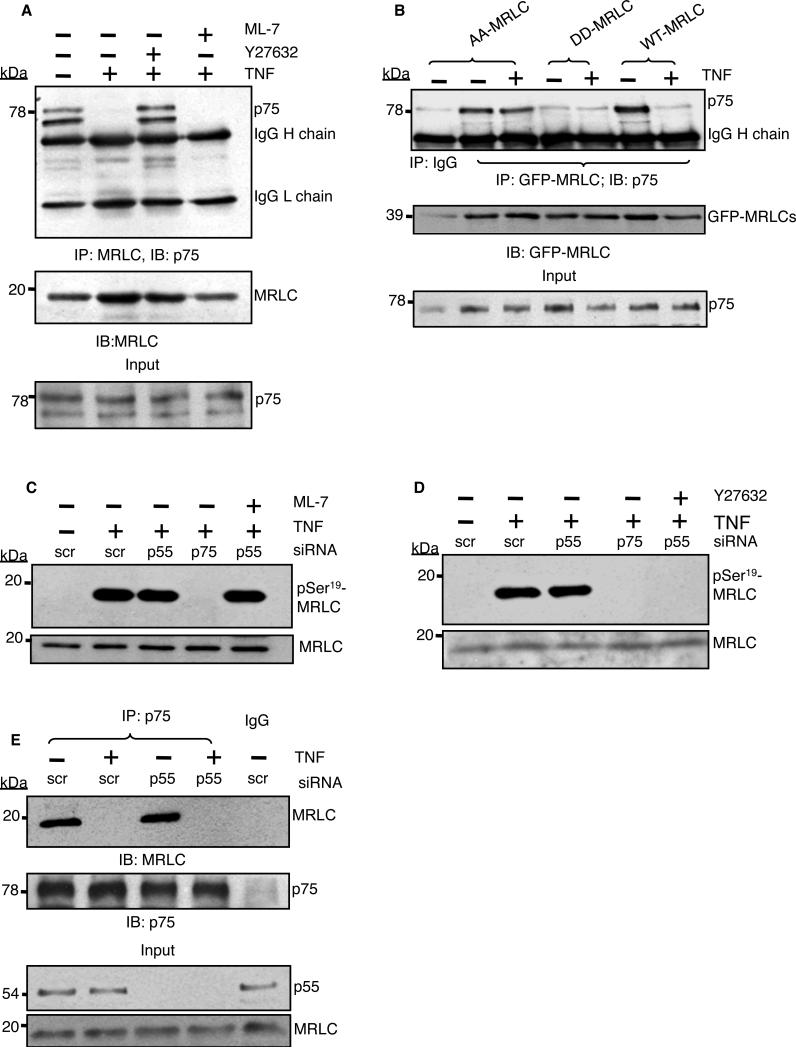
Fig. 3. p75-induced ROCK activity is required to disrupt the p75-myosin interaction.
(A) MRLC was immunoprecipitated from untreated or TNF-α-stimulated endothelial cells (2 ng/ml, 5 min) in the presence of 10 μM ML-7 or 20 μM Y27632, and samples were analyzed by Western blotting for coimmunoprecipitated p75. (B) Cells transfected with plasmids encoding GFP-tagged wild-type (WT) MRLC or the AA-MRLC or DD-MRLC mutants were subjected to immunoprecipitation with antibody against GFP-MRLC and Western blotting analysis for the presence of p75. (C and D) Western blotting analysis of TNF-α-mediated phosphorylation of MRLC Ser19 in p75- or p55-depleted cells in the presence of (C) 10 μM ML-7 or (D) 20 μM Y27632. Total MRLC protein was used as a loading control. (E) Human endothelial cells transfected with scrambled siRNA (scr) or p55-specific siRNA were left untreated or were treated with TNF-α (2 ng/ml) for 5 min before being subjected to immunoprecipitation with an antibody against p75 and then analyzed by Western blotting to detect coimmunoprecipitated MRLC. Inputs: 5% of the total lysates. Data are representative of three independent experiments.
To confirm the role of MRLC phosphorylation in the regulation of the p75-myosin interaction, we tested whether a nonphosphorylatable (Thr18/Ser19-mutated) form of MRLC (23, 24) would be resistant to TNF-α-induced myosin release. We transfected endothelial cells with plasmids encoding green fluorescent protein (GFP)-tagged wild-type MRLC, a nonphosphorylatable mutant MRLC (Thr18/Ser19 to Ala18/Ala19; AA-MRLC), or a phosphomimetic mutant MRLC (Thr18/Ser19 to Asp18/Asp19; DD-MRLC), and we used an anti-GFP antibody to coimmunoprecipitate recombinant MRLCs. Similar to endogenous MRLC, exogenous wild-type MRLC coimmunoprecipitated with p75 from the lysates of resting cells, but not of TNF-α-treated cells (Fig. 3B). The phosphorylation-resistant mutant AA-MRLC coimmunoprecipitated with p75 from resting and TNF-α–stimulated cells, whereas the phosphomimetic mutant DD-MRLC failed to bind to p75 under either condition.
We used RNA interference (RNAi) to determine the individual roles of p75 and p55 in the phosphorylation of MRLC. After 5 min of treatment with TNF-α, we detected substantial phosphorylation of Ser19 of MRLC in human endothelial cells transfected with scrambled siRNA (Fig. 3C). Whereas we detected MRLC phosphorylation in p55-depleted cells, we did not detect MRLC phosphorylation in p75-deficient cells. The MLCK specific inhibitor ML-7 was ineffective, but the ROCK inhibitor Y27632 blocked p75-mediated phosphorylation of MRLC at Ser19 (Fig. 3, C and D). Loss of p55 did not affect the association of p75 with myosin or the TNF-α-dependent dissociation of the complex, ruling out the involvement of p55 in mediating the p75-myosin interaction (Fig. 3E). Our results suggest that TNF-α, through the activation of p75 and ROCK, stimulated the phosphorylation of MRLC at Ser19 and consequently disrupted the physical interaction between p75 and myosin.
ROCK1 activity is necessary for the release of myosin from the cytosolic domain of p75
TNF-α activates both isoforms of ROCK in cultured human microvascular endothelial cells; however, at 24 hours after stimulation with TNF-α, ROCK2, but not ROCK1, is responsible for MRLC phosphorylation (25). Because we observed ROCK-mediated phosphorylation of MRLC at 5 min after treatment with TNF-α, we chose relatively short time points at which to measure ROCK isoform activity. We immunoprecipitated ROCK1 or ROCK2 from TNF-α-stimulated cells with isoform-specific antibodies and then measured ROCK activity in vitro with MYPT-1 as a substrate. TNF-α activated ROCK1 as early as 1 min (~2.5-fold increase in ROCK1 activity compared to that in unstimulated cells), which peaked at 5 min (with a ~3-fold increase in activity), and returned to basal activity at 30 min (Fig. 4A). The response of ROCK2 to TNF-α was relatively modest; it was increased in activity only ~1.5-fold 1 min after stimulation with TNF-α, increasing to ~2-fold at 5 min and ~3-fold at 15 min, and its activity persisted for at least 30 min (Fig. 4B).
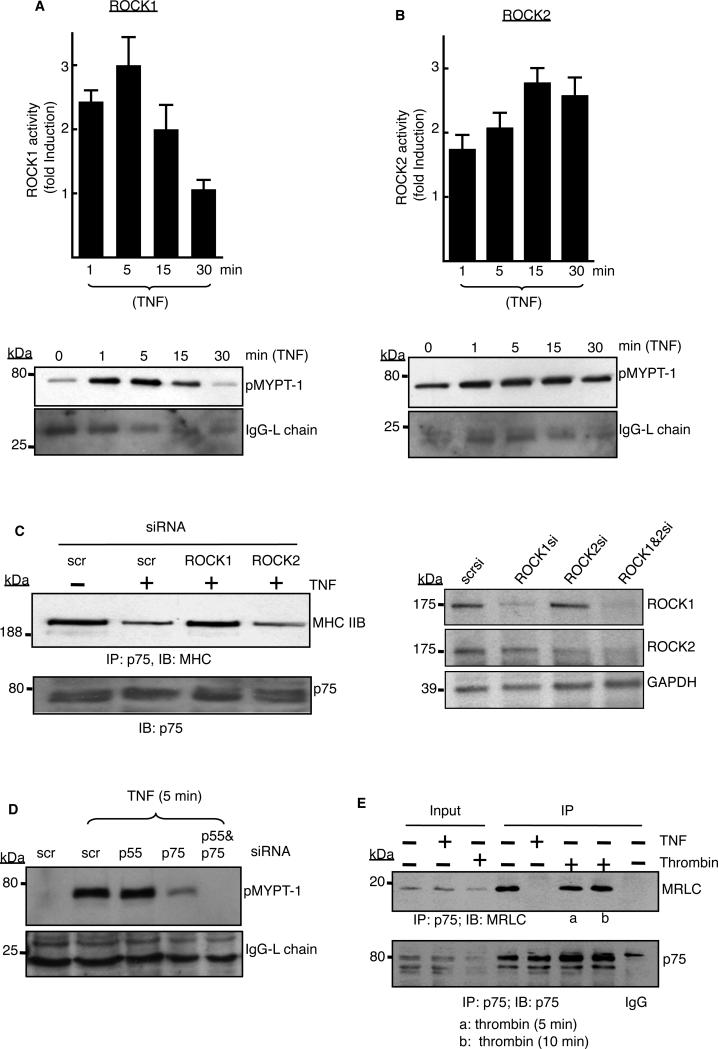
Fig. 4. TNF-α–activated ROCK1 is necessary to release myosin from p75.
(A and B) Endothelial cells were stimulated with TNF-α (2 ng/ml) for the indicated times, ROCK1 or ROCK2 were immunoprecipitated with isoform-specific antibodies, and assays with the precipitated enzymes were performed as described in the Materials and Methods. The extent of phosphorylation of the purified ROCK substrate MYPT-1 (pMYPT-1) was determined by Western blotting analysis. The relative activities of (A) ROCK1 and (B) ROCK2 are shown as the fold-increase in activity with respect to that of untreated cells (defined as one). ROCK activity assays were performed with immunoprecipitates from three independent experiments, and data are expressed as means ± SD. Western blots below the graphs are from representative experiments. The IgG light chain was used as a loading control. (C) Cells transfected with the indicated siRNAs were left untreated or were treated with TNF-α (2 ng/ml) for 5 min before being subjected to immunoprecipitation with antibody against p75, and samples were analyzed by Western blotting with an antibody specific for MHC IIB. Right panel shows Western blotting analysis of the extent of depletion of ROCK1 and ROCK2 by their respective siRNAs. Data are representative of three independent experiments. (D) Cells transfected with the indicated siRNAs were stimulated with TNF-α (2 ng/ml) for 5 min before being subjected to immunoprecipitation with ROCK1-specific antibody. ROCK1 activity was then determined as described earlier. Data are representative of three independent experiments. (E) Cells treated with TNF-α (2 ng/ml, 5 min) or thrombin (5 U/ml) were subjected to immunoprecipitation with antibody against p75 and Western blotting analysis with an MRLC-specific antibody. 5% of the total lysates were used as the inputs. Data are representative of two independent experiments.
Because both ROCK isoforms were active when myosin was released from p75 (Fig. 4, A and B), either isoform might be responsible for disrupting the p75-myosin interaction. We performed p75-myosin coimmunoprecipitation studies with cells deficient in either ROCK1 or ROCK2. RNAi-mediated depletion of ROCK1 substantially blocked the TNF-α-dependent release of myosin from p75 (Fig. 4C). Although knockdown of ROCK2 was less effective in disrupting dissociation of the complex, (Fig. 4C, right panel), the presence of ROCK2 in the ROCK1-depleted cells was insufficient to disrupt the p75-myosin interaction. Through in vitro activity assays with ROCK isoforms specifically immunoprecipitated from p75- or p55-depleted cells, we showed that ROCK1 activation at 5 min after stimulation with TNF-α was predominantly through p75, because ROCK1 activation remained intact in p55-deficient cells (Fig. 4D). Thrombin, an activator of Rho kinases in endothelial cells (18) that activated ROCK1 (fig. S3) failed to stimulate dissociation of p75 from myosin (Fig. 4E). We conclude that in TNF-α-stimulated cells, p75-mediated activation of ROCK1 and its subsequent phosphorylation of MRLC destabilizes the physical interaction between p75 and myosin.
Release of myosin from p75 is required for p75-dependent expression of target genes
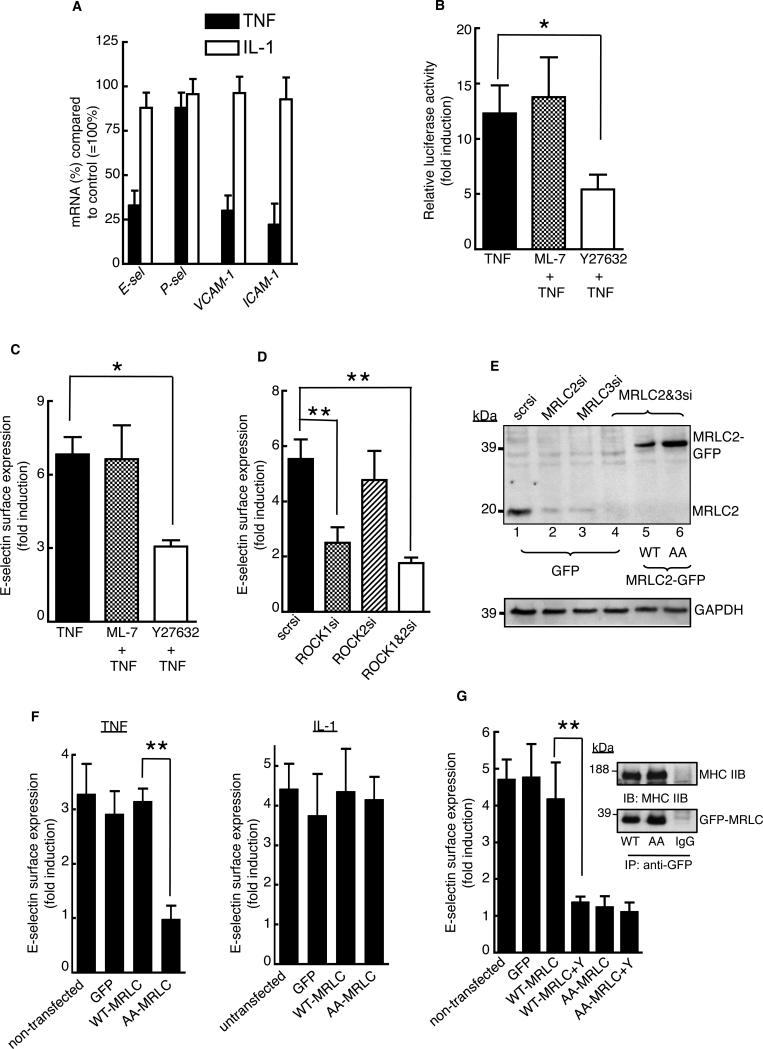
To determine the regulatory role of ROCK activity in the TNF-α-dependent expression of target genes, we pretreated endothelial cells with the ROCK inhibitor Y27632 for 30 min before treating them with TNF-α, and then we measured the abundances of given mRNAs with quantitative, real-time polymerase chain reaction (PCR) assays. We found that Y27632 decreased the extent of TNF-α-induced expression of p75-dependent genes, including E-selectin, VCAM-1, and ICAM-1, whereas the expression of P-selectin, a p75-independent gene (19) was unaffected (Fig. 5A). Y27632 did not inhibit interleukin-1β (IL-1β)-mediated expression of such proinflammatory genes, which suggested the specificity of ROCK activity in regulating p75 function.
Fig. 5. Release of myosin from p75 is required for induction of TNF-α target gene expression.
(A) Quantitative real-time PCR analysis was performed with total RNA isolated from TNF-α- or IL-1β-stimulated cells (2 ng/ml, 2 hours) in the presence or absence of 20 μM Y27632. The y-axis represents the percentage of the indicated mRNA (%) in the inhibitor-treated samples compared to that in the control cells (without inhibitor), which was considered as 100%. (B) Human endothelial cells were cotransfected with the E-selectin promoter-luciferase reporter plasmid (61) and the CMV promoter β-galactosidase reporter plasmid. Cells were stimulated with TNF-α (2 ng/ml, 5 hours) in the presence of 10 μM ML-7 or 20 μM Y27632, and relative luciferase activity was measured. (C and D) Cells treated with TNF-α (2 ng/ml, 5 hours) in the presence of (C) the indicated inhibitors or (D) the indicated siRNAs were analyzed by cell-based ELISA to determine the cell-surface abundance of E-selectin. Data are expressed as the fold-increase in the cell-surface abundance of E-selectin compared to that of untreated cells. (E) Cells transfected were the indicated siRNAs, including MRLC2-specific (3′UTR-specific) and MRLC3-specific siRNAs either alone or in combination, were analyzed by Western blotting to determine the abundance of endogenous MRLC proteins, which correspond to the ~20-kD band in lanes 4 to 6. The ~39-kD band in lanes 5 and 6 corresponds to recombinant MRLC2-GFP protein. (F and G) MRLC-depleted cells reconstituted with WT-MRLC, AA-MRLC, or GFP were sorted on a flow cytometer, cultured in 96-well plates, and stimulated for 5 hours with (F) TNF-α or IL-1 (2 ng/ml) or (G) TNF-α (2 ng/ml) in the presence of Y27632. Inset shows Western blotting analysis of coimmunoprecipitated MHC IIB and GFP-tagged MRLCs. The cell-surface abundance of E-selectin was measured by ELISA, and data are shown as the fold-increase in abundance compared to that of untreated cells. Quantitative PCR analysis, measurement of E-selectin abundance, and luciferase assays were performed in triplicate with independent endothelial cell isolates. Data are means ± SD of three independent experiments. *P < 0.05, **P < 0.01.
We then focused on E-selectin as a model to further characterize the consequence of the p75-myosin interaction in the induction of proinflammatory gene expression by TNF-α. We found that Y27632 blocked ~60% of the TNF-α-induced activity of the E-selectin promoter (P < 0.05), whereas the MLCK inhibitor ML-7 had no effect (Fig. 5B). Similarly, Y27632, but not ML-7, inhibited the TNF-α-induced increase in the cell-surface abundance of E-selectin by ~60% (Fig. 5C, P < 0.05). To determine the ROCK isoform involved, we compared the extent of the TNF-α-dependent increase in cell-surface abundance of E-selectin in cells deficient in either ROCK1 or ROCK2. Cells transfected with control scrambled siRNA showed a ~6-fold increase in the cell-surface abundance of E-selectin in response to TNF-α, which was reduced to a ~2-fold increase in ROCK1-depleted cells (Fig. 5D, P < 0.01). However, loss of ROCK2 did not substantially inhibit the TNF-α-dependent increase in cell-surface E-selectin abundance, and simultaneous loss of both ROCK isoforms had no more effect on the TNF-α-dependent increase in E-selectin abundance that did depletion of ROCK-1 alone.
We directly tested the relevance of the release of myosin from p75 in the TNF-α-dependent increase in E-selectin expression by reconstituting endothelial cells with the AA-MRLC mutant. We used an MRLC2-specific siRNA targeted to the 3’ untranslated region (UTR) in combination with an siRNA targeting the coding region of MRLC3 to deplete the human endothelial cells of endogenous MRLC2 and MRLC3 simultaneously. Because human umbilical vein endothelial cells express MRLC2 and MRLC3, but not MRLC1 (fig. S4), this strategy enabled us to reconstitute MRLC-depleted cells with GFP-tagged wild-type MRLC2 or the dissociation-defective mutant MRLC2, AA-MRLC in. Western blotting analysis with an antibody that recognizes all three MRLC isoforms showed reconstitution of the cells with recombinant MRLC (Fig. 5E). MRLC-depleted cells appeared normal under the light microscope (fig. S5). To determine the effect on target gene expression of the defective TNF-α-dependent dissociation of p75 and myosin, we measured the TNF-α-dependent abundance of E-selectin on the surface of endothelial cells reconstituted with wild-type MRLC or the mutant AA-MRLC protein. Cells with GFP-tagged wild-type MRLC or GFP-tagged AA-MRLC were sorted by flow cytometric analysis, cultured in a 96-well plate, stimulated with TNF-α for 5 hours, and determined the cell-surface abundance of E-selectin. The extent of the TNF-α-dependent increase in the cell-surface abundance of E-selectin in cells expressing wild-type MRLC (~3-fold) was similar to that in untransfected cells or in GFP-expressing cells (Fig. 5F). In cells expressing the AA-MRLC mutant, the increase in E-selectin abundance stimulated by TNF-α, but not IL-1, was statistically significantly blocked (P < 0.01, Fig. 5F). Coimmunoprecipitation studies showed that GFP-tagged MRLC proteins bound to endogenous MHC proteins (Fig. 5G, inset). The ROCK inhibitor Y27632 blocked the TNF-α-dependent increase in cell-surface E-selectin abundance in human endothelial cells reconstituted with wild-type MRLC (Fig. 5G). Together, these results demonstrate the requirement of the TNF-α-dependent dissociation of myosin from p75 for target gene expression.
Depletion of myosin prolongs p75 signaling and target gene expression
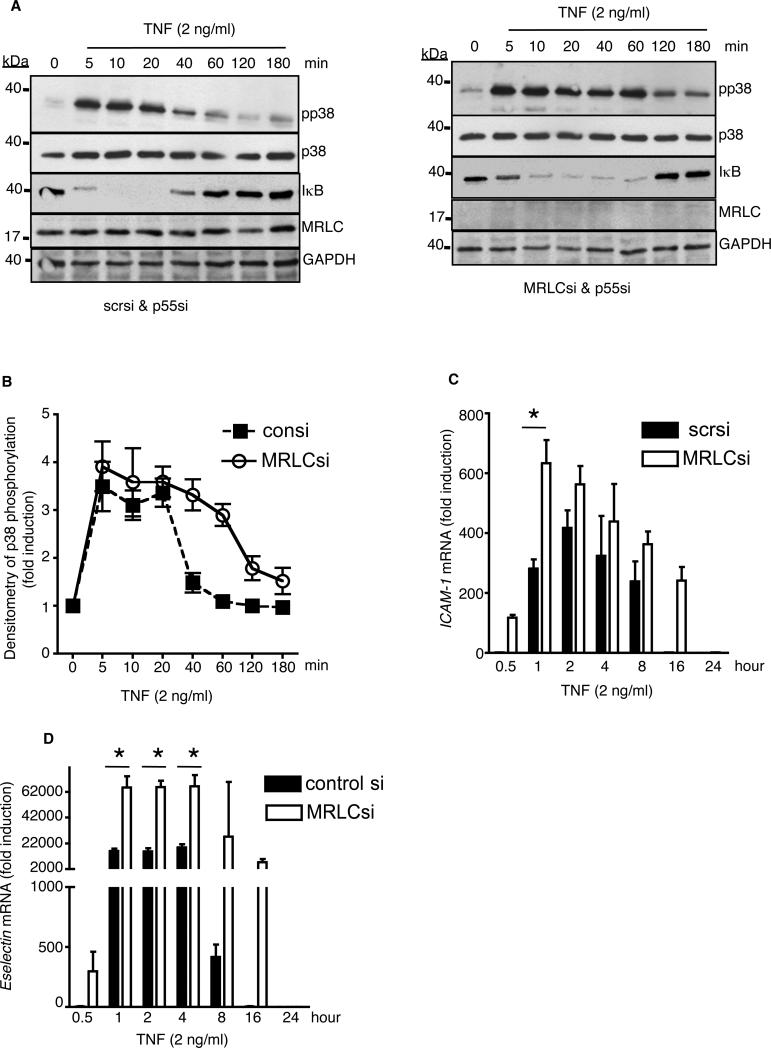
Our finding that the binding of myosin to p75 leads to receptor inhibition prompted us to determine whether depletion of myosin had a positive effect on p75-dependent signaling and target gene expression. We observed a substantial p75-mediated phosphorylation (and activation) of p38 MAPK as early as 5 min after stimulation of either MRLC-sufficient or MRLC-deficient cells with TNF-α (Fig. 6, A and B). In MRLC-intact cells, the phosphorylation of p38 subsided substantially within 40 min of stimulation, whereas p38 phosphorylation persisted for at least 60 min in MRLC-deficient cells. The magnitude of maximal p38 phosphorylation did not alter with loss of MRLC (Fig. 6B). In both sets of cells, p55 was depleted by siRNA to restrict TNF signaling to p75. Similarly, loss of MRLC altered p75-dependent degradation of inhibitor of κB (IκB), a critical step in the activation of NF-κB. In MRLC-sufficient cells, p75-dependent degradation of IκB was restricted to 20 min after stimulation with TNF-α, and, as reported earlier (26), we detected resynthesized IκB within 40 min (Fig. 6A). In MRLC-depleted cells, IκB degradation persisted up to 60 min after stimulation with TNF-α.
Fig. 6. Loss of MRLC prolongs p75 signaling and TNF-α-dependent gene expression.
(A) Human endothelial cells depleted of p55 (left panel) or p55 and MRLC (right panel) were stimulated with TNF-α (2 ng/ml) for the indicated times and phosphorylation of p38 MAPK or IκB was detected by Western blotting analysis with specific antibodies. Data are representative of three independent experiments. (B) The extent of p38 phosphorylation in the cells shown in (A) was determined by densitometric analysis. Data are mean fold-changes ± SD in the intensities of the bands corresponding to pp38 (normalized to that of total p38 protein) from three independent experiments. (C) Human endothelial cells depleted of p55 alone (filled bars) or of both p55 and MRLC (empty bars) were treated with TNF-α (2 ng/ml) for the indicated times and then analyzed by quantitative real-time PCR to determine the abundance of ICAM-1 mRNA. (D) Human endothelial cells transfected with scrambled siRNA (filled bars) or with MRLC-specific siRNA (empty bars) were treated with TNF-α (2 ng/ml) for the indicated times and then analyzed by quantitative real-time PCR to determine their abundance of E-selectin mRNA. In (D), we did not deplete p55 because TNF-α-dependent induction of E-selectin expression requires activation of both p55 and p75 (19). Data in the graph are means ± SD of three independent experiments. *P < 0.05
Next, we determined the effect of MRLC depletion on TNF-α-mediated expression of proinflammatory genes. We observed prolonged TNF-α-dependent expression of ICAM-1 mRNA in endothelial cells deficient in both p55 and MRLC compared to that in cells deficient in p55 alone(Fig. 6C). In MRLC-replete cells, we observed ICAM-1 expression between 1 and 8 hours after stimulation with TNF-α, with peak induction at 2 hours (~400-fold greater than basal expression). Loss of MRLC resulted in p75-mediated ICAM-1 expression as early as 30 min after stimulation with TNF-α, which peaked at 1 hour (to ~ 600-fold greater than basal), and persisted for up to 16 hours. Depletion of MRLC had a similar effect on the TNF-α-dependent expression of E-selectin in endothelial cells (Fig. 6D). Thus, our results suggest that the association between myosin and p75 is important in the “on-off” regulation of p75 signaling and expression of target genes.
The region of p75 that interacts with myosin is required for induction of E-selectin expression
To identify the myosin-binding region in p75, we performed coimmunoprecipitation studies with mutant p75 proteins with successively larger deletions of the cytosolic tail (Fig. 7A). The N-termini of all constructs were tagged with the myc epitope. Deletion of the Etk/Bmx-binding site did not affect the ability of the mutant p75 to bind to myosin (Fig. 7B). Deletion of a further 26 amino acid residues that encompass the canonical TRAF2-binding site prevented the p75-myosin interaction. Similarly, a mutant p75 from which the Etk/Bmx-binding site was deleted (Etkdel) rescued the increase in E-selectin abundance to a similar extent as did full-length p75 (~4-fold, Fig. 7C), suggesting that Etk/Bmx binding is not required for p75-mediated increases in E-selectin abundance. The stretch of 26 amino acid residues required for the interaction between p75 and myosin (residues 420 to 447) was also responsible for the increase in cell-surface E-selectin abundance. The amounts of full-length and mutant p75 proteins on the cell surface were comparable, as measured with a biotin-conjugated anti-p75 antibody that binds to the extracellular domain of p75 and a 125I-streptavidin-based assay (fig. S6). Our results suggest the functional importance of the myosin-interacting region in p75 for TNF-α-dependent gene expression.
Fig. 7. The myosin-interacting region of p75 overlaps with the TRAF2-binding region.
(A) Depiction of the organization of the cytosolic domain of p75 (p75cyto) and its various deletion mutants. Numbering is based on the amino acid sequence of the full-length p75 protein. (B) Cells transfected with plasmids expressing p75cyto or its deletion mutants were subjected to immunoprecipitation with an anti-myc antibody and were analyzed by Western blotting with an MRLC-specific antibody or an anti-myc antibody. The construct p75cytoCK1del did not produce a stable protein. Data are representative of three independent experiments. (C) Aortic endothelial cells from p75-deficient mice were transfected with plasmids encoding full-length p75 (p75-full) or various p75 deletion mutants, cells were stimulated with human TNF-α (2 ng/ml) for 5 hours, and the cell-surface abundance of E-selectin was measured by ELISA. Experiments were performed in triplicate with independent EC isolates and data are means ± SD. (D) Human endothelial cells expressing p75cyto or a mutant p75 protein in which the TRAF2-binding site in the cytosolic tail was mutated (424SKEE427 to 424AAAA427, TRAF2mut) were subjected to immunoprecipitation with an anti-myc antibody and analysis by Western blotting with an MHC IIB–specific antibody. (E) Cells transfected with the indicated siRNAs were also transfected with plasmid encoding a myc-tagged p75 mutant protein from which the C-terminal TRAF2-binding site was deleted (T2bs-Cdel). Cells were then left untreated or were treated with TNF-α (2 ng/ml) for 5 min before being subjected to immunoprecipitation with an anti-myc antibody and analysis by Western blotting to detect coimmunoprecipitated TRAF2 or MRLC. (F) Cells transfected with scrambled siRNA or p55-specific siRNA were transfected with plasmid encoding T2bs-Cdel before being treated with TNF-α (2 ng/ml) for the indicated times and then were subjected to immunoprecipitation with an anti-myc antibody and analysis by Western blotting with antibodies against TRAF2 or MRLC. Western blotting analysis of the input samples used for the experiments in (F) is shown in fig. S8. Inputs: 5% of the total lysates. Representative in (D) to (F) are representative of three independent experiments.
To test whether the TRAF2- and myosin-binding sites of p75 overlapped, we examined the interaction between myosin and p75 in a mutant p75 protein in which the TRAF2-binding site was mutated; 424SKEE427 of p75 was mutated to 424AAAA427 (TRAF2mut). The TRAF2mut protein interacted much less efficiently with myosin that did wild-type p75, which suggested that the canonical TRAF2-binding site indeed overlapped with the binding site for myosin (Fig. 7D). We next asked whether the interaction between myosin and p75 affected the recruitment of TRAF2 to p75. Because myosin constitutively binds to p75 in resting cells and TRAF2 recruitment occurs in response to TNF-α, we postulated that dissociation of myosin from p75 was a prerequisite for the binding of TRAF2 to p75. If myosin and TRAF2 compete for binding to the same site, we reasoned that the TNF-α-induced recruitment of TRAF2 would be blocked in ROCK1-deficient cells. To test this hypothesis, we immunoprecipitated endogenous p75 from ROCK1-deficient cells that had been left untreated or were stimulated with TNF-α, and then we looked for coimmunoprecipitated TRAF2 and myosin. Unexpectedly, we found that p75 was bound to TRAF2 in untreated and in TNF-α-treated cells, but that depletion of ROCK1 or ROCK2 had little effect on the extent of this binding interaction (fig. S7). In contrast, depletion of ROCK1 blocked the release of myosin from p75 in response to TNF-α, as evidenced by the coimmunoprecipitation of MRLC from ROCK1-deficient cells as opposed to ROCK2-deficient cells (fig. S7).
TRAF2 may bind to an alternate C-terminal site in p75 (T2bs-C) even in the absence of TNF-α (12, 13). It was possible in our Western blotting analysis that the TNF-α-regulated recruitment of TRAF2 to the canonical site, 420QVPFSKEE427, was masked by the constitutive binding of TRAF2 to the T2bs-C site. To investigate the 420QVPFSKEE427 site, we used a T2bs-C-deleted recombinant p75 protein (T2bs-Cdel) in coimmunoprecipitation experiments. We immunoprecipitated Myc-tagged T2bs-Cdel protein from untreated and TNF-α-treated cells and looked for coimmunoprecipitated TRAF2 and myosin. Unlike the endogenous p75-TRAF2 interaction, the extent of basal TRAF2 binding to T2bs-Cdel was weak, whereas there was substantial association between TRAF2 and T2bs-Cdel in response to TNF-α (Fig. 7E). Depletion of ROCK1 blocked the TNF-α-induced binding of TRAF2 to T2bs-Cdel. Next, we determined the kinetics of the p75-myosin interaction and, as expected, we found that myosin dissociated from p75 by no later than 5 min after stimulation with TNF-α (Fig. 7F, and fig. S8). We detected the reassociation of myosin and p75 at 60 min after stimulation with TNF-α. The time during which TRAF2 was recruited to p75 overlapped with the time during which myosin disengaged from p75. Collectively, these data suggest that ROCK1-mediated phosphorylation of myosin and its subsequent release from p75 are necessary for the recruitment of TRAF2 to the 420QVPFSKEE427 site in p75.
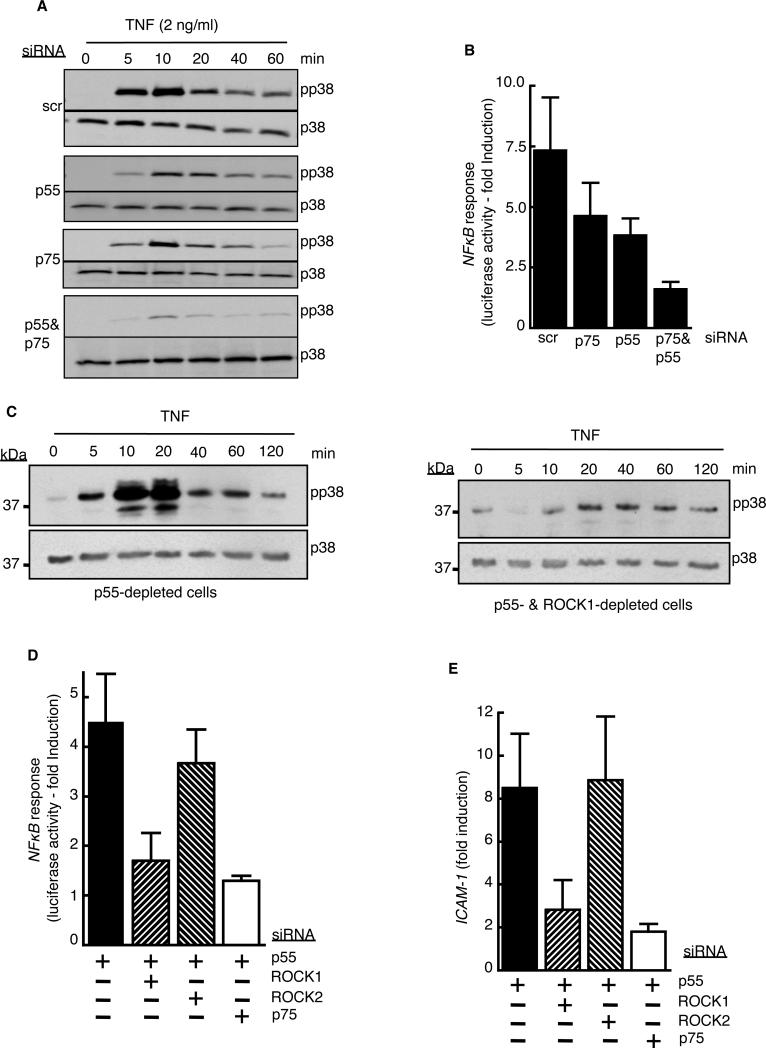
ROCK1 activity is critical for p75-mediated activation of MAPK and NF-κB signaling in TNF-α-stimulated cells
Members of the family of TNFRs, including p75, recruit TRAF proteins to their cytosolic tails upon ligand binding. Upstream kinases required for the activation of MAPKs and NF-κB, in turn, bind to the receptor-TRAF2 complex (27). If ROCK1-mediated phosphorylation of myosin and its release from the receptor was a prerequisite for the recruitment of TRAF2 to p75, we hypothesized that ROCK1-deficient cells would not activate MAPKs or NF-κB through p75 in response to TNF-α. Both p55 and p75 receptors independently activate MAPKs and NF-κB in other cell types (28, 29). In agreement with those findings, we found that TNF-α activated MAPKs and NF-κB in both p55-depleted and p75-depleted endothelial cells, including p38, a member of the MAPK family predominantly involved in TNF-α induction of leukocyte adhesion molecules in endothelial cells (Fig. 8A) (30). Similarly, either p75 or p55 was sufficient to activate an NF-κB–responsive promoter upon stimulation with TNF-α (Fig. 8B). Furthermore, loss of ROCK1 blocked p75-mediated activation of p38 MAPK and NF-κB (Fig. 8, C and D) in p55-depleted cells. In addition, in p55-depleted cells, loss of ROCK1 depletion inhibited the TNF-α-dependent induction of the expression of ICAM-1, a p75-specifc gene (Fig. 8E) (19). Collectively, our results suggest that the ROCK1-mediated phosphorylation and subsequent release of p75-bound myosin is critical for p75-specific signaling and target gene expression. Although p75 physically interacted with myosin II A (fig. S9) and II B (Fig. 2A), the two isoforms expressed in endothelial cells, a myosin ATPase inhibitor (blebbistatin) did not block the dissociation of myosin from p75 (fig. S10), suggesting that ROCK1-mediated myosin release is independent of myosin motor activity.
Fig. 8. ROCK1 activity is required for p75-mediated activation of MAPK and NF-κB.
(A) Human endothelial cells transfected with the indicated siRNAs were left untreated or were stimulated with TNF-α (2 ng/ml) for the indicated times before being analyzed by Western blotting to determine the extent of activation of p38 MAPK. Data are representative of three independent experiments. (B) Human endothelial cells treated with the indicated siRNAs were transfected with an NF-κB-dependent luciferase reporter, and cells were stimulated with TNF-α (2 ng/ml) for 5 hours before the luciferase activity in each sample was determined. Data are means ± SD of three independents. (C) Cells treated with p55-specific siRNA alone or with p55- and ROCK1-specific siRNAs were treated with TNF-α (2 ng/ml) for the indicated times before being analyzed by Western blotting to determine the extent of p75-dependent phosphorylation of p38 MAPK. Data are representative of three independent experiments. (D and E) Cells treated with the indicated siRNAs were treated with TNF-α (2ng/ml) for 5 hours before being assessed for the extent of p75-mediated (D) NF-κB activation, as measured by luciferase reporter assay, and (E) ICAM-1 expression, as determined by measurement of mRNA abundance. Data are means ± SD of three independent experiments.
Discussion
With mouse models, we and others have implicated p75 as a proinflammatory and proatherogenic molecule (19, 31-35). Pircher et al., demonstrated that the prothrombotic activity of TNF-α in vivo is mediated through p75, whereas, p55 shows an anti-thrombotic effect (36). The activity of p75 is also involved in inflammatory angiogenesis (37, 38) and T-cell proliferation (39), processes that contribute to the pathogenesis of atherosclerosis, Crohn's disease, arthritis, and psoriasis. These studies collectively revealed p75 as a potential anti-inflammatory therapeutic target. Understanding the signaling mechanisms by which p75 elicits its proinflammatory activity is important to develop p75-specific therapeutic strategies. Here, with biochemical and molecular biology approaches, we have identified a previously uncharacterized molecular mechanism that is essential for p75-specific signaling to lead to gene induction.
Both p75 and p55 participate in the TNF-α-dependent induction of many proinflammatory genes, including E-selectin, in a cell type– and context-dependent manner (19, 40). It has been proposed that p75 functions as an auxiliary receptor by passing TNF-α to p55 (41). If the role of p75 is to simply pass the ligand to p55, a mutant p75 protein from which the cytosolic domain is deleted but in which the extracellular domain is preserved would be expected to function similarly to the full-length receptor. However, our rescue studies showed that a p75 protein lacking the cytosolic tail was incapable of inducing E-selectin expression in cells expressing p55. Furthermore, TNF-α activated NF-κB and MAPK signaling, as well as the expression of target genes, in p55-deficient cells, demonstrating the independent function of p75 as a signaling receptor.
Myosin reversibly interacts with actin filaments and uses ATP hydrolysis to power its motor activity. The motor activity of nonmuscle myosin II is essential for cell motility, cell division, and cell-cell or cell-matrix interactions (21). Evidence has accumulated of some non-conventional functions of myosin, including its ability to stimulate gene transcription and act as a receptor for herpes simplex virus entry (42, 43). Nonmuscle myosin II (or its subunits) participate in the trafficking of, and positively or negatively regulate, the signaling of multiple receptors, including the chemokine receptors CXCR4 and CXCR5 (44, 45), the N-methyl-D-aspartate (NMDA) receptor (46, 47), and multi-drug resistance protein 1 (48). Kim et al., identified nonmuscle myosin II as a binding partner of the epidermal growth factor receptor and showed that it plays an important role in receptor internalization and signaling (49). In all such cases, myosin binds to the given receptor under basal conditions, and upon ligand activation, myosin translocated the receptors to the cell membrane or endosomal compartments by virtue of its motor activity. In contrast, the p75-myosin interaction that we describe here was independent of myosin motor activity, because blebbistatin, a myosin ATPase inhibitor, did not prevent TNF-α-dependent dissociation of myosin from p75. Moreover, we did not detect actin, an integral partner for myosin-mediated transport, in the p75-myosin complex. Finally, we showed that latrunculin, which inhibits actin polymerization, failed to prevent the interaction between p75 and myosin. When unphosphorylated, nonmuscle myosin folds into a compact conformation in which the two myosin heads interact with the tail (50). Phosphorylation of MRLC destabilizes this head-tail interaction, converting the folded myosin to an elongated form. It is possible that only the folded form of myosin stably binds to p75 and that ROCK1-mediated phosphorylation of MRLC induces a conformational change in myosin that destabilizes its physical association with p75.
TNF-α activates both ROCK1 and ROCK2 in primary endothelial cells (25). ROCK activity is implicated in NF-κB activation and gene expression in cells stimulated with TNF-α and other agonists (16, 51); however, the role of ROCK activation in p75 signaling and target gene expression has not been investigated. We have identified a regulatory mechanism in the p75 signaling pathway whereby TNF-α-dependent activation of ROCK1 resulted in the phosphorylation of p75-bound myosin at Ser19 of MRLC, which resulted in the dissociation of myosin from p75. ROCKs are the primary effectors of the Rho family of GTPases, which can be activated by TNF-α (52). One study demonstrated that TNF-α-mediated activation of MAPK is mediated through a Rho GTPase signaling pathway (53); however, this study did not address which of the two TNFRs was responsible for Rho GTPase activation. Activated ROCKs increase the extent of myosin phosphorylation either directly by phosphorylating MRLC at Thr18 and Ser19 or indirectly through the inhibitory phosphorylation of the myosin phosphatase MYPT1 (54). Either or both mechanisms may be playing a role in the mechanism that we have described.
TRAF2 was first identified as a p75-binding protein (55). Later studies revealed the pivotal role of TRAF proteins in the activation of NF-κB and MAPKs by members of the TNFR family (9, 27). Receptor-bound TRAF2 functions as an E3 ubiquitin ligase by itself or recruits proteins with ubiquitinating activity that, in turn, facilitate the recruitment of the IKK complex and MEK kinase 1 to the receptor, which leads to NF-κB and MAPK activation. We found that in resting cells, myosin occupied the canonical TRAF2-binding site on p75. Displacement of myosin from p75 in response to TNF-α was a prerequisite for the recruitment of TRAF2 to p75 and consequent downstream signaling. We detected the reassociation of myosin with p75 60 min after stimulation with TNF-α, which coincided with the loss of detectable binding of TRAF2 to p75. The different kinetics of interaction between p75 and myosin and between p75 and TRAF2 indicates that the reassociation of myosin may function as one of the regulatory mechanisms required to terminate p75 signaling. TRAF2 also binds to p55 indirectly, through the adaptor protein TRADD, and it activates NF-κB and MAPK signaling (27); however, we failed to detect p55 in complexes immunoprecipitated with anti-MRLC antibody from unstimulated or TNF-α-stimulated cells (Fig. 2D). Furthermore, the ROCK inhibitor Y26732, which prevented the release of myosin from p75, had no effect on ability of TNF-α to induce the expression of P-selectin, a p55-specific, TNF-α-responsive gene. Together, our results indicate that the regulatory activity of myosin is limited to p75, not p55, signaling. Our time course studies further demonstrated the importance of the regulatory role of myosin in p75 signaling. Depletion of the MRLC subunit altered p75 signaling and enabled prolonged expression of genes encoding pro-inflammatory factors. We believe that approaches that prevent the displacement of myosin from p75, including the inhibition of ROCK1 activity or the use of small molecules to stabilize the p75-myosin interaction, could be used as a p75-specific therapeutic strategy to target inflammatory diseases in a manner that has fewer side effects than do the currently used anti- TNF-α blockers.
The mammalian genome contains three genes encoding MHC isoforms, MHC IIA, MHC IIB, and MHC IIC, of which endothelial cells express the first two (56). We coimmunoprecipitated MHC IIA and MHC IIB, as well as the light chains MRLC and MELC, together with p75 Further studies are required to unravel the residues in myosin that are responsible for its binding to p75. Similarly, the role of the myosin-p75 interaction on the subcellular distribution, integrity, or both of p75 warrant further investigation.
Here, we identified a distinct role for myosin in the regulation of p75 signaling. ROCK1-mediated release of myosin from p75 was critical for TNF-α-dependent gene expression. Because p75 and p55 stimulate NF-κB and MAPK activation to similar extents and with similar kinetics, we propose that a p75-ROCK1-TRAF2 axis activates a specific signaling pathway that participates with p55 signaling to induce expression of genes encoding proinflammatory factors. Our study has revealed a previously uncharacterized signaling mechanism that may lead to the development of p75-specific therapeutic strategies against inflammatory diseases in which TNF-α is a major contributory cytokine.
Materials and Methods
Endothelial cell isolation, culture, and transfection
Human umbilical vein endothelial cells were isolated by trypsinization as previously described (19). Endothelial cells were plated on fibronectin-coated cell culture dishes and maintained in MCDB/F12 medium containing 15% fetal bovine serum (FBS), 0.009% heparin, and .015% endothelial cell growth supplement. All experiments were performed with cells between the third and fifth passage. Mouse aortic endothelial cells (MAECs) were isolated by explant technique (57). The authenticity of the MAECs was verified by staining for Willibrand factor. Endothelial cells were transfected with Targefect reagents (Targeting Systems) according to the manufacturer's protocol.
Quantitative real-iime PCR
Total RNA was isolated with Qiagen RNeasy spin columns. First-stand complementary DNA (cDNA) was synthesized with TaqMan reverse transcription reagents (Roche). cDNA reactions were diluted 6-fold in deionized water and used as templates for qRT-PCR analysis. Reactions were performed with Sybr Green Master Mix (Applied Biosystems). The extent of induction of gene expression was calculated relative to that of untreated controls with the 2−ΔΔCt method.
Cytokines
TNF-α and IL-1β were purchased from R&D Systems.
Analysis of the cell-surface abundance of E-selectin
The cell-surface abundance of E-selectin was determined with a biotin-labeled antibody/125I-streptavidin technique (19) or by ELISA with an anti-E-selectin primary antibody and a horseradish peroxidase (HRP)-conjugated secondary antibody (58).
RNA interference
The sources and product identification of the siRNAs used in this study are as follows: p55-specific siRNA: ID 42862; p75-specific siRNA: ID 110832; ROCK1-specific siRNA: ID 12099; ROCK2-specific siRNA: ID 18163 (all from Ambion, Life technologies). The MRLC2-specifc siRNA (3’UTR-targeted, 5′-CCAGTTACATTGTCTTACTCTCTTT-3′) was custom made by Invitrogen, Life technologies. The MRLC3-specific siRNA (sc-106238) was purchased from Santa Cruz biotechnologies. Alternative sets of siRNAs were used to verify the effects of the primary sets of siRNAs are as follows: p55-specific siRNA (sc 29507) and p75-specific siRNA (sc-1072), both from Santa Cruz biotechnologies; and ROCK1- and ROCK2-specific siRNA (On-Target Smart pool) from Dharmacon. Transfections of cells with siRNA were performed with Targefect F-2 and peptide enhancer (Targeting Systems). Targefect F-2 (5 μl/ml), peptide enhancer (5 μl/ml), and 50 nM siRNA were successively added to Dulbecco's modified Eagle medium, thoroughly mixed, and kept at 37°C for 30 min. Endothelial cells (> 90% confluency) were incubated with the transfection mixture for 4 hours. Cells were replenished with fresh endothelial cell culture medium and, after a 24- to 36-hour incubation period, cells were harvested for protein analysis to verify the target protein or RNA depletion.
Coimmunoprecipitations and mass spectrometry
Endothelial cells transfected with a plasmid encoding a myc-tagged cytosolic fragment of p75 (p75cyto) were lysed in a buffer with 50 mM Tris-HCl, 150 mM NaCl, 1% Triton X100, 5 mM sodium orthovanadate, 10 mM sodium pyrophosphate, and 10 mM sodium fluoride containing 1X complete protease inhibitor cocktail (Roche Applied Science). Anti-myc-antibody (Millipore) together with protein AG agarose was used to immunoprecipitate p75cyto and associated proteins. Proteins were resolved by SDS-PAGE and stained with silver stain. Tryptic peptides of the specific bands were analyzed by LC-ESI/MALDI-TOF mass spectrometry. A Finnigan LTQ ion trap mass spectrometer (ThermoFinnigan) equipped with an Eksigent nano-ID HPLC was used for the LC-MS system.
Coimmunoprecipitations and Western blotting analysis
The Myc-tagged cytosolic fragment (p75cyto) or p75cyto-mutants were immunoprecipitated as described earlier. We followed the method described by Pan et al. (59) for the coimmunoprecipitation studies involving full-length, endogenous, or recombinant p75 and various p75 mutants. ImageJ software (NIH) was used to quantify Western blotting signals.
ROCK activity assay
Cells were lysed in 50 mM Tris (pH 7.5), 10 mM MgCl2, 0.5 M NaCl and 1% Triton X100. Cell lysates were precleared with isoform-specific control antibodies. ROCK1 or ROCK2 was immunoprecipitated with anti-ROCK1 or anti-ROCK2 antibody (Santa Cruz Biotechnology Inc.), respectively, in conjunction with protein A/G agarose. The beads were washed and incubated with pure MYPT-1 (substrate) in kinase reaction buffer provided by the ROCK Activity Immunoblot kit (Cell Biolabs, Inc.). Immunoprecipitates were then diluted to achieve the enzyme activity in a linear range before the activity assay. Pure MYPT-1 (~80 kD) was used as a substrate. The extent of phosphorylation of MYPT-1 was determined by Western blotting analysis. Except for fig. S3, the immunoglobulin G (IgG) light chain was used as loading control, because the antibodies used were not sensitive enough to detect ROCK1 or ROCK2 from the diluted samples.
Analysis of E-selectin cell-surface abundance on endothelial cells reconstituted with AA-MRLC2
Endothelial cells cultured in 100-mm dishes were transfected with GFP-tagged MRLC2 cDNA in pcDNA3 (2.5 μg) together with MRLC2- and MRLC3-specific siRNAs (50 nM each). Twenty-four hours later, cells were trypsinized, washed, and resuspended in cell sorting buffer [25 mM HEPES (pH 7.5), 1 mM EDTA, 1% fetal bovine serum (FBS) in phosphate-buffered saline (PBS)] at a concentration of 6 × 106 cells/ml. The cell suspension was filtered with a CellTrics filter (PARTEC) with a pore size of 30 μM. The GFP-expressing cells were sorted with a BD FACSAriaII cell sorter (Becton-Dickinson) and distributed into a 96-well plate at a density of 10,000 cells/well. Twenty-four hours later, cells were treated with TNF-α (2 ng/ml) or IL-1 (2 ng/ml) for 5 hours and then the cell-surface abundance of E-selectin was measured by ELISA. In vitro transcription and translation of cDNAs were performed with the TNT-coupled reticulocyte system (Promega) according to the manufacturer's protocol.
Statistical analysis
Data are expressed as mean ± SD. Differences between groups were assessed by Student's t-test or analysis of variance (ANOVA), as appropriate, with P values for post hoc pairwise comparisons adjusted with the Bonferroni method. P < 0.05 was considered statistically significant. Prism (GraphPad Inc.) was used to perform the statistical analysis.
Supplementary Material
Acknowledgments
We thank J. Boerckel for his constructive criticism of the manuscript, M. Rajabi for standardizing the ELISA to measure E-selectin abundance, and B. Willard and M. Kinter for mass spectrometry analyses. We acknowledge Chad Braley for his technical help in performing Western blotting analysis and cell culture. Funding: This study was supported by NIH Grant HL29582 (to P.E.D). Umbilical vein endothelial cells were harvested from human umbilical cords collected from the Birthing Services Department at the Cleveland Clinic (Hillcrest Hospital) and the Perinatal Clinical Research Center (PCRC) at the Cleveland MetroHealth Hospital. The PCRC has been supported by a grant (UL1TR000439) awarded to the Clinical and Translational Science Collaborative of Cleveland by the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: * This manuscript has been accepted for publication in Science Signaling. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the copyright Act without the prior, written permission of AAAS.
Author contributions: U.M.C. and P.E.D. designed the research, analyzed the data, and wrote the manuscript; U.M.C., L.D., U.I.D., L.M., and K.L. performed experiments; J.B. and T.T.E. contributed critical reagents for the study; M.W., T.T.E., X.L., and P.L.F. analyzed the data, suggested experiments, and contributed to writing the manuscript.
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P. Tumornecrosis factor-mediated cell death: to break or to burst, that's the question. Cell Mol Life Sci. 2010;67:1567–1579. doi: 10.1007/s00018-010-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, Min W. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem. 2003;278:51267–51276. doi: 10.1074/jbc.M310678200. [DOI] [PubMed] [Google Scholar]
- 3.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 4.Rao P, Hsu KC, Chao MV. Upregulation of NF-kappa B-dependent gene expression mediated by the p75 tumor necrosis factor receptor. J Interferon Cytokine Res. 1995;15:171–177. doi: 10.1089/jir.1995.15.171. [DOI] [PubMed] [Google Scholar]
- 5.Kalb A, Bluethmann H, Moore MW, Lesslauer W. Tumor necrosis factor receptors (Tnfr) in mouse fibroblasts deficient in Tnfr1 or Tnfr2 are signaling competent and activate the mitogen-activated protein kinase pathway with differential kinetics. J Biol Chem. 1996;271:28097–28104. doi: 10.1074/jbc.271.45.28097. [DOI] [PubMed] [Google Scholar]
- 6.Read MA, Whitley MZ, Gupta S, Pierce JW, Best J, Davis RJ, Collins T. Tumor necrosis factor alpha-induced E-selectin expression is activated by the nuclear factor-kappaB and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:2753–2761. doi: 10.1074/jbc.272.5.2753. [DOI] [PubMed] [Google Scholar]
- 7.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 8.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 9.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 10.Ha H, Han D, Choi Y. TRAF-mediated TNFR-family signaling. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im1109ds87. Chapter 11: Unit11 19D. [DOI] [PubMed] [Google Scholar]
- 11.Wajant H, Scheurich P. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int J Biochem Cell Biol. 2001;33:19–32. doi: 10.1016/s1357-2725(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 12.Grech AP, Gardam S, Chan T, Quinn R, Gonzales R, Basten A, Brink R. Tumor necrosis factor receptor 2 (TNFR2) signaling is negatively regulated by a novel, carboxyl-terminal TNFR-associated factor 2 (TRAF2)-binding site. J Biol Chem. 2005;280:31572–31581. doi: 10.1074/jbc.M504849200. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez M, Cabal-Hierro L, Carcedo MT, Iglesias JM, Artime N, Darnay BG, Lazo PS. NF-kappaB signal triggering and termination by tumor necrosis factor receptor 2. J Biol Chem. 2011;286:22814–22824. doi: 10.1074/jbc.M111.225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mong PY, Petrulio C, Kaufman HL, Wang Q. Activation of Rho kinase by TNF-alpha is required for JNK activation in human pulmonary microvascular endothelial cells. J Immunol. 2008;180:550–558. doi: 10.4049/jimmunol.180.1.550. [DOI] [PubMed] [Google Scholar]
- 15.Matoba K, Kawanami D, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun. 2010;402:725–730. doi: 10.1016/j.bbrc.2010.10.093. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Liu P, Liang L, Danesh FR, Yang X, Ye Y, Zhan Z, Yu X, Peng H, Sun L. RhoA-mediated, tumor necrosis factor alpha-induced activation of NF-kappaB in rheumatoid synoviocytes: inhibitory effect of simvastatin. Arthritis Rheum. 2006;54:3441–3451. doi: 10.1002/art.22169. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrasekharan UM, Siemionow M, Unsal M, Yang L, Poptic E, Bohn J, Ozer K, Zhou Z, Howe PH, Penn M, DiCorleto PE. Tumor necrosis factor alpha (TNF-alpha) receptor-II is required for TNF-alpha-induced leukocyte-endothelial interaction in vivo. Blood. 2007;109:1938–1944. doi: 10.1182/blood-2006-05-020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci. 2008;121:11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]
- 21.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alphainduced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 23.Fumoto K, Uchimura T, Iwasaki T, Ueda K, Hosoya H. Phosphorylation of myosin II regulatory light chain is necessary for migration of HeLa cells but not for localization of myosin II at the leading edge. Biochem J. 2003;370:551–556. doi: 10.1042/BJ20021559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beach JR, Licate LS, Crish JF, Egelhoff TT. Analysis of the role of Ser1/Ser2/Thr9 phosphorylation on myosin II assembly and function in live cells. BMC Cell Biol. 2011;12:52. doi: 10.1186/1471-2121-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182:2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 26.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karin M, Gallagher E. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-and-go for MAPK signaling complexes. Immunol Rev. 2009;228:225–240. doi: 10.1111/j.1600-065X.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 28.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 29.Laegreid A, Medvedev A, Nonstad U, Bombara MP, Ranges G, Sundan A, Espevik T. Tumor necrosis factor receptor p75 mediates cell-specific activation of nuclear factor kappa B and induction of human cytomegalovirus enhancer. J Biol Chem. 1994;269:7785–7791. [PubMed] [Google Scholar]
- 30.Chandrasekharan UM, Yang L, Walters A, Howe P, DiCorleto PE. Role of CL-100, a dual specificity phosphatase, in thrombin-induced endothelial cell activation. J Biol Chem. 2004;279:46678–46685. doi: 10.1074/jbc.M406441200. [DOI] [PubMed] [Google Scholar]
- 31.Hildebrandt GC, Olkiewicz KM, Corrion L, Clouthier SG, Pierce EM, Liu C, Cooke KR. A role for TNF receptor type II in leukocyte infiltration into the lung during experimental idiopathic pneumonia syndrome. Biol Blood Marrow Transplant. 2008;14:385–396. doi: 10.1016/j.bbmt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douni E, Kollias G. A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin alpha, or the p55TNF-R. J Exp Med. 1998;188:1343–1352. doi: 10.1084/jem.188.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115:1199–1209. doi: 10.1172/JCI23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizoguchi E, Mizoguchi A, Takedatsu H, Cario E, de Jong YP, Ooi CJ, Xavier RJ, Terhorst C, Podolsky DK, Bhan AK. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekharan UM, Mavrakis L, Bonfield TL, Smith JD, DiCorleto PE. Decreased atherosclerosis in mice deficient in tumor necrosis factor-alpha receptor-II (p75). Arterioscler Thromb Vasc Biol. 2007;27:e16–17. doi: 10.1161/01.ATV.0000255551.33365.22. [DOI] [PubMed] [Google Scholar]
- 36.Pircher J, Merkle M, Wornle M, Ribeiro A, Czermak T, Stampnik Y, Mannell H, Niemeyer M, Vielhauer V, Krotz F. Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF-alpha receptor subtype 1 and require TNF-alpha receptor subtype 2. Arthritis Res Ther. 2012;14:R225. doi: 10.1186/ar4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, Zak V, Mehta S, Burg A, Thorne T, Kishore R, Losordo DW. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, Xu Z, Wan T, He Y, Jones D, Zhang H, Min W. Endothelialspecific transgenesis of TNFR2 promotes adaptive arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1307–1314. doi: 10.1161/ATVBAHA.110.204222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 40.Bertok S, Wilson MR, Dorr AD, Dokpesi JO, O'Dea KP, Marczin N, Takata M. Characterization of TNF receptor subtype expression and signaling on pulmonary endothelial cells in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L781–789. doi: 10.1152/ajplung.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993;268:18542–18548. [PubMed] [Google Scholar]
- 42.de Lanerolle P, Serebryannyy L. Nuclear actin and myosins: life without filaments. Nat Cell Biol. 2011;13:1282–1288. doi: 10.1038/ncb2364. [DOI] [PubMed] [Google Scholar]
- 43.Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, Kawaguchi Y. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature. 2010;467:859–862. doi: 10.1038/nature09420. [DOI] [PubMed] [Google Scholar]
- 44.Rey M, Valenzuela-Fernandez A, Urzainqui A, Yanez-Mo M, Perez-Martinez M, Penela P, Mayor F, Jr., Sanchez-Madrid F. Myosin IIA is involved in the endocytosis of CXCR4 induced by SDF-1alpha. J Cell Sci. 2007;120:1126–1133. doi: 10.1242/jcs.03415. [DOI] [PubMed] [Google Scholar]
- 45.Rey M, Vicente-Manzanares M, Viedma F, Yanez-Mo M, Urzainqui A, Barreiro O, Vazquez J, Sanchez-Madrid F. Cutting edge: association of the motor protein nonmuscle myosin heavy chain-IIA with the C terminus of the chemokine receptor CXCR4 in T lymphocytes. J Immunol. 2002;169:5410–5414. doi: 10.4049/jimmunol.169.10.5410. [DOI] [PubMed] [Google Scholar]
- 46.Amparan D, Avram D, Thomas CG, Lindahl MG, Yang J, Bajaj G, Ishmael JE. Direct interaction of myosin regulatory light chain with the NMDA receptor. J Neurochem. 2005;92:349–361. doi: 10.1111/j.1471-4159.2004.02869.x. [DOI] [PubMed] [Google Scholar]
- 47.Bajaj G, Zhang Y, Schimerlik MI, Hau AM, Yang J, Filtz TM, Kioussi C, Ishmael JE. N-methyl-D-aspartate receptor subunits are non-myosin targets of myosin regulatory light chain. J Biol Chem. 2009;284:1252–1266. doi: 10.1074/jbc.M801861200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajaj G, Rodriguez-Proteau R, Venkataraman A, Fan Y, Kioussi C, Ishmael JE. MDR1 function is sensitive to the phosphorylation state of myosin regulatory light chain. Biochem Biophys Res Commun. 2010;398:7–12. doi: 10.1016/j.bbrc.2010.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JH, Wang A, Conti MA, Adelstein RS. Nonmuscle Myosin II Is Required for Internalization of the Epidermal Growth Factor Receptor and Modulation of Downstream Signaling. J Biol Chem. 2012;287:27345–27358. doi: 10.1074/jbc.M111.304824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milton DL, Schneck AN, Ziech DA, Ba M, Facemyer KC, Halayko AJ, Baker JE, Gerthoffer WT, Cremo CR. Direct evidence for functional smooth muscle myosin II in the 10S self-inhibited monomeric conformation in airway smooth muscle cells. Proc Natl Acad Sci U S A. 2011;108:1421–1426. doi: 10.1073/pnas.1011784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–12542. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew SJ, Haubert D, Kronke M, Leptin M. Looking beyond death: a morphogenetic role for the TNF signalling pathway. J Cell Sci. 2009;122:1939–1946. doi: 10.1242/jcs.044487. [DOI] [PubMed] [Google Scholar]
- 53.Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, Davis RJ. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–2078. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 56.Chantler PD, Wylie SR, Wheeler-Jones CP, McGonnell IM. Conventional myosins – unconventional functions. Biophysical Reviews. 2010;2:67–82. doi: 10.1007/s12551-010-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- 58.Bandyopadhyay S, Harris DP, Adams GN, Lause GE, McHugh A, Tillmaand EG, Money A, Willard B, Fox PL, Dicorleto PE. HOXA9 Methylation by PRMT5 Is Essential for Endothelial Cell Expression of Leukocyte Adhesion Molecules. Mol Cell Biol. 2012;32:1202–1213. doi: 10.1128/MCB.05977-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan S, An P, Zhang R, He X, Yin G, Min W. Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: role in endothelial cell migration and angiogenesis. Mol Cell Biol. 2002;22:7512–7523. doi: 10.1128/MCB.22.21.7512-7523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darnay BG, Singh S, Aggarwal BB. The p80 TNF receptor-associated kinase (p80TRAK) associates with residues 354-397 of the p80 cytoplasmic domain: similarity to casein kinase. FEBS Lett. 1997;406:101–105. doi: 10.1016/s0014-5793(97)00251-2. [DOI] [PubMed] [Google Scholar]
- 61.Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Mol Cell Biol. 2007;27:4207–4216. doi: 10.1128/MCB.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.