Abstract
Background
Despite the availability of established guidelines for measuring platelet serotonin, these guidelines may be difficult to follow in a hospital setting where time to processing may vary from sample to sample.
Purpose
The purpose of this study was to evaluate the effect of the time to processing of human blood samples on the stability of the enzyme-linked immunosorbent assay (ELISA) for the determination of platelet serotonin levels in human plasma.
Method
Human blood samples collected from a convenience sample of eight healthy volunteers were analyzed to determine platelet serotonin levels from plasma collected in ethylene diamine tetra acetic acid (EDTA) tubes and stored at 4°C for 3 hr, 5 hr, 8 hr, and 12 hr.
Results
Refrigeration storage at 4°C for 3 hr, 5 hr, 8 hr, and 12 hr altered the platelet serotonin measurement when compared to immediate processing. The bias for the samples stored at 4°C for 3 hr was 102.3 (±217.39 ng/109 platelets), for 5 hr was 200.1 (±132.76 ng/109 platelets), for 8 hr was 146.9 (±221.41 ng/109 platelets), and for 12 hr was –67.6 (±349.60 ng/109 platelets).
Discussion
Results from this study show that accurate measurement of platelet serotonin levels is dependent on time to processing. Researchers should therefore follow a standardized laboratory guideline for obtaining immediate platelet serotonin levels after blood sample collection.
Keywords: platelet serotonin, acute coronary syndrome, laboratory methods
Tryptophan hydroxylase-1 located in the gastrointestinal tract converts tryptophan into serotonin. Available peripheral serotonin is then secreted into the vascular system and transported into the platelets for storage by the serotonin transporter gene (SERT; Cote, Fligny, Fromes, Mallet, & Vodjdani, 2004). In the periphery, the majority of the serotonin is stored inside the platelets. Stored platelet serotonin is released and bound to available serotonin receptors. The effects of this peripheral platelet serotonergic system produce marked physiological changes in the cardiovascular system. A number of these potential physiological changes are also associated with acute coronary syndrome (ACS), including (a) morphological platelet changes, (b) serotonin-mediated platelet aggregation, and (c) thrombus formation (Cote et al., 2004; Parissis et al., 2007; Steptoe & Brydon, 2008). Knowledge about the relationship between peripherally available stored platelet serotonin levels and ACS may help identify patients at risk of adverse cardiac events associated with serotonin-mediated platelet aggregation.
Measurement of platelet serotonin levels, however, may pose difficulties for researchers, especially in the hospital setting. Established guidelines for the handling, processing, and storage of human blood samples for measurement of platelet serotonin levels are available, yet they are not definitive regarding time to processing (Immuno-Biological Laboratories [IBL], 2009). The standardized IBL Immuno-Biological Laboratory guidelines require “immediate” sample processing after blood sample collection followed by either conducting of an assay or frozen storage until the assay can be conducted. “Immediate” processing in this case is not defined, and is, therefore, open to interpretation. While immediate sample processing is easy to achieve in a laboratory site or with a stable population, it is more difficult to accomplish in a hospital setting where there may be less control, for example, when patients are hospitalized for ACS. It is not always feasible to transport a blood sample immediately from the catheterization laboratory or the emergency department to a research lab. As a result, processing times in patients admitted around the clock in a hospital setting for ACS may vary from sample to sample. While authors have reported problems with measurement of platelets themselves (e.g., unreliable laboratory results due to the effects of delayed time to processing after blood sample collection, exposure to anticoagulant during blood sample collection, storage temperature of the blood), we found no published papers on the effect of time to processing on platelet serotonin levels. It is, therefore, possible that time to processing influences the in vitro diagnostic quantitative determination of platelet serotonin. We conducted the present study as part of the preparation for a larger ACS trial in order to evaluate the impact of time to processing on the stability of blood samples collected for platelet serotonin analysis.
Materials and Methods
Design and Sample
We collected blood samples from a nonfasting convenience sample of eight healthy volunteers between 07:30 a.m. and 08:00 a.m. at The University of Texas Health Science Center at Houston School of Nursing (UTHSC-SON) laboratory. The number of samples required to test an existing established laboratory protocol is dependent on the experience and recommendation of the laboratory. In this case, the recommended sample size was 5–12 specimens. We performed a venipuncture in the antecubital vein with a 21-gauge needle and collected a total of 10 ml (filling one 6 cc tube [6 ml] and one 4 cc [4 ml] ethylene diamine tetra acetic acid [EDTA] tube) of blood from each volunteer to assess platelet serotonin levels. The University of Texas Health Science Center at Houston Institutional Review Board approved the study and all participants provided written informed consent. We obtained no identifiers from participants.
The study was designed to evaluate the effect of time to processing of human blood samples on the stability of the enzyme-linked immunosorbent assay (ELISA) for the in vitro diagnostic quantitative determination of platelet serotonin in human plasma samples (Laboratory Protocol Part A) and to confirm the accuracy of Laboratory Protocol Part A by performing a dose–response trial (Laboratory Protocol Part B). For Part B, we examined three individual samples that had been stored at 4°C for 3 hr. For Part A, we used five individual samples processed immediately and after storage at 4°C for 3 hr, 5 hr, 8 hr, and 12 hr. We also included in Part A analysis the three individual samples processed immediately and after 3 hr of refrigerated storage in Part B at the same 2 ml concentration as those used in Part A. For both Part A and Part B, the initial laboratory processing time point began approximately 30 min after venipuncture and was labeled as the reference measurement.
Laboratory Protocol Part A
We processed the samples of the five individuals included in Part A in sterile conditions using a tissue culture hood. First, we combined the 6 ml and 4 ml EDTA tubes into one 15 ml conical tube and gently inverted it 20 times to ensure an even mixture. We transferred 2 ml of blood to a new 15 ml conical tube for immediate processing and divided the remaining 8 ml of blood into 4 aliquots of 2 ml each for storage at 4°C for 3 hr, 5 hr, 8 hr, or 12 hr, labeling each tube based on length of storage time.
At each time point (immediate, 3 hr, 5 hr, 8 hr, and 12 hr), we placed each 2 ml sample into a 15 ml conical tube and gently tapped the tube to ensure an even mixture. We centrifuged samples at 200 relative centrifugal force (RCF) × g at ambient temperature for 10 min. After centrifugation, we removed the samples and placed them back in the hood. We then removed 500 μl of plasma supernatant from each 15 ml conical tube and placed it in a new 15 ml conical tube for platelet purification. We added 2 ml of saline solution to the conical tubes containing platelet-rich plasma (PRP) and centrifuged them at 4,500 RCF × g at 4°C for 10 min. After centrifugation, we discarded the supernatant, being careful not to disturb the platelet pellets. Next, we vortexed the platelet pellets and added 500 μl of distilled water to each sample. We made two separate aliquots from each sample by transferring the suspended platelets to 2.0 ml microcentrifuge tubes prelabeled with bar codes. For each sample, Aliquot 1 contained 200 μl and Aliquot 2 contained 300 μl of purified platelets. We placed the aliquots in cardboard storage boxes in the –80°C freezer, where they remained until they were needed for quantitative determination of platelet serotonin levels (IBL, 2009).
Laboratory Protocol Part B
For Laboratory Protocol Part B, we stored the three 6 ml EDTA tubes at 4°C for 3 hr and began processing the samples in the 4 ml EDTA tubes immediately. Under the biological hood, we gently inverted each 4 ml tube 20 times to ensure an even mixture and transferred 2 ml of blood to new 15 ml conical tubes for immediate processing. We then followed the same procedure as described above in Laboratory Protocol Part A and, as in Part A, we placed Aliquot 1, containing 200 μl, and Aliquot 2, containing 300 μl, of purified platelets in cardboard storage boxes in the –80°C freezer.
From the 6 ml EDTA samples that had been stored at 4°C for 3 hr, we transferred 1 ml, 2 ml, and 3 ml of blood to separate 15 ml conical tubes. We tapped each tube to ensure an even mixture and followed the steps described above for immediate processing through the separation of purified platelets and storage of the aliquots at –80°C until they were needed for the in vitro diagnostic quantitative determination of serotonin in human plasma (IBL, 2009).
Data Analysis
Data analysis included comparison of the reference measurement (immediate processing) to the 3-hr, 5-hr, 8-hr, and 12-hr delayed laboratory processing measurements for Laboratory Protocol Part A and comparison of the reference measurement (immediate processing) to the 3-hr delayed processing measurements of different platelet concentrations for Laboratory Protocol Part B. We constructed Bland and Altman (1986) plots to assess the repeatability of platelet serotonin measurement by comparing the difference between the reference and the delayed laboratory processing value to the mean of these two values. We completed a separate plot for each comparison with the reference measurement (3 hr, 5 hr, 8 hr, and 12 hr). We used MedCalc for Windows, version 9.3.8.0 for statistical analyses.
Results
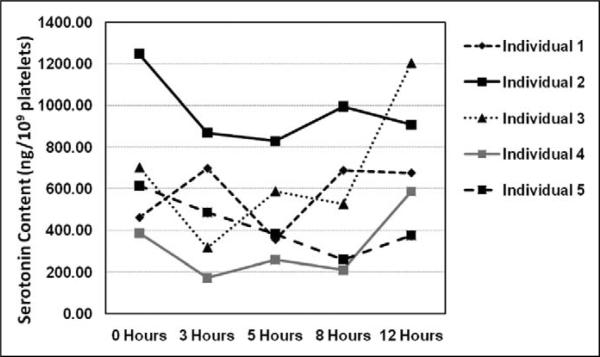
We expressed calculated serotonin content in platelets in ng/109 platelets. Immediate processing, or time point 0 hr, served as the reference measurement for all comparisons. For Laboratory Protocol Part A, we constructed a line graph to show the data points for the five individuals for all five time points, which is shown in Figure 1. Platelet serotonin levels show wide variation across individuals and time points, from 172.06 ng/109 platelets to 1248.76 ng/109 platelets.
Figure 1.
Comparison of serotonin content in platelets from five individuals across five refrigeration time points. The x-axis displays the five measured time points (0, 3, 5, 8, and 12 hr). The y-axis displays the values for the five individuals at each time point in increments of 200 ng/109 platelets.
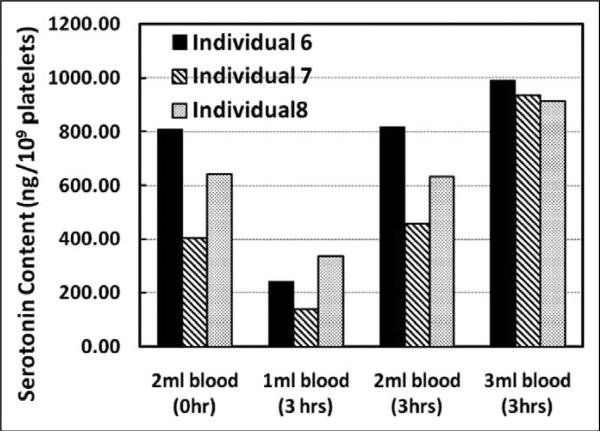
For Laboratory Protocol Part B, we constructed a bar graph to show the dose–response data points for the three individuals at 0 and 3 hr, which is shown in Figure 2. Platelet serotonin content increases as sample volume increases.
Figure 2.
Dose–response data for the serotonin content in platelets from 3 individuals at different sample volumes for the reference time point (0 hr) and after 3 hr of refrigerated storage. The x-axis displays serotonin concentrations. The bars represent the serotonin content in ng/109 platelets.
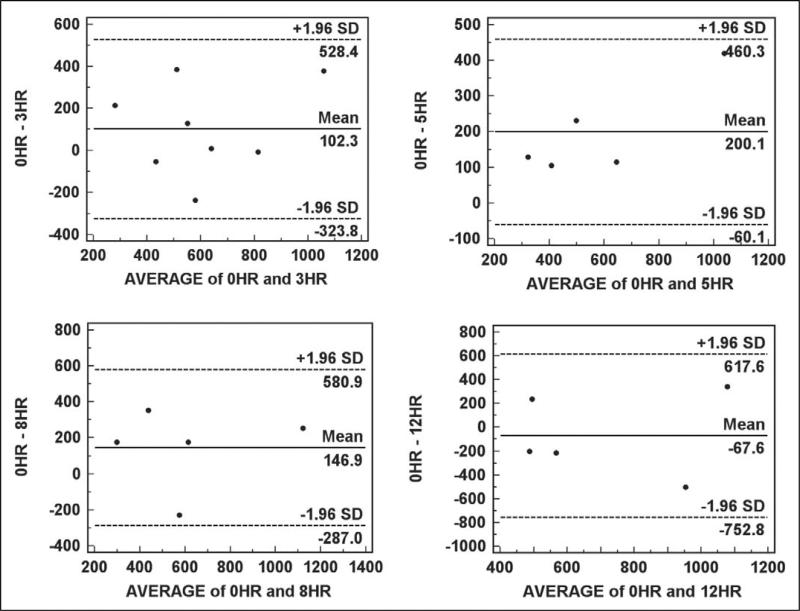
The Bland–Altman plots we constructed are displayed in Figure 3. The bias for the samples stored for 3 hr is 102.3 (±217.39 ng/109 platelets), for 5 hr is 200.1 (±132.76 ng/109 platelets), and for 8 hr is 146.9 (±221.41 ng/109 platelets), indicating that delays in laboratory processing of these durations result in higher platelet serotonin levels when compared to immediate laboratory processing. The bias for the samples stored for 12 hr is –67.6 (±349.60 ng/109 platelets), indicating that a delay in processing of 12 hr results in lower platelet serotonin levels when compared to immediate laboratory processing.
Figure 3.
Bland–Altman plots of reference samples stored for 0 hr compared to samples stored at 4°C for 3, 5, and 12 hr. The plot for the comparison with the 3-hr time point includes all five of the individuals included in Laboratory Protocol Part A in addition to the three individuals included in Laboratory Protocol Part B with 2 ml blood samples. The remaining time points include only samples from the five individuals included in Laboratory Protocol Part A.
Discussion
Researchers have established that platelet measurements are dependent on a variety of laboratory factors, including time to processing, instrumentation utilized during processing, exposure to anticoagulant during blood sample collection, and storage temperature, and are prone to in vitro activation when manipulated (Jackson & Carter, 1993). While studies have shown that platelet measurements are variable and dependent on a wide variety of laboratory factors, investigators have not clearly defined the influence of laboratory standards on the measurement of platelet serotonin levels. It is likely that the instability of platelet measurements, themselves, impacts the stability of platelet serotonin levels, and potential problems may occur with the reproducibility and accuracy of platelet serotonin measurements.
Our findings in the current study indicate that delayed time to processing results in platelet serotonin levels that are inconsistent and unpredictable over time when compared to immediate laboratory processing after blood sample collection. Given this demonstrated instability, researchers should work closely with their laboratory personnel to ensure that they are using standardized methods of sample collection and processing to measure platelet serotonin levels. Available literature suggests that time to processing is important when measuring platelet parameters, with authors often recommending that platelet measurements occur within 2 hr of venipuncture (Lance, van Oerle, Henskens, & Marcus, 2010). While we found no published papers on the effect of time to processing on platelet serotonin levels, our data indicate that, because of the unstable nature of platelet serotonin, it is not possible to recommend a specific delayed time point in which to process platelet serotonin. Rather, the ideal setup in a clinical setting would be to have a laboratory facility on-site that is available for research protocols and immediate laboratory processing.
Acknowledgments
The authors express their appreciation to Stanley Cron, MSPH, Nikhil Padhye, PhD, and Fang Liu, MA, for critique of the manuscript and Facheng Luo, BDM, and the laboratory staff at The University of Texas Health Science Center at Houston, School of Nursing.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institute of Nursing Research (1R01NR010235-01A1), the Clinical and Translational Science Awards (CTSA) Administrative Supplement Award from the CTSA Consortium Biobank (3 UL 1 RR024148-03S1), and the Sigma Theta Tau International, Zeta Pi Chapter.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Cote F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends in Molecular Medicine. 2004;10:232–238. doi: 10.1016/j.molmed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Immuno-Biological Laboratories Instructions for use. 2009 Serotonin ELISA. IB89546. Retrieved from http://www.ibl-america.com/pdf/newIBLelisa/IB89546.pdf.
- Jackson SR, Carter JM. Platelet volume: Laboratory measurement and clinical application. Blood Reviews. 1993;7:104–113. doi: 10.1016/s0268-960x(05)80020-7. [DOI] [PubMed] [Google Scholar]
- Lance MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Laboratory Hematology: Official Publication of the International Society for Laboratory Hematology. 2010;16:28–31. doi: 10.1532/LH96.10011. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Fountoulaki K, Filippatos G, Adamopoulos S, Paraskevaidis I, Kremastinos D. Depression in coronary artery disease: Novel pathophysiologic mechanisms and therapeutic implications. International Journal of Cardiology. 2007;116:153. doi: 10.1016/j.ijcard.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Brydon L. Emotional triggering of cardiac events. Neuroscience and Biobehavioral Reviews. 2008;33:63–70. doi: 10.1016/j.neubiorev.2008.04.010. [DOI] [PubMed] [Google Scholar]