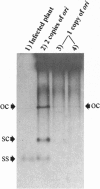
Abstract
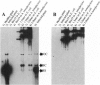
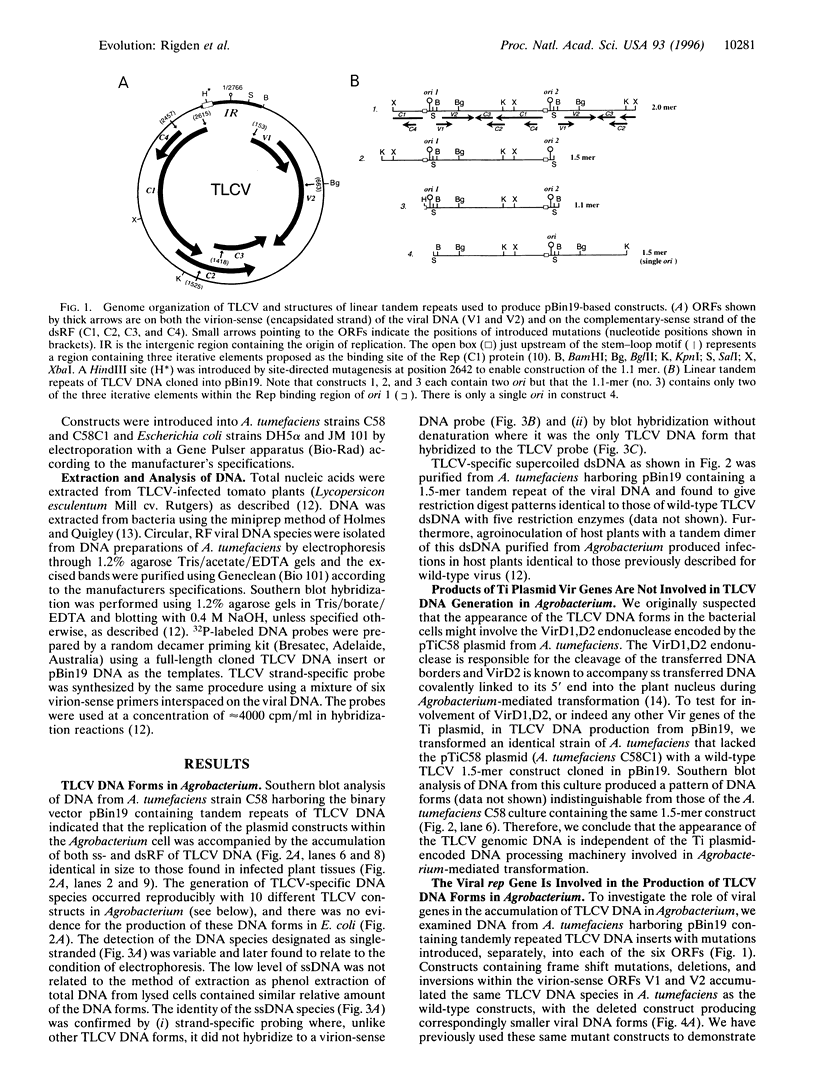
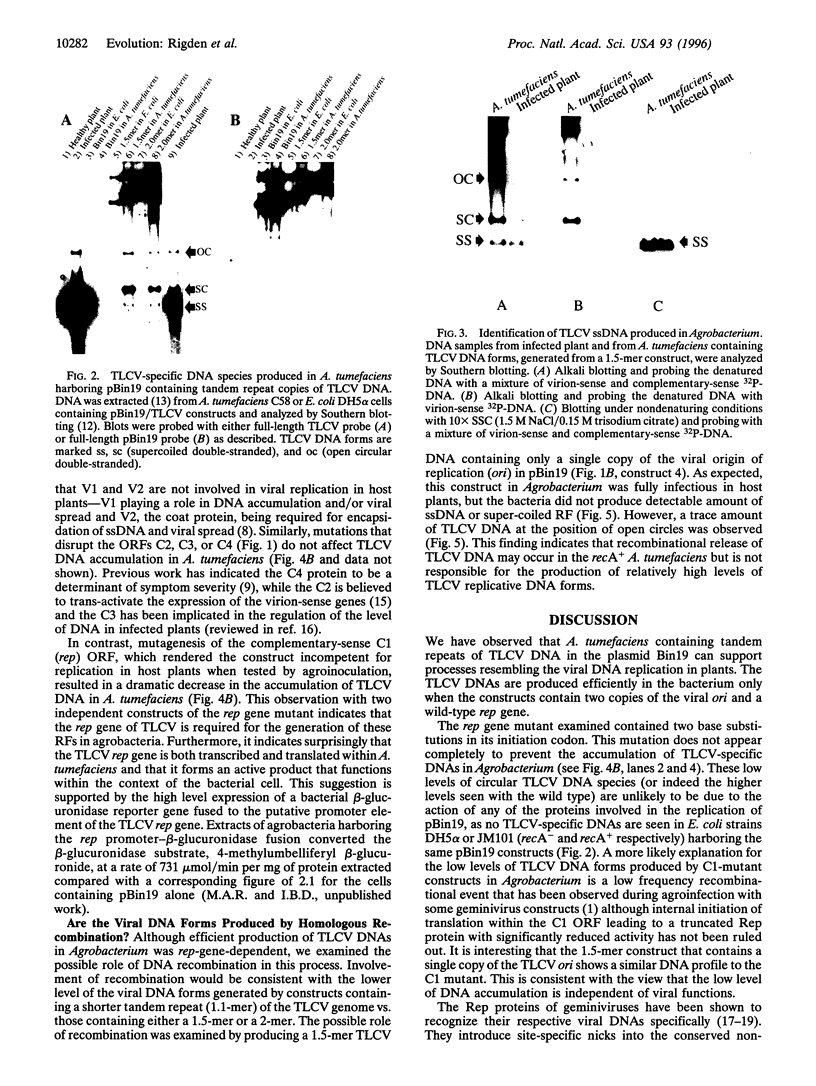
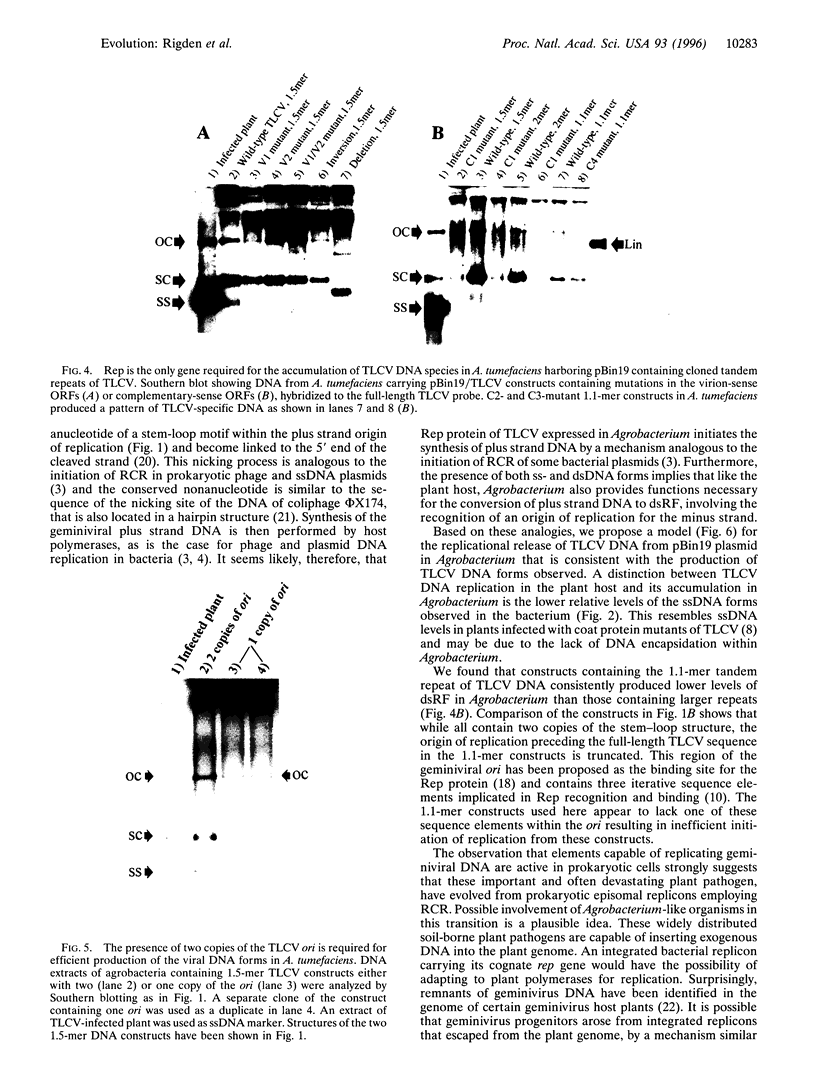
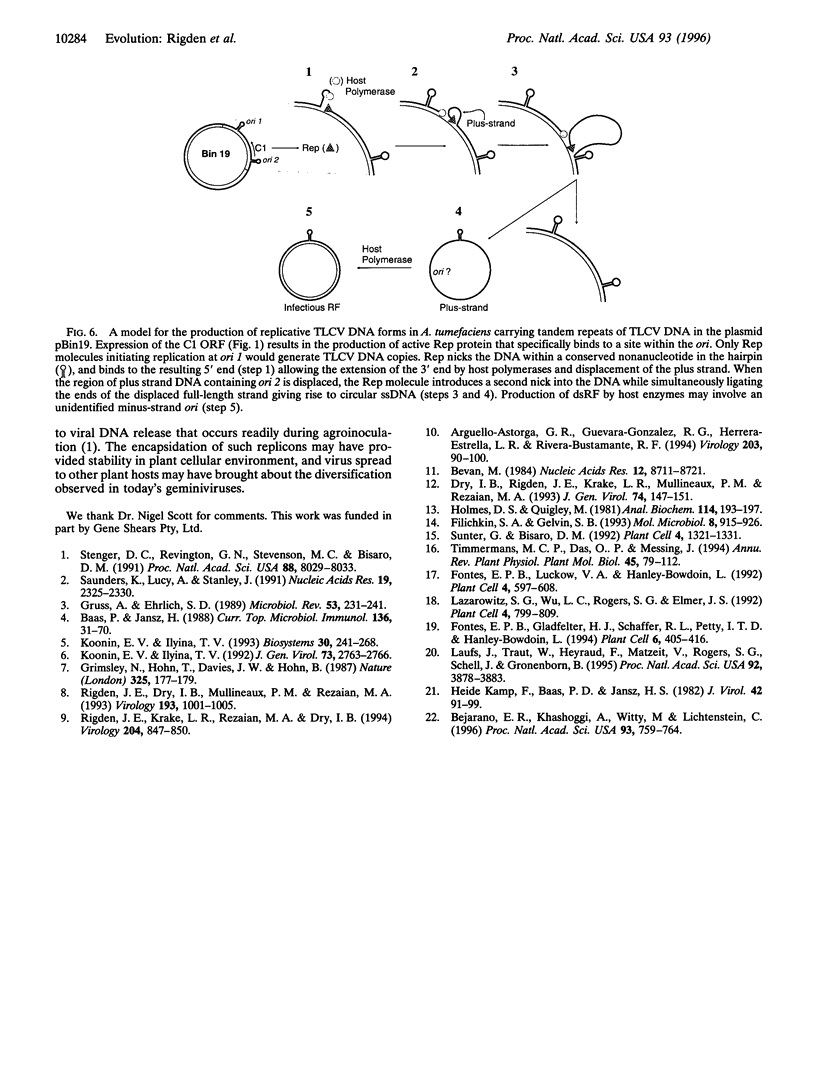
Agrobacterium tumefaciens, a bacterial plant pathogen, when transformed with plasmid constructs containing greater than unit length DNA of tomato leaf curl geminivirus accumulates viral replicative form DNAs indistinguishable from those produced in infected plants. The accumulation of the viral DNA species depends on the presence of two origins of replication in the DNA constructs and is drastically reduced by introducing mutations into the viral replication-associated protein (Rep or C1) ORF, indicating that an active viral replication process is occurring in the bacterial cell. The accumulation of these viral DNA species is not affected by mutations or deletions in the other viral open reading frames. The observation that geminivirus DNA replication functions are supported by the bacterial cellular machinery provides evidence for the theory that these circular single-stranded DNA viruses have evolved from prokaryotic episomal replicons.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argüello-Astorga G. R., Guevara-González R. G., Herrera-Estrella L. R., Rivera-Bustamante R. F. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication. Virology. 1994 Aug 15;203(1):90–100. doi: 10.1006/viro.1994.1458. [DOI] [PubMed] [Google Scholar]
- Baas P. D., Jansz H. S. Single-stranded DNA phage origins. Curr Top Microbiol Immunol. 1988;136:31–70. doi: 10.1007/978-3-642-73115-0_3. [DOI] [PubMed] [Google Scholar]
- Bejarano E. R., Khashoggi A., Witty M., Lichtenstein C. Integration of multiple repeats of geminiviral DNA into the nuclear genome of tobacco during evolution. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):759–764. doi: 10.1073/pnas.93.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry I. B., Rigden J. E., Krake L. R., Mullineaux P. M., Rezaian M. A. Nucleotide sequence and genome organization of tomato leaf curl geminivirus. J Gen Virol. 1993 Jan;74(Pt 1):147–151. doi: 10.1099/0022-1317-74-1-147. [DOI] [PubMed] [Google Scholar]
- Filichkin S. A., Gelvin S. B. Formation of a putative relaxation intermediate during T-DNA processing directed by the Agrobacterium tumefaciens VirD1,D2 endonuclease. Mol Microbiol. 1993 May;8(5):915–926. doi: 10.1111/j.1365-2958.1993.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Fontes E. P., Gladfelter H. J., Schaffer R. L., Petty I. T., Hanley-Bowdoin L. Geminivirus replication origins have a modular organization. Plant Cell. 1994 Mar;6(3):405–416. doi: 10.1105/tpc.6.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes E. P., Luckow V. A., Hanley-Bowdoin L. A geminivirus replication protein is a sequence-specific DNA binding protein. Plant Cell. 1992 May;4(5):597–608. doi: 10.1105/tpc.4.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidekamp F., Baas P. D., Jansz H. S. Nucleotide sequences at the phi X gene A protein cleavage site in replicative form I DNAs of bacteriophages U3, G14, and alpha 3. J Virol. 1982 Apr;42(1):91–99. doi: 10.1128/jvi.42.1.91-99.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Ilyina T. V. Computer-assisted dissection of rolling circle DNA replication. Biosystems. 1993;30(1-3):241–268. doi: 10.1016/0303-2647(93)90074-m. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Ilyina T. V. Geminivirus replication proteins are related to prokaryotic plasmid rolling circle DNA replication initiator proteins. J Gen Virol. 1992 Oct;73(Pt 10):2763–2766. doi: 10.1099/0022-1317-73-10-2763. [DOI] [PubMed] [Google Scholar]
- Laufs J., Traut W., Heyraud F., Matzeit V., Rogers S. G., Schell J., Gronenborn B. In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3879–3883. doi: 10.1073/pnas.92.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Wu L. C., Rogers S. G., Elmer J. S. Sequence-specific interaction with the viral AL1 protein identifies a geminivirus DNA replication origin. Plant Cell. 1992 Jul;4(7):799–809. doi: 10.1105/tpc.4.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden J. E., Dry I. B., Mullineaux P. M., Rezaian M. A. Mutagenesis of the virion-sense open reading frames of tomato leaf curl geminivirus. Virology. 1993 Apr;193(2):1001–1005. doi: 10.1006/viro.1993.1215. [DOI] [PubMed] [Google Scholar]
- Rigden J. E., Krake L. R., Rezaian M. A., Dry I. B. ORF C4 of tomato leaf curl geminivirus is a determinant of symptom severity. Virology. 1994 Nov 1;204(2):847–850. doi: 10.1006/viro.1994.1606. [DOI] [PubMed] [Google Scholar]
- Saunders K., Lucy A., Stanley J. DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 1991 May 11;19(9):2325–2330. doi: 10.1093/nar/19.9.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger D. C., Revington G. N., Stevenson M. C., Bisaro D. M. Replicational release of geminivirus genomes from tandemly repeated copies: evidence for rolling-circle replication of a plant viral DNA. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8029–8033. doi: 10.1073/pnas.88.18.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter G., Bisaro D. M. Transactivation of geminivirus AR1 and BR1 gene expression by the viral AL2 gene product occurs at the level of transcription. Plant Cell. 1992 Oct;4(10):1321–1331. doi: 10.1105/tpc.4.10.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]