Abstract
Importance
Excess urinary albumin excretion is more common in black individuals than in white individuals and is more strongly associated with incident stroke risk in blacks than whites. Whether similar associations extend to coronary heart disease (CHD) is unclear.
Objective
To determine whether the association of urinary albumin excretion with CHD events differs by race.
Design, Setting and Participants
Within the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a prospective cohort of black and white US adults ≥45 years of age enrolled between 2003 and 2007 with follow-up through December 31 2009, we examined race-stratified associations of urinary albumin to creatinine ratio (ACR) with (1) incident CHD among 23,273 participants free of CHD at baseline, and (2) first recurrent CHD event among 4,934 participants with CHD at baseline.
Main Outcome Measure
Expert-adjudicated incident and recurrent myocardial infarction (MI) and acute CHD death.
Results
A total of 616 incident CHD events (421 non-fatal MIs and 195 CHD deaths) and 468 recurrent CHD events (279 non-fatal MIs and 189 CHD deaths) were observed over a mean 4.4 years of follow-up. Among those free of CHD at baseline, age- and sex-adjusted incidence rates of CHD per 1000 person-years of follow-up increased with increasing categories of ACR in blacks and whites, with rates being nearly 1.5-fold higher in the highest category of ACR (>300 mg/g) in blacks vs. whites (20.59, 95% confidence interval [14.36,29.51] in blacks vs. 13.60 [7.60,24.25] in whites). In proportional hazards models adjusted for traditional cardiovascular risk factors and medications, higher baseline urinary ACR was associated with higher risk of incident CHD among blacks (hazard ratio [HR] comparing ACR >300 vs. <10 mg/g, 3.21 [2.02,5.09]) but not whites (HR comparing ACR >300 vs. <10 mg/g, 1.49 [0.80,2.76]) (P-interaction=0.03). Among those with CHD at baseline, fully-adjusted associations of baseline urinary ACR with first recurrent CHD event were similar in blacks and whites (HR comparing ACR >300 vs. <10 mg/g, 2.21 [1.22,4.00] in blacks vs. 2.48 [1.61,3.78] in whites) (P-interaction=0.53).
Conclusions
Higher urinary ACR was associated with higher risk of incident but not recurrent CHD in blacks compared to whites.
INTRODUCTION
Increased urinary albumin excretion (generally defined as an albumin to creatinine ratio [ACR] ≥30 mg/g) is an important marker of kidney injury and a strong risk factor for cardiovascular disease.1 Black individuals have higher levels of urinary albumin excretion than white individuals,2–6 which may contribute to racial disparities in cardiovascular outcomes. In support of this, in participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study free of stroke at baseline, we previously showed that the association of urinary ACR with incident stroke differed by race, such that higher urinary ACR was independently associated with a higher risk of incident stroke in blacks but not in whites.6 While these data suggest that higher urinary albumin excretion may be a stronger risk factor for cardiovascular disease events in blacks than in whites, little is known about racial differences in the association of urinary ACR with cardiovascular outcomes apart from stroke events. In particular, no study to our knowledge has specifically examined whether the association of urinary ACR and coronary heart disease (CHD) differs by race. Accordingly, we examined the associations of ACR with both incident and recurrent CHD events in black and white participants of REGARDS.
METHODS
Study Participants
The REGARDS study is a population-based investigation of stroke incidence in black and white US adults ≥45 years of age. Details of the study design have been reviewed elsewhere.7 Briefly, the study was designed to provide approximately equal representation of men and women, and oversampled blacks and persons living in the “stroke belt” of the US. Trained interviewers conducted computer-assisted telephone interviews to obtain information including participants’ sociodemographics, cardiovascular risk factors, cigarette smoking, physical activity, and use of medications. Following this call, certified health professionals conducted an in-home study visit that included an electrocardiograph (ECG) recording and inventory of medications. In addition, fasting blood and urine samples were collected during the in-home visit and shipped to the University of Vermont Central Laboratory using standardized procedures. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided informed consent.
Primary Exposure of Interest
The exposure of interest was baseline urinary ACR. Urine albumin was measured by nephelometry using the BNII ProSpec nephelometer (Now Siemens AG), and urine creatinine was measured by the rate Jaffé method using the Modular-P chemistry analyzer (Roche/Hitachi, Basel, Switzerland).
Ascertainment of Outcomes
The outcomes of interest were incident and recurrent CHD events, defined as acute myocardial infarction (MI) or acute CHD death. Suspected CHD events were detected via telephone follow-up with participants every 6 months with corresponding medical records reviewed by two expert adjudicators blinded to ACR results to validate potential events using published guidelines,8, 9 as detailed previously.10 Briefly, for suspected MIs, records were examined for signs or symptoms of ischemia; a rising and/or falling pattern in cardiac troponin or creatine phosphokinase-MB concentration over six or more hours with a peak concentration greater than twice the upper limit of normal; and ECG changes consistent with ischemia, guided by the Minnesota code and classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia.11 Definite MIs were defined as those with diagnostic enzymes or ECG, and probable MIs were defined as those with equivocal diagnostic enzymes with a positive but not diagnostic ECG; or, if enzymes were missing, with a positive ECG in the presence of ischemic signs or symptoms. Only definite or probable MIs were included as CHD events in this study; since racial contrasts were a major interest of this study, coronary revascularizations were not included because of the known relatively low utilization of these procedures among blacks.12 For fatal events, the medical history, hospital records, interviews with next of kin or proxies, and death certificates or National Death Index data were reviewed to adjudicate the cause of death, with definite or probable CHD death used in this analysis (see Supplemental Table 1 for expanded definition). In pre-specified analyses, we tested for and did not detect a statistically significant difference in the association of ACR with CHD type (fatal vs. non-fatal) by race; therefore, we examined the composite of fatal or non-fatal CHD events as the main outcome.
Covariates of Interest
Age, sex, smoking history, annual family income, and educational attainment were determined by self-report. Systolic blood pressure was defined as the average of two seated measures taken after a 3 minute rest. History of CHD was defined as having any of the following: evidence of MI on the baseline ECG, self-report of a prior history of a cardiac procedure (coronary artery bypass surgery or percutaneous angioplasty), or self-reported history of MI. Diabetes was defined as self-reported use of insulin or oral hypoglycemic agents, fasting serum glucose concentration of 126 mg/dL or higher, or a non-fasting serum glucose concentration of 200 mg/dL or higher. Using height and weight measured during the in-home study visit, body mass index (BMI) was calculated. Waist circumference (in centimeters) was measured during the in-home visit using a tape measure midway between the lowest rib and the iliac crest with the participant standing. Use of lipid-lowering and anti-hypertensive medications was based on self-report and pill bottle review during the home visit. Serum concentrations of total cholesterol, high-density lipoprotein (HDL), triglycerides and high-sensitivity c-reactive protein (hsCRP) were measured using established assays, and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease (CKD) Epidemiology Collaboration formula.13
Statistical Analyses
The end of follow-up for this analysis was December 31st, 2009. Follow-up time for each participant was calculated from the date of the in-home visit to the date of first incident or first recurrent CHD event after study enrollment, death, or last telephone follow-up. Age- and sex-adjusted incidence rates for CHD were calculated by strata of ACR in participants with and without CHD at baseline, stratified by race. After confirming the proportionality of hazards, Cox proportional hazards models were used to estimate the hazard ratio of incident or recurrent CHD, separately, as a function of ACR in sequential models, stratified by race. Model 1 adjusted for age, sex and geographic region of residence (stroke belt, stroke buckle or other). Model 2 adjusted for variables in model 1 plus annual family income (< vs. ≥$20,000 per year), educational achievement (< or ≥ a high school diploma), health insurance (yes/no), waist circumference (continuous), systolic blood pressure (continuous), total and HDL-cholesterol (continuous), triglycerides (continuous), eGFR (continuous), natural log-transformed hsCRP (continuous), diabetes (yes/no), smoking status (current vs. non-current tobacco use), physical activity (none vs. any), use of statins (yes/no), use of aspirin (yes/no), use of any antihypertensive medications (yes/no) and use of angiotensin II receptor blockers or angiotensin converting enzyme inhibitors (yes/no). In all models, urinary ACR was analyzed as a categorical variable (<10 mg/g, 10–29.99, 30–300, and >300),14 with the lowest category (<10 mg/g) serving as the referent group, or as a continuous, natural log-transformed variable. All multivariable-adjusted Cox proportional hazards models were fitted with imputed data to account for missing covariate data, using multiple imputation by chained equations with five datasets. The decision to stratify all analyses by race was made a priori. We also formally examined for effect modification by race by testing the statistical significance of ACR category x race interaction terms in multivariable-adjusted Cox models using a post-estimation Wald test to obtain an omnibus P-value for interaction between ACR categories and race. In addition, we tested the statistical significance of ACR x race interaction terms modeling ACR as a continuous, natural log-transformed variable. In sensitivity analyses, death from non-CHD-related deaths was analyzed as a competing risk. Finally, point estimates and confidence intervals for the Harrell’s c-index and change in c-indices for the fully adjusted models with and without ACR were calculated from 1000 bootstrap replicates; each replicate included the same number of observations in the original data sets. To account for the additional variability due to multiple imputation, bootstrap replicates were drawn randomly with replacement from the pool of all five imputed datasets. A two-sided P-value of 0.05 was considered statistically significant for all analyses, which were conducted using SAS software version 9.2 (SAS Institute, Cary, NC), STATA version 12 (STATA incorporated, College Station, TX) and Rv3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Participants
Overall, 30,239 black and white adults were enrolled between January 2003 and October 2007. We excluded 1,400 individuals missing urinary ACR at baseline, 569 individuals missing follow-up data and 63 participants receiving hemodialysis at baseline, leaving a total of 28,207 participants in the final analyzed sample. Of these, 5,076 were missing data for demographic, clinical or lifestyle covariates, 5,306 for biochemical covariates, and 587 for medication use. Characteristics of the 23,273 study participants without CHD and the 4,934 participants with CHD at baseline, stratified by categories of urinary ACR and race, are depicted in Tables 1 and 2, respectively. In general, participants who had higher urinary ACR values at baseline were more likely to be older, male, have lower education and annual family income, have higher systolic blood pressure and waist circumference, be current smokers, have a history of diabetes, hypertension, and dyslipidemia, be currently taking statins and anti-hypertensive medications and have lower mean eGFR and higher median hsCRP concentrations, irrespective of race and CHD status at baseline.
Table 1.
Baseline characteristics of REGARDS participants, by categories of albumin to creatinine ratio and race in those without CHD at baseline.†
| Whites (n=13,526) | Blacks (n= 9,747) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ACR (mg/g) categories | < 10 n=9105 |
10 – 29.99 n=3015 |
30 – 300 n=1254 |
> 300 n=152 |
< 10 n=5869 |
10 – 29.99 n=2110 |
30 – 300 n=1350 |
> 300 n=368 |
| Age, M, (SD) | 63.1(9.0) | 66.7(9.4) | 67.9(9.8) | 67.2(10.0) | 62.6(8.9) | 65.0(9.4) | 65.4(9.8) | 65.1(9.0) |
| Female, N(%) | 4723(51.9) | 1889(62.7) | 649(51.8) | 53(34.9) | 3734(63.6) | 1440(66.7) | 819(60.7) | 206(56.0) |
| Education < HS, N(%) | 495(5.4) | 234(7.8) | 110(8.8) | 9(5.9) | 947(16.2) | 444(20.6) | 334(24.8) | 85(23.1) |
| Income < $20,000/yr, N(%) | 807(8.9) | 389(12.9) | 212(16.9) | 30(19.7) | 1352(23.0) | 596(27.6) | 411(30.4) | 121(32.9) |
| Health Insurance, N(%) | 8670(95.3) | 2879(95.6) | 1188(94.9) | 142(93.4) | 5270(89.9) | 1931(89.5) | 1203(89.2) | 326(88.6) |
| BMI (kg/m2), M, (SD) | 28.0(5.4) | 28.2(5.8) | 29.0(6.4) | 29.8(6.2) | 30.6(6.4) | 30.9(6.9) | 31.5(7.1) | 31.7(7.5) |
| Waist circumference (cm), M, (SD) | 93.1(14.6) | 93.6(16.0) | 97.6(17.6) | 101.4(16.3) | 96.5(14.7) | 98.4(15.8) | 100.7(16.7) | 102.9(16.7) |
| SBP (mmHg), M, (SD) | 122.7(14.3) | 127.5(16.7) | 131.4(17.5) | 137.0(19.2) | 127.3(15.4) | 132.8(17.3) | 136.0(18.7) | 143.2(20.8) |
| DBP (mmHg), M, (SD) | 75.0(8.8) | 76.1(9.6) | 76.8(10.1) | 79.3(10.6) | 77.5(9.2) | 79.6(10.4) | 80.4(11.1) | 81.6(11.3) |
| Lifestyle Habits | ||||||||
| Current smoker, N(%) | 1035(11.4) | 388(12.9) | 187(15.0) | 27(17.9) | 924(15.8) | 342(15.9) | 270(20.1) | 87(23.7) |
| Exercise (none), N(%) | 2541(28.3) | 1017(34.3) | 502(40.8) | 64(43.0) | 1957(33.8) | 782(36.8) | 535(40.1) | 168(46.7) |
| Co-morbidities | ||||||||
| Diabetes, N(%) | 767(8.6) | 494(16.8) | 328(27.0) | 78(52.4) | 1168(20.7) | 676(32.6) | 571(43.6) | 231(64.9) |
| Hypertension, N(%) | 3732(41.1) | 1629(54.2) | 821(65.7) | 121(80.1) | 3709(63.3) | 1613(74.9) | 1078(80.0) | 327(88.9) |
| Dyslipidemia, N(%) | 4946(55.7) | 1721(58.8) | 785(64.2) | 115(77.2) | 2854(50.7) | 1052(50.9) | 741(57.4) | 236(67.2) |
| Medication use | ||||||||
| HMG CoA reductase Inhibitors, N(%) | 2464(27.4) | 916(30.7) | 401(32.5) | 66(43.7) | 1500(25.8) | 575(26.8) | 422(31.5) | 144(39.3) |
| Anti-hypertensives N, (%) | 3147(34.9) | 1345(45.1) | 652(52.5) | 106(70.2) | 3271(56.4) | 1386(65.1) | 944(70.6) | 306(83.4) |
| ARB/ACEi, N(%) | 2197(24.1) | 928(30.8) | 497(39.6) | 85(55.9) | 1990(33.9) | 920(42.6) | 650(48.2) | 223(60.6) |
| Aspirin, N(%) | 3617(39.8) | 1238(41.1) | 534(42.6) | 71(46.7) | 1882(32.1) | 776(36.0) | 496(36.8) | 165(45.0) |
| eGFR (ml/min/1.73m2), median, [IQR] | 88.1 [76.5–96.2] | 86.4 [73.2–95.5] | 83.0 [64.8–94.1] | 66.0 [53.3–87.2] | 94.2 [79.2–108.2] | 94.6 [77.4–108.5] | 88.7 [68.4–106.8] | 64.1 [40.0–90.6] |
| C-reactive protein (mg/L), median, [IQR] | 1.7 [0.8–3.8] | 2.0 [0.9–4.4] | 2.6 [1.1–5.4] | 2.8 [1.1–6.5] | 2.6 [1.1–5.8] | 3.1 [1.2–6.6] | 3.6 [1.4–7.7] | 3.7 [1.6–8.0] |
Abbreviations: CHD – coronary heart disease, HS—high school, BMI—body mass index, SBP – systolic blood pressure, DBP—diastolic blood pressure, HMG CoA—hydroxymethyl glutamate Coenzyme A, eGFR—estimated glomerular filtration rate, ACR—urinary albumin to creatinine ratio, ARB/ACEi – angiotensin II receptor blockers or angiotensin converting-enzyme inhibitors
Baseline CHD is defined as history of self-reported myocardial infarction (MI), coronary artery bypass graft, angioplasty, stenting, or evidence of MI via electrocardiograph.
Table 2.
Baseline characteristics of REGARDS participants, by categories of albumin to creatinine ratio and race in those with CHD at baseline.†
| Whites (n= 3,236) | Blacks (n= 1,698) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ACR (mg/g) categories | < 10 n=1697 |
10 – 29.99 n=892 |
30 – 300 n=516 |
> 300 n=131 |
< 10 n=797 |
10 – 29.99 n=418 |
30 – 300 n=343 |
> 300 n=140 |
| Age, M, (SD) | 68.0(8.6) | 71.1(8.9) | 71.3(8.8) | 69.7(8.7) | 65.6(8.6) | 66.6(9.2) | 68.8(9.2) | 66.6(8.7) |
| Female, N(%) | 520(30.6) | 340(38.1) | 142(27.5) | 28(21.4) | 428(53.7) | 219(52.4) | 174(50.7) | 62(44.3) |
| Education < HS, N(%) | 162(9.6) | 108(12.1) | 80(15.5) | 21(16.2) | 186(23.4) | 113(27.0) | 109(31.8) | 37(26.4) |
| Income < $20,000/yr, N(%) | 222(13.1) | 169(19.0) | 105(20.4) | 30(22.9) | 235(29.5) | 136(32.5) | 119(34.7) | 55(39.3) |
| Health Insurance, N(%) | 1653(97.5) | 867(97.2) | 505(97.9) | 129(99.2) | 743(93.2) | 388(92.8) | 324(94.5) | 131(94.2) |
| BMI (kg/m2), M, (SD) | 28.5(5.2) | 28.4(5.3) | 29.2(6.0) | 29.6(6.6) | 30.5(6.4) | 31.3(7.6) | 31.1(6.2) | 30.8(6.5) |
| Waist circumference (cm), M, (SD) | 97.9(14.2) | 98.4(16.7) | 101.8(16.1) | 102.9(16.2) | 98.5(14.5) | 101.0(15.7) | 102.3(16.0) | 103.5(17.3) |
| SBP (mmHg), M, (SD) | 124.9(15.0) | 130.2(17.2) | 132.5(18.4) | 136.9(18.2) | 129.2(16.1) | 133.4(17.3) | 138.3(20.0) | 144.0(19.4) |
| DBP (mmHg), M, (SD) | 73.6(9.1) | 74.4(9.3) | 75.1(10.0) | 77.0(10.7) | 77.2(10.0) | 77.9(10.4) | 79.3 (11.4) | 80.8(12.7) |
| Lifestyle Habits | ||||||||
| Current smoker, N(%) | 189 (11.2) | 129(14.5) | 87(16.9) | 35(26.7) | 152(19.2) | 80(19.3) | 70(20.5) | 27(19.3) |
| Exercise (none), N(%) | 528(31.5) | 333(37.8) | 213(42.3) | 56(43.4) | 293(37.3) | 191(46.3) | 143(42.7) | 84(60.9) |
| Co-morbidities | ||||||||
| Diabetes, N(%) | 306(18.5) | 267(31.1) | 223(44.0) | 76(59.8) | 233(30.4) | 178(43.5) | 186(56.9) | 96(69.1) |
| Hypertension, N(%) | 1004(59.5) | 661(74.2) | 393(76.3) | 110(85.3) | 620(77.9) | 348(83.3) | 308(90.1) | 129(92.1) |
| Dyslipidemia, N(%) | 1346(80.6) | 687(78.9) | 411(81.4) | 111(87.4) | 539(69.6) | 301(73.8) | 225(70.3) | 105(77.2) |
| Medication use | ||||||||
| HMG CoA reductase inhibitors, N(%) | 1029(61.1) | 545(61.7) | 306(59.9) | 83(63.4) | 417(52.7) | 228(55.6) | 172(50.6) | 81(57.9) |
| Anti-hypertensives, N(%) | 920(55.2) | 594(67.0) | 360(70.7) | 102(78.5) | 578(73.5) | 331(80.3) | 279(82.8) | 122(87.8) |
| ARB/ACEi, N(%) | 805(47.4) | 478(53.6) | 288(55.8) | 76(58.0) | 433(54.3) | 230(55.0) | 205(60.4) | 98(70.0) |
| Aspirin, N(%) | 1264(74.5) | 651(73.0) | 366(70.9) | 97(74.6) | 497(62.4) | 271(64.8) | 218(63.6) | 87(62.1) |
| eGFR (ml/min/1.73m2), median, [IQR] | 82.2 [69.0–91.9] | 78.1 [62.4–89.8] | 69.8 [53.4–86.1] | 61.0 [36.4–81.6] | 87.2 [70.3–102.8] | 86.8 [69.8–104.6] | 77.8 [60.6–98.1] | 51.5 [32.7–76.9] |
| C-reactive protein (mg/L), median, [IQR] | 1.6 [0.8–3.8] | 1.9 [0.9–4.6] | 2.9 [1.3–6.8] | 3.6 [1.5–8.5] | 2.8 [1.1–6.3] | 3.3 [1.4–7.4] | 3.4 [1.3–8.8] | 3.9 [1.8–9.7] |
Abbreviations: CHD – coronary heart disease, SBP – systolic blood pressure, DBP—diastolic blood pressure, HMG CoA—hydroxymethyl glutamate Coenzyme A, eGFR—estimated glomerular filtration rate, ARB/ACEi – angiotensin II receptor blockers or angiotensin-converting-enzyme inhibitors, ACR—urinary albumin to creatinine ratio
Baseline CHD is defined as history of self-reported myocardial infarction (MI), coronary artery bypass graft, angioplasty, stenting, or evidence of MI via electrocardiograph.
Associations of ACR with Incident CHD
Over a median 4.5 (interquartile range 3.1, 5.5) years of follow-up, a total of 616 incident CHD events (260 among blacks and 356 among whites) were observed. Of these, 421 were non-fatal MIs (153 among blacks and 268 among whites) and 195 were CHD-related deaths (106 among blacks and 89 among whites) (Supplemental Table 2). Age- and sex-adjusted incidence rates [IRs] of CHD per 1,000 person-years of follow-up increased with increasing categories of urinary ACR in both blacks and whites (Table 3). The adjusted IRs in the two highest categories of ACR were ~1.5-fold higher in blacks as compared to whites (30–300 mg/g, IR 11.23, 95% confidence interval [CI] 8.64,14.58 in blacks vs. 8.04, 95% CI 6.03,10.73 in whites; > 300 mg/g, IR 20.59, 95% CI 14.36,29.51 in blacks vs. 13.60, 95% CI 7.64,24.26 in whites).
Table 3.
Coronary heart disease (CHD) events, incidence rates per 1000 person-years (95% confidence intervals) and incidence rate ratios (95% confidence interval) by level of urinary albumin to creatinine ratio in participants without and with CHD at baseline.†
| Without CHD at baseline
| ||||
|---|---|---|---|---|
| ACR, mg/g | < 10 | 10 – 29.99 | 30–300 | > 300 |
|
Age- and sex-adjusted incidence rates (95% CI)
| ||||
| CHD events (white/black) | 193/86 | 96/73 | 55/68 | 12/33 |
|
| ||||
| Whites (n=13,526) | 4.41 (3.72–5.24) | 6.53 (5.21–8.19) | 8.04 (6.03–10.73) | 13.60 (7.64–24.26) |
| Blacks (n=9,747) | 3.39 (2.68– 4.30) | 7.48 (5.78–9.67) | 11.23 (8.64–14.58) | 20.59 (14.36–29.51) |
|
| ||||
|
Age- and sex-adjusted incidence rate ratios (95% CI)
| ||||
| Whites (n=13,526) | 1 (ref) | 1.48(1.15–1.90) | 1.82(1.35–2.47) | 3.08(1.72–5.53) |
| Blacks (n=9,747) | 1 (ref) | 2.20(1.61–3.02) | 3.31(2.40–4.56) | 6.07 (4.06–9.07) |
| With CHD at baseline
| ||||
|---|---|---|---|---|
| ACR, mg/g | < 10 | 10 – 29.99 | 30–300 | > 300 |
|
Age- and sex-adjusted incidence rates (95% CI)
| ||||
| CHD events (white/black) | 119/49 | 96/28 | 81/39 | 35/21 |
| Whites (n=3,236) | 15.86 (12.64–19.89) | 24.77 (19.38–31.65) | 36.65 (28.12–47.75) | 70.06 (48.66–100.7) |
| Blacks (n=1,698) | 14.92 (10.89–20.43) | 15.88 (11.69–23.58) | 27.74 (19.48–39.53) | 40.73 (25.86–64.17) |
|
| ||||
|
Age- and sex-adjusted incidence rate ratios (95% CI)
| ||||
| Whites (n=3,236) | 1 (ref) | 1.56(1.19–2.05) | 2.31(1.74–3.07) | 4.42(3.03–6.44) |
| Blacks (n=1,698) | 1 (ref) | 1.06(0.67–1.70) | 1.86(1.22–2.84) | 2.73(1.64–4.56) |
Abbreviations: ACR—urinary albumin to creatinine ratio, CHD—coronary heart disease
Baseline CHD is defined as history of self-reported myocardial infarction (MI), coronary artery bypass graft, angioplasty, stenting, or evidence of MI via electrocardiograph.
The adjusted hazard ratios [HR] of incident CHD according to categories of urinary ACR are depicted in Table 4, stratified by race. Among both black and white participants, higher urinary ACR was associated with higher risk of incident CHD in analyses adjusted for age, sex, and geographic region of residence. After further adjustment for traditional cardiovascular risk factors and medication use, higher categories of ACR remained associated with higher risk of incident CHD among blacks such that in the fully-adjusted model, black participants with an ACR >300 mg/g had a 3-fold higher risk of incident CHD as compared to black participants with an ACR <10 mg/g (HR 3.21, 95% CI 2.02,5.09). In contrast, in the fully adjusted model among whites, the association of higher ACR with incident CHD was attenuated and no longer statistically significant (HR comparing ACR >300 vs. <10 mg/g, 1.49[0.80, 2.76]). Similarly, when modeling ACR as a continuous variable in fully-adjusted models, higher ACR was significantly associated with higher risk of incident CHD in blacks (HR per doubling of ACR 1.18[1.12, 1.24]) but not whites (HR per doubling of ACR 1.06[1.00, 1.13]) (P-interaction=0.03). In fully-adjusted models, addition of ACR increased the c-index of the model from 0.723 (95% CI 0.698,0.749) to 0.746 (95% CI 0.723,0.770) among blacks (mean difference 0.023, 95% CI 0.011,0.036), whereas there was no change among whites (0.745[0.726,0.765] without vs. 0.745[0.725, 0.765] with ACR; mean difference −0.000 [−0.002,0.002]). In sensitivity analyses using death as a competing risk, the results did not meaningfully change (Supplemental Table 3).
Table 4.
Hazard ratios for CHD events by level of urinary albumin to creatinine ratio among participants without and with CHD at baseline stratified by race.†
| Without CHD at baseline
| |||||
|---|---|---|---|---|---|
| ACR, mg/g | < 10 | 10 – 29.99 | 30–300 | > 300 | P for interaction* |
|
Age, sex, region adjusted hazard ratio (95% CI)
|
|||||
| Whites (n=13,526) | 1 (ref) | 1.45(1.13–1.86) | 1.76(1.30–2.39) | 2.98(1.66–5.35) | 0.03 |
| Blacks (n= 9,747) | 1 (ref) | 2.17(1.58–2.97) | 3.27(2.37–4.50) | 6.14(4.11–9.18) | |
|
|
|||||
|
Multivariable-adjusted hazard ratio††
(95% CI)
|
|||||
| Whites (n=13,526) | 1 (ref) | 1.23(0.96–1.59) | 1.19(0.86–1.64) | 1.49(0.80–2.76) | 0.03 |
| Blacks (n= 9,747) | 1 (ref) | 1.84(1.34–2.53) | 2.40(1.72–3.36) | 3.21(2.02–5.09) | |
| With CHD at baseline
| |||||
|---|---|---|---|---|---|
| ACR, mg/g | < 10 | 10 – 29.99 | 30–300 | > 300 | P for interaction* |
|
Age, sex, region adjusted hazard ratio (95% CI)
|
|||||
| Whites (n=3,236) | 1 (ref) | 1.53(1.17–2.01) | 2.32(1.74–3.08) | 4.50(3.08–6.57) | 0.39 |
| Blacks (n=1,698) | 1 (ref) | 1.07(0.67–1.70) | 1.84(1.21–2.82) | 2.74(1.64–4.58) | |
|
|
|||||
|
Multivariable-adjusted hazard ratio††
(95% CI)
|
|||||
| Whites (n=3,236) | 1 (ref) | 1.27(0.96–1.67) | 1.54(1.13–1.67) | 2.45(1.59–3.77) | 0.53 |
| Blacks (n= 1,698) | 1 (ref) | 1.05(0.66–1.69) | 1.73(1.11–2.69) | 2.16(1.18–3.95)* | |
Baseline CHD is defined as history of self-reported myocardial infarction (MI), coronary artery bypass graft, angioplasty, stenting, or evidence of MI via electrocardiograph.
Multivariable adjustment includes age, sex, geographic region of residence, income, education, health insurance coverage, waist circumference, systolic blood pressure, total and HDL-cholesterol, triglycerides, estimated glomerular filtration rate, C-reactive protein, diabetes, smoking status, physical activity, use of HMG-CoA reductase inhibitors, use of any antihypertensive medications, use of angiotensin II receptor blockers or angiotensin converting enzyme inhibitors and regular aspirin use.
P-values for interaction were obtained using a post-estimation Wald test to generate an omnibus P-value for the interaction term between ACR categories and race.
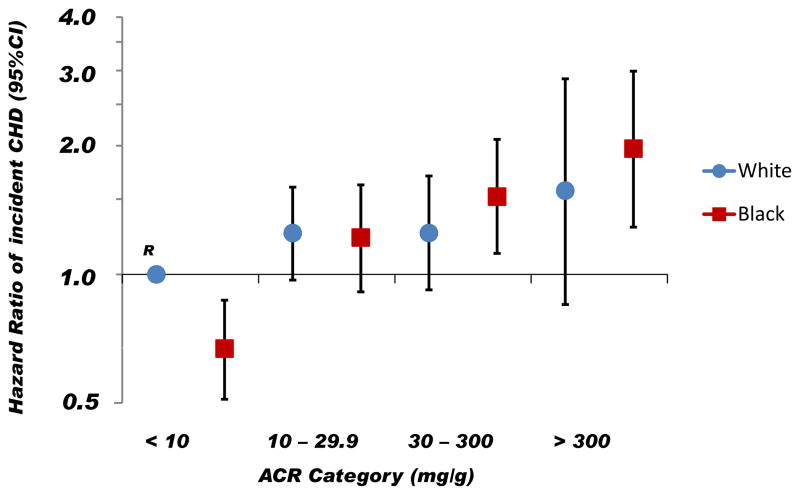
The Figure depicts the HRs of incident CHD according to race and baseline ACR, with white participants in the lowest ACR risk category serving as the referent group. In fully adjusted models, compared to white participants with an ACR <10 mg/g, the HR of incident CHD was significantly increased in blacks with an ACR ≥30 mg/g, whereas there was no statistically significant association of increasing ACR with incident CHD among white participants.
Figure. Hazard ratios of incident coronary heart disease by race and albumin to creatinine ratio.
Blue circles represent white participants and red squares represent black participants. Vertical lines represent 95% confidence intervals. White participants with an albumin to creatinine ratio (ACR) < 10mg/g was the referent group. The multivariable model was adjusted for age, sex, geographic region of residence, income, education, health insurance coverage, waist circumference, systolic blood pressure, total and HDL-cholesterol, triglycerides, estimated glomerular filtration rate, C-reactive protein, diabetes, smoking status, physical activity, use of statins, use of any antihypertensive medications, use of angiotensin II receptor blockers or angiotensin converting enzyme inhibitors and regular aspirin use.
Associations of ACR with First Recurrent CHD
Over 4.4 (interquartile range 2.8,5.4) years of follow-up, 468 recurrent CHD events (137 among blacks and 331 among whites) were observed. Of these, 279 were non-fatal MIs (86 among blacks and 193 among whites) and 189 were CHD-related deaths (51 among blacks and 138 among whites). Age- and sex-adjusted incidence rates of CHD increased with increasing categories of urinary ACR in both blacks and whites (Table 3). In Cox models adjusted for age, sex, and geographic region of residence, higher urinary ACR was associated with higher risk of recurrent CHD among blacks and whites (Table 4). After further adjustment for traditional cardiovascular risk factors and medication use, higher ACR remained associated with higher risk of recurrent CHD among both blacks and whites, with no statistically significant difference in these relationships by race whether modeling ACR as a categorical (P-interaction=0.53) or continuous variable (P-interaction=0.44). Addition of ACR to the fully-adjusted model resulted in an increase in the c-index of the model in whites (0.691[0.667,0.715] without vs. 0.703[0.679,0.727] with ACR, mean difference 0.012[0.004,0.022]) but not blacks (0.710[0.679,0.742] without vs. 0.721[0.689,0.751] with ACR, mean difference 0.011[−0.000,0.026]).
COMMENT
In this national cohort of community-dwelling adults, higher urinary albumin excretion was associated with a higher risk of incident CHD, with the strength and magnitude of this association being greater in blacks than in whites. In contrast, no racial differences in the association of urinary ACR with recurrent CHD were noted. These findings confirm the results of prior studies showing that urinary ACR is an important biomarker for CHD risk in the general population, even among individuals with ACR values below the current threshold for defining microalbuminuria (30 mg/g).15–17 Additionally, to our knowledge, this is the first study to demonstrate that the higher risk of incident CHD associated with excess ACR differs by race.
A growing body of evidence suggests that blacks are more susceptible to vascular injury and its consequences such as stroke and CHD than whites. In a recent analysis from the REGARDS study, racial differences in the impact of systolic blood pressure on stroke risk were observed, such that the risk of incident stroke associated with a 10-mm Hg difference in systolic blood pressure was 3-fold higher in black compared to white participants.18 Similarly, we previously showed that the risk of incident stroke associated with any given strata of urinary ACR above the normal range was significantly higher in blacks than in whites even after accounting for traditional cardiovascular risk factors and eGFR.6 The results of the current study extend these findings by showing that higher urinary ACR confers a 2-fold higher relative risk of incident CHD in blacks compared to whites with an ACR ≥30mg/g. While the absolute risk differences in the two highest categories of ACR were relatively small (~4 and 7 extra events per 1,000 person years of follow-up in blacks vs. whites, respectively), given the link between elevated urinary ACR and systemic microvascular injury,19–22 these data suggest that blacks have greater susceptibility to vascular disease than whites, which in turn may account for much of their excess risk of cardiovascular disease events such as stroke and CHD.
Given that elevated urinary ACR is also an important marker of kidney injury, a further potential explanation for these findings is that kidney disease itself may confer a higher risk of CHD in blacks than in whites. Arguing against this possibility is the finding that black-white differences in the association of urinary ACR with incident CHD were observed even after adjusting for baseline eGFR. Nonetheless, creatinine-based eGFR measurements are known to have relatively poor sensitivity for detecting chronic kidney disease in its earliest stages,23 whereas albuminuria is thought to be among the earliest biomarkers of kidney injury. Given that blacks tend to have faster rates of kidney function decline than whites,24, 25 faster CKD progression among blacks could potentially explain the difference in the relationship between excess urinary ACR and CHD risk by race in this study.
The reason for the absence of racial differences in the association of urinary ACR and first recurrent CHD events was unclear. It is conceivable that racial differences in the management of CHD risk factors before as opposed to after a CHD event could account for these findings. Studies have shown that blacks are less likely to receive primary preventive care for CHD, including maintaining normal cholesterol levels, controlling blood pressure, and stopping tobacco use.26 This likely plays a key role in the higher incidence of CHD events in blacks vs. whites in the US since the higher burden of traditional CHD risk factors among blacks almost completely accounted for their increased risk of fatal CHD in the REGARDS cohort.10 Following a CHD event, however, blacks (like whites) are more likely to receive secondary preventive therapies such as lipid-lowering medications, aspirin and tobacco cessation counseling, perhaps mitigating racial differences in the risk for recurrent CHD events associated with higher urinary ACR (and by extension systemic vascular injury).
Our study had limitations. We only had a single measurement of ACR, which may have led to exposure misclassification for some study participants. Next, some ACR categories had relatively few events, which may have reduced power to detect significant associations with CHD outcomes in these categories. Furthermore, we had limited information with respect to disease progression over time, which may play a role in explaining racial differences in these outcomes. In addition, it is possible that unmeasured differences in lifetime exposure to cultural, lifestyle and environmental factors in blacks vs. whites residing in the Southeastern US may have impacted the results.27 Finally, the inclusion of only black or white participants may limit the generalizability of these results to other races/ethnicities in the US.
In conclusion, as compared to white participants in this large national cohort, higher urinary ACR was a stronger risk factor for incident but not recurrent CHD events among black participants. Future studies should examine whether addition of ACR can improve the diagnosis and management of CHD in black individuals.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. The authors would also like to thank Dr. Joshua S. Richman, MD, PhD (University of Alabama at Birmingham) for his assistance with the analyses. Drs. Gutiérrez and Khodneva had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
SOURCES OF FUNDING: This study was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS) and R01 HL080477 from the National Heart, Lung and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NHLBI or the National Institutes of Health. In addition, OMG was supported by grants K23DK081673 and R03DK095005 from the NIDDK and R01NS080850 from the NINDS. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. The manuscript was sent to Amgen Corporation for review prior to submission for publication.
ROLE OF SPONSORS: Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation of the manuscript.
Footnotes
FINANCIAL DISCLOSURES: DGW is a member of the Amgen National Nephrology Advisory Board. OMG and DGW have received research support from the Amgen Corporation and DGW has received honoraria from Amgen and Sanofi-Genzyme. No other authors report financial disclosures.
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanevold CD, Pollock JS, Harshfield GA. Racial differences in microalbumin excretion in healthy adolescents. Hypertension. 2008;51(2):334–338. doi: 10.1161/HYPERTENSIONAHA.107.098095. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X, Srinivasan SR, Radhakrishnamurthy B, Dalferes ER, Jr, Bao W, Berenson GS. Microalbuminuria in young adults related to blood pressure in a biracial (black-white) population. The Bogalusa Heart Study. Am J Hypertens. 1994;7(9 Pt 1):794–800. doi: 10.1093/ajh/7.9.794. [DOI] [PubMed] [Google Scholar]
- 4.Jolly SE, Burrows NR, Chen SC, et al. Racial and ethnic differences in albuminuria in individuals with estimated GFR greater than 60 mL/min/1.73 m(2): results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010;55(3 Suppl 2):S15–22. doi: 10.1053/j.ajkd.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClellan WM, Warnock DG, Judd S, et al. Albuminuria and racial disparities in the risk for ESRD. J Am Soc Nephrol. 2011;22(9):1721–1728. doi: 10.1681/ASN.2010101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez OM, Judd SE, Muntner P, et al. Racial differences in albuminuria, kidney function, and risk of stroke. Neurology. 2012;79(16):1686–1692. doi: 10.1212/WNL.0b013e31826e9af8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 8.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 10.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: Wright-OSG; 1982. [Google Scholar]
- 12.Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353(7):671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 15.Shara NM, Wang H, Valaitis E, et al. Comparison of estimated glomerular filtration rates and albuminuria in predicting risk of coronary heart disease in a population with high prevalence of diabetes mellitus and renal disease. Am J Cardiol. 2011;107(3):399–405. doi: 10.1016/j.amjcard.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waheed S, Matsushita K, Sang Y, et al. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012;60(2):207–216. doi: 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waheed S, Matsushita K, Astor BC, Hoogeveen RC, Ballantyne C, Coresh J. Combined Association of Creatinine, Albuminuria, and Cystatin C with All-Cause Mortality and Cardiovascular and Kidney Outcomes. Clin J Am Soc Nephrol. 2013;8(3):434–442. doi: 10.2215/CJN.04960512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard G, Lackland DT, Kleindorfer DO, et al. Racial Differences in the Impact of Elevated Systolic Blood Pressure on Stroke Risk. Arch Intern Med. 2012:1–6. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster MC, Keyes MJ, Larson MG, et al. Relations of measures of endothelial function and kidney disease: the Framingham Heart Study. Am J Kidney Dis. 2008;52(5):859–867. doi: 10.1053/j.ajkd.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva AM, Schaan BD, Signori LU, et al. Microalbuminuria is associated with impaired arterial and venous endothelium-dependent vasodilation in patients with Type 2 diabetes. J Endocrinol Invest. 2010;33(10):696–700. doi: 10.1007/BF03346672. [DOI] [PubMed] [Google Scholar]
- 21.Solbu MD, Jenssen TG, Eriksen BO, Toft I. Changes in insulin sensitivity, renal function, and markers of endothelial dysfunction in hypertension--the impact of microalbuminuria: a 13-year follow-up study. Metabolism. 2009;58(3):408–415. doi: 10.1016/j.metabol.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 23.Wu I, Parikh CR. Screening for kidney diseases: older measures versus novel biomarkers. Clin J Am Soc Nephrol. 2008;3(6):1895–1901. doi: 10.2215/CJN.02030408. [DOI] [PubMed] [Google Scholar]
- 24.Peralta CA, Katz R, DeBoer I, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1327–1334. doi: 10.1681/ASN.2010090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 26.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123(8):850–857. doi: 10.1161/CIRCULATIONAHA.110.980151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plantinga L, Howard VJ, Judd S, et al. Association of duration of residence in the southeastern United States with chronic kidney disease may differ by race: the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Int J Health Geogr. 2013;12:17. doi: 10.1186/1476-072X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.