Abstract
We describe the development of a silver-catalyzed carbonyl olefination employing electron rich siloxy alkynes. This process constitutes an efficient synthesis of trisubstituted unsaturated esters, and represents an alternative to the widely utilized Horner-Wadsworth-Emmons reaction. Excellent diastereoselectivities are observed for a range of aldehydes using either 1-siloxy-1-propyne or 1-siloxy-1-hexyne. This mild catalytic process also enables chemoselective olefination of aldehydes in the presence of either ester or ketone functionality. Furthermore, since no by-products are generated, this catalytic process is perfectly suited for development of sequential reactions that can be carried out in a single flask.
Keywords: Olefination, Silver, Siloxy Alkyne, Chemoselectevity
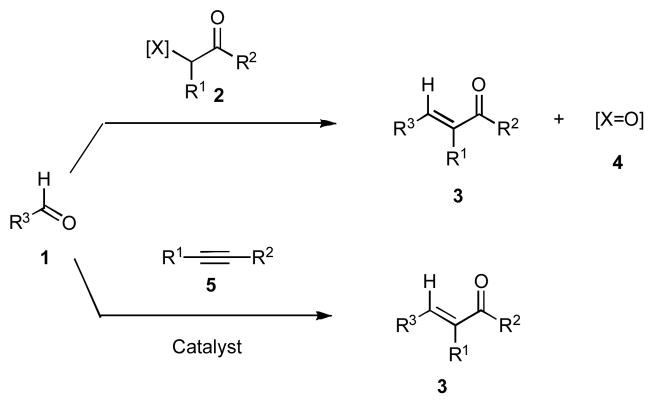
The majority of carbonyl olefinations are typically based on the reaction of an aldehyde 1 with a suitable reagent 2, which transfers the alkylidene moiety to give unsaturated product 3 with a concomitant formation of a stoichiometric amount of oxygenated product 4 (Scheme 1).1 The Arens carbonyl olefination represents a highly effective and atom-economical alternative to this process, which produces exclusively the desired product 3 by employing a combination of a carbonyl compound 1, alkyne 5 and an appropriate catalyst. 2 While extensive efforts have been devoted to advancing this olefination method, the reported transformations typically employ either large amounts of highly reactive Lewis acids3 or must be carried out at elevated temperatures.4 The photochemical version of this process proceeds under mild conditions albeit with low efficiency.5 The use of highly nucleophilic ynolates to affect this transformation has also been extensively investigated.6 While significant advances have been made, the development of a chemoselective and diastereoselective Arens olefination, which would proceed under mild conditions and display high catalytic turnover, has not been achieved. Such transformation would have significant potential for broad implementation in organic synthesis.
Scheme 1.
Alternative Carbonyl Olefination Strategies
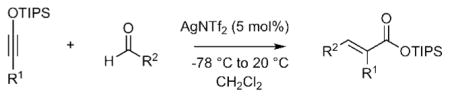
We recently identified AgNTf2 as a highly effective catalyst for [2+2] cycloaddition and hydroamination of siloxy alkynes.7 Such reactions were found to proceed under exceedingly mild conditions typically employing 1–5 mol% of this catalyst. In continuation of our systematic investigation of reactivity of electron rich alkynes,8 we report herein the development of a silver-catalyzed Arens carbonyl olefination. Promoted by substoichiometric amount of AgNTf2, this transformation represents a mild, efficient and highly chemoselective method for conversion of aldehydes into the corresponding unsaturated esters using siloxy alkynes. Furthermore, excellent diastereoselectivities were observed for a range of aldehydes using either 1-siloxy-1-propyne or 1-siloxy-1-hexyne.
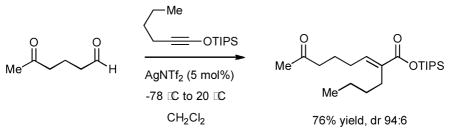
Our initial studies began with examination of the reaction of hexanal with 1-siloxy-1-propyne (Table 1, entry 1). We found that substoichiometric amount of AgNTf2 was uniquely effective to enable the olefination process. The optimum conditions entailed the treatment of siloxy alkyne with a slight excess of aldehyde (1.1 equiv.) and 5 mol% of AgNTf2 at −78 °C, followed by warming the reaction mixture to ambient temperature and chromatographic purification to give trisubstituted siloxy ester 6 in 87% yield as a single detectable diastereomer. The efficiency and catalytic turnover of this reaction compared favorably to the protocol developed by Kowalski and co-workers,3c which required the use of stoichiometric amount of TiCl4, and olefination of aldimines reported by Shindo and co-workers, which employed lanthanides.9. A range of other possible reaction promoters, including AgOTf, AuCl, AuCl3, PdCl2 and CuCl proved significantly less effective (0–9% conversion). The use of Tf2NH (10 mol%) resulted in diminished diastereoselectivity (2:1 dr), as well as lower reaction efficiency (42% yield).
Table 1.
Ag-Catalyzed olefination of aldehydes using 1-siloxy-1-propyne or 1-siloxy-1-hexyne
| |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yieid, % | E:Z Ratio |
| 1 | Me |
|
6 | 87 | >95:5 |
| 2 | Me |
|
7 | 75 | >95:5 |
| 3 | Me |
|
8 | 74 | >95:5 |
| 4 | Me |
|
9 | 75 | >95:5 |
| 5 | Me |
|
10 | 84 | >95:5 |
| 6 | n-Bu |
|
11 | 85 | 92:8 |
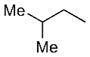
| 7 | n-Bu |
|
12 | 93 | 94:6 |
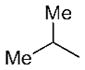
| 8 | n-Bu |
|
13 | 94 | 92:8 |
| 9 | n-Bu |
|
14 | 66 | >95:5 |
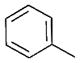
| 10 | n-Bu |
|
15 | 73 | 83:17 |
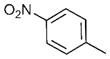
| 11 | n-Bu |
|
16 | 84 | >95:5 |
Having established the general catalytic protocol, we next examined the substrate scope of this process. The reactions of 1-siloxy-1-propyne with other aliphatic aldehydes proceeded equally efficiently in the presence of 5 mol% of AgNTf2 to produce the corresponding siloxy esters 7–8 with excellent diastereoselectivities (Table 1, entries 2 and 3). Olefinations of crotonaldehyde and p-nitro benzaldehyde also proceeded smoothly to give the expected products 9 and 10 as single geometrical isomers (Table 1, entries 4 and 5). Similarly, olefination of a range of aldehydes using 1-siloxy-1-hexyne proved highly effective. The corresponding products 11–16 (Table 1, entries 6–11) were produced in excellent yields and high levels of diastereoselectivity.
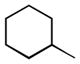
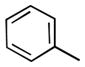
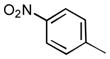
We also examined the effect of increasing steric bulk of the siloxy alkyne on the efficiency and diastereoselectivity of the olefination process. While treatment of 2-cyclohexyl-1-siloxyacetylene with hexanal produced the expected ester 17 in 72% yield (Table 2, entry 1), the increased size of the siloxy alkyne contributed to a notable decrease of in the diastereoselectivity of this reaction. A similar trend was observed during olefinations of aliphatic, unsaturated or aromatic aldehydes using 2-phenyl-1-siloxyacetylene (Table 2, entries 2–4). The products were produced efficiently albeit with only moderate diastereoselectivity.
Table 2.
Ag-Catalyzed olefination using cyclohexyl and phenyl substituted siloxy alkynes
| |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield, % | E:Z Ratio |
| 1 |
|
|
17 | 72 | 54:46 |
| 2 |
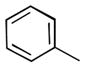
|
|
18 | 76 | 83:17 |
| 3 |
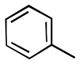
|
|
19 | 74 | 50:50 |
| 4 |
|
|
20 | 75 | 86:14 |
The mild reaction conditions, which were employed during olefination of a range of aldehydes, suggested to us that this catalytic process could be suitable for chemoselective olefination of aldehydes in the presence of other carbonyl groups, such as esters or even ketones. To test this hypothesis, we prepared aldehydes 21 and 22 bearing such functionalities (Scheme 2). Subjection of both compounds to 1-siloxy-1-hexyne under our standard conditions resulted in efficient and diastereoselective formation of the corresponding silyl esters 23 and 24 arising from selective functionalization of the aldehyde moiety. The ability to effect chemoselective olefination of aldehydes in the presence of a ketone is particularly attractive in the context of potential applications of this process in the area of complex molecule synthesis.
Scheme 2.
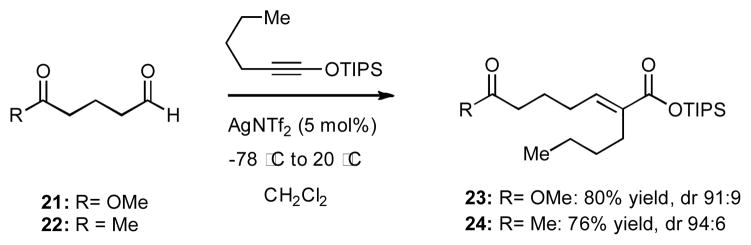
Chemoselective Olefinations
The working mechanistic hypothesis is outlined in Scheme 3. Our previous studies indicated that AgNTf2 undergoes rapid and reversible coordination to electron rich alkynes. Even though aldehyde activation can not be ruled out at this point, we propose that the olefination begins with complexation of AgNTf2 with siloxy alkyne 25 to give alkyne-silver complex, which can be represented as two resonance structures 26 and 27.7a Subsequent addition to an aldehyde 28 affords an intermediate 29, which is next converted to siloxy oxetene 30.10 While the intermediacy of 29 has not been proven experimentally thus far, our previous work on [2+2] cycloadditions of siloxy alkynes,7a as well as relevant theoretical studies11 suggest that formation of the oxetene 30 is most likely a step-wise process. Once formed, siloxy oxetene 30 is expected to undergo rapid conrotatory electocyclic ring-opening to give preferentially E-enoate 31 due to predominant torquoselective outward rotation of the R2 group.12 This mechanism provides an explanation for high diastereoselectivity of the olefination in the case of a small R1 substituent, which originates from siloxy alkyne. Furthermore, the increased size of the R1 substituent is expected to decrease the energetic preference for the torquoselective outward rotation of R2 group due to increased steric repulsion between R1 and R2. The result is lowering of E-selectivity of the reaction when bulky siloxy alkynes are employed.
Scheme 3.
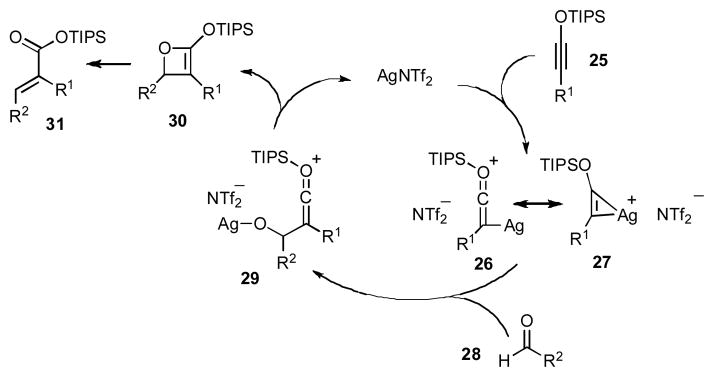
Mechanistic Hypothesis
In closing, we described the development of a mild, catalytic olefination of aldehydes using siloxy alkynes. Following our previous studies, we found that AgNTf2 proved to be uniquely effective at promoting this transformation with high efficiency and excellent chemoselectivity. Furthermore, high levels of diastereoselection were observed for a range of aldehydes using either 1-siloxy-1-propyne or 1-siloxy-1-hexyne. This process represents an important advance in the area of catalytic Arens olefination and constitutes an alternative to the widely practiced Horner-Wadsworth-Emmons reaction.
Experimental Section
General Olefination Procedure. A solution of siloxy alkyne (0.20 mmol) and aldehyde (0.22 mmol) in CH2Cl2 (4 mL) was cooled to −78 °C under inert nitrogen atmosphere and treated with AgNTf2 (3.9 mg, 0.01 mmol). The reaction mixture was stirred at the same temperature for 30 min, allowed to warm to room temperature and stirred for another 30 min. The solvent was removed under reduced pressure. Crude product was purified directly by silica gel column chromatography. See Supporting Information for additional experimental protocols and analytical data.
Supplementary Material
Acknowledgments
Financial support of this work was provided by NSF CAREER (CHE-0447751) and NIH 1P50GM086145.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/adsc.200######.
References
- 1.Tanaka T, editor. Modern Carbonyl Olefination: Methods and Applications. Wiley-VCH; Weinheim: 2004. [Google Scholar]
- 2.a) Veiregge H, Bos HJT, Arens JF. Rec Trav Chim. 1959;78:664–666. [Google Scholar]; b) Veiregge H, Schmidt HM, Renema J, Bos HJT, Arens JF. Rec Trav Chim. 1966;85:929–951. [Google Scholar]
- 3.a) Pannanen SI. Tetrahedron Lett. 1980;21:657–658. [Google Scholar]; b) Pornet J, Rayadh A, Miginiac L. Tetrahderon Lett. 1986;27:5479–5482. [Google Scholar]; c) Kowalski CJ, Sakdarat S. J Org Chem. 1990;55:1977–1979. [Google Scholar]; d) Sisko J, Balog A, Curran DP. J Org Chem. 1992;57:4341–4342. [Google Scholar]; e) Zakarya D, Rayadh A, Samih M, Lakhlihi T. Tetrahedron Lett. 1994;35:2345–2348. [Google Scholar]; f) Hayashi A, Yamaguchi M, Hirama M. Synlett. 1995:195–196. [Google Scholar]; g) Oblin M, Parrain J-L, Rajzmann M, Pons J-M. Chem Commun. 1998:1619–1620. [Google Scholar]; h) Viswanathan G, Li CJ. Tetrahedron Lett. 2002;43:1613–1615. [Google Scholar]; i) Kurtz KCM, Hsung RP, Zhang Y. Org Lett. 2006;8:231–234. doi: 10.1021/ol052487s. [DOI] [PubMed] [Google Scholar]; j) You L, Figueroa ZFA, Ghosh SK, Li G, Lu T, Hsung RP. Synlett. 2007:1656–1662. [Google Scholar]
- 4.a) Curini M, Epifano F, Maltese F, Rosati O. Synlett. 2003:552–554. [Google Scholar]; b) Rhee JU, Krische MJ. Org Lett. 2005;7:2493–2495. doi: 10.1021/ol050838x. [DOI] [PubMed] [Google Scholar]; c) Jin T, Yamamoto Y. Org Lett. 2007;9:5259–5262. doi: 10.1021/ol702455v. [DOI] [PubMed] [Google Scholar]; d) Saito A, Umakoshi M, Yagyu N, Hanzawa Y. Org Lett. 2008;10:1783–1785. doi: 10.1021/ol800539a. [DOI] [PubMed] [Google Scholar]
- 5.a) Büchi G, Kofron JT, Koller E, Rosenthal D. J Am Chem Soc. 1956;78:876–877. [Google Scholar]; b) Friedrich LE, Bower JD. J Am Chem Soc. 1973;95:6869–6870. [Google Scholar]
- 6.a) Kowalski CJ, Fields KW. J Am Chem Soc. 1982;104:321–323. [Google Scholar]; b) Shindo M, Sato Y, Shishido K. Tetrahedron Lett. 1998;39:4857–4860. [Google Scholar]; c) Shindo M, Sato Y, Shishido K. J Org Chem. 2000;65:5443–5445. doi: 10.1021/jo000650l. [DOI] [PubMed] [Google Scholar]; d) Shindo M, Matsamoto K, Mori S, Shishindo K. J Am Chem Soc. 2002;124:6840–6841. doi: 10.1021/ja026275r. [DOI] [PubMed] [Google Scholar]; e) Shindo M, Sato Y, Yoshikawa T, Koretsune R, Shishindo K. J Org Chem. 2004;69:3912–3916. doi: 10.1021/jo0497813. [DOI] [PubMed] [Google Scholar]; f) Shindo M, Yoshikawa T, Itou Y, Mori S, Nishii T, Shishindo K. Chem Eur J. 2006;12:524–536. doi: 10.1002/chem.200500574. [DOI] [PubMed] [Google Scholar]; g) Shindo M, Kita T, Kumagai T, Matsumoto K, Shishindo K. J Am Chem Soc. 2006;128:1062–1063. doi: 10.1021/ja0561082. [DOI] [PubMed] [Google Scholar]; h) Yoshikawa T, Mori S, Shindo M. J Am Chem Soc. 2009;131:2092–2093. doi: 10.1021/ja809592q. [DOI] [PubMed] [Google Scholar]
- 7.a) Sweis R, Schramm MP, Kozmin SA. J Am Chem Soc. 2004;126:7442–7443. doi: 10.1021/ja048251l. [DOI] [PubMed] [Google Scholar]; b) Sun J, Kozmin SA. Angew Chem Int Ed. 2006;45:4991–4993. doi: 10.1002/anie.200601276. [DOI] [PubMed] [Google Scholar]
- 8.a) Schramm MP, Reddy DS, Kozmin SA. Angew Chem, Int Ed. 2001;40:4274–4277. doi: 10.1002/1521-3773(20011119)40:22<4274::AID-ANIE4274>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; b) Reddy DS, Kozmin SA. J Org Chem. 2004;69:4860–4862. doi: 10.1021/jo049431g. [DOI] [PubMed] [Google Scholar]; c) Zhang L, Kozmin SA. J Am Chem Soc. 2004;126:10204–10205. doi: 10.1021/ja046586x. [DOI] [PubMed] [Google Scholar]; d) Zhang L, Kozmin SA. J Am Chem Soc. 2004;126:11806–11807. doi: 10.1021/ja046112y. [DOI] [PubMed] [Google Scholar]; e) Sun J, Kozmin SA. J Am Chem Soc. 2005;127:13512–13513. doi: 10.1021/ja055054t. [DOI] [PubMed] [Google Scholar]; f) Sun J, Conley M, Zhang L, Kozmin SA. J Am Chem Soc. 2006;128:9705–9710. doi: 10.1021/ja063384n. [DOI] [PubMed] [Google Scholar]
- 9.Shindo M, Soichiro O, Sato Y, Shishindo K. Heterocycles. 2000;52:545–548. [Google Scholar]
- 10.Middleton WJ. J Org Chem. 1965;30:1307. [Google Scholar]
- 11.Oblin M, Rajzmann M, Pons J-M. Tetrahedron. 2001;57:3099–3104. [Google Scholar]
- 12.Dolbier WR, Koroniak H, Houk KN, Sheu C. Acc Chem Res. 1996;29:471–477. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.