Abstract
Introduction
While high HPV 16 viral load measured at a single time point is associated with cervical disease outcomes, few studies have assessed changes in HPV 16 viral load on viral clearance.
Objective
To measure the association between changes in HPV 16 viral load and viral clearance in a cohort of Thai women infected with HPV 16.
Study design
Fifty women (n = 50) between the ages of 18–35 years enrolled in a prospective cohort study were followed up every three months for two years. Women positive for HPV 16 DNA by multiplex TaqMan© assay at two or more study visits were selected for viral load quantitation using a type-specific TaqMan© based real-time PCR assay. The strength of the association of change in viral load between two visits and viral clearance at the subsequent visit was assessed using a GEE model for binary outcomes.
Results
At study entry, HPV 16 viral load did not vary by infection outcome. A >2 log decline in viral load across two study visits was found to be strongly associated with viral clearance (AOR: 5.5, 95% CI: 1.4–21.3). HPV 16 viral load measured at a single time point was not associated with viral clearance.
Conclusions
These results demonstrate that repeated measurement of HPV 16 viral load may be a useful predictor in determining the outcome of early endpoints of viral infection.
Keywords: HPV, DNA, Viral load, Epidemiology, Thailand
1. Background and objectives
Human papillomavirus (HPV) infections of the female anogen-ital tract are the established cause of cervical cancer. Infection with HPV type 16 is responsible for >50% of cervical cancer cases worldwide.1,2 Additionally, among HPV 16 infected women, elevated DNA viral load measured at a single time-point using either semi- or fully quantitative methods is positively associated with a cross-sectional diagnosis of cervical squamous intraepithelial lesions (SIL) or cervical intraepithelial neoplasia (CIN).3–27 Higher HPV 16 viral load measured has also been shown prospectively to be associated with development of high-grade cervical pre-cancer (CIN 2+),28 carcinoma in situ,29–31 and cervical carcinoma.32
In a long term prospective study conducted among women in Colombia, an increased risk of viral clearance was observed in women with lower peak viral load over the course of an incident infection.33 Risk of clearance by changes in viral load over time was not reported. The impact of viral load change has been assessed in only a few studies.28,34 An investigation of a hospitalized population of HPV 16 positive, cytologically normal women demonstrated that increases in HPV 16 viral load measured at six month intervals were associated with progression to CIN2/3+, while women who remain cytologically normal were more likely to have decreasing viral load over time.34 Changes in viral load and their associations with disease risk may have implications on understanding the complex interaction of HPV with the human host as well as potentially serving as an additional predictive marker for outcomes of infection.
Currently, there are no studies to our knowledge that have assessed changes in HPV 16 viral load on early endpoints of natural history such as viral clearance. We compared the association of HPV 16 viral load either at a single time point or repeatedly every three months for 2 years on viral clearance in a cohort of young women from Thailand.
2. Study design
Women attending family planning clinics throughout the Northern (Chiang Mai), Northeastern (Khon Kaen), Central (Bangkok) and Southern (HatYai) regions in Thailand between 2002 and 2003 were recruited into a prospective study to assess the natural history of HPV and CIN 2/3, and were between 18 and 35 years of age. These women were originally enrolled in a study designed to evaluate the effects of hormonal contraceptive use on HIV acquisition (HC-HIV). Selection criteria are described in detail elsewhere.35 Inclusion criteria for enrollment in the HC-HIV study included: (1) HIV negative; (2) not pregnant; (3) intact uterus; (4) used some form of modern contraception within 3 months prior to enrollment; and (5) willing to adhere to self-selected contraceptive method for at least one consecutive year during a two year follow-up. At enrollment, women self-selected combined oral contraception (ethyl estradiol and levongestrel, COC), progesterone only contraception (depomedroxyprogesterone acetate, DMPA), or non-hormonal contraceptive methods (NHC) for at least 1 year. The study protocols were reviewed and approved by the committees on human subject research at Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, Merck Research Laboratories, West Point, PA, and the Institutional Review Board of the Thailand Ministry of Health (MOH), Thailand, and by the seven collaborating hospitals.
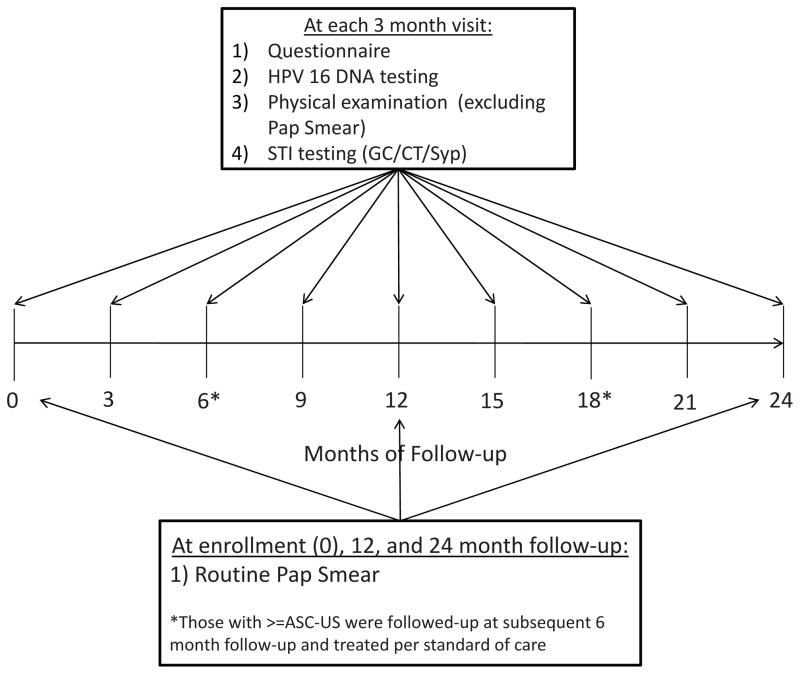
At enrollment and at each follow-up visit, information on sociodemographic characteristics, sexual risk behavior, partner sexual behavior, reproductive and contraceptive history, current contraceptive use status, and self-reported medical history were collected at each study site by trained interviewers using a standardized questionnaire (Fig. 1).
Fig. 1.
Schematic of the timing of data collection including Pap smears in the HC-HIV study.
Endo/ecto cervical swab specimens were collected and stored in specimen transport medium at −20 °C until time of HPV 16 genotyping as well as gonorrhea (GC)/chlamydia (CT) detection. A Thin Prep Papanicolaou smear was performed at the enrollment, 12 month and 24 month study visits and was classified as normal, inflammation, atypical squamous cells of unknown significance (ASC-US), low grade squamous intraepithelial lesions (LSIL), or high grade intraepithelial lesions (HSIL). Repeat Pap smears were performed after six months for women with abnormal cytology >ASC-US (Fig. 1). Women with an abnormal cytological diagnosis were referred for colposcopy to confirm the presence of pre-cancerous lesions. Women with a diagnosis of cervical intraepithelial neoplasia 2+ (CIN 2+) were referred for treatment.
At the end of follow-up of this initial study, women were invited to enroll into an observational study for an additional 18-months of follow-up with 6 month sampling. At the enrollment visit in this follow-up study all women received a Pap smear and were referred for colposcopy and treatment as stated above. Thirty-six women (72%) included in this analysis were enrolled in this follow-up study with an average total follow-up of 17.6 months (SD: 2.3).
2.1. HPV 16 DNA detection and viral load quantification
DNA was extracted from cervical swab specimens (Digene© standard transport medium) using the QiaAmp Blood kit (Qiagen, Courtaboeuf, France). Real-time PCR was used to detect the E6, E7 and L1 genes of the HPV 16 genome.36,37 Specimens were considered positive for HPV 16 if two out of three genes measured were detected, or if the same single gene was detected twice upon repeat testing.
Viral load measurements were performed on all samples determined to be HPV 16 positive by a type-specific TaqMan based real-time PCR assay targeting the E7 ORF of the HPV 16 genome.38 The assay used in this analysis was shown to have a high level of agreement with another previously described, HPV 16 quantitative viral load assay that targets the E6 ORF.38 To control for sampling heterogeneity, viral load measures were normalized to 104 human cells through quantification of the human β-globin gene.
2.2. Statistical analysis
Women who were HPV 16 DNA positive at enrollment (prevalent infections) or became detectable during follow-up (incident infections) and had at least two subsequent study visits were included in the analysis. The primary outcome of this analysis is the loss of detectability of HPV 16 DNA (i.e., clearance) over two consecutive visits. Intercurrent negative visits (a single visit of HPV 16 DNA negativity flanked by two positive visits) were treated as visits in which HPV 16 DNA load has fallen below the limit of detection for the assay and assigned a value of 1.6 copies/104 CE, the lower limit of detection of the RT-PCR assay. HPV 16 viral load was assessed as either (1) a single absolute value at the visit of first detectability of HPV 16 DNA (i.e., study entry or first visit with incident HPV16 infection), or (2) the relative change (i.e., log-fold change) over two consecutive visits (visit-pairs), or (3) the first visit of a given visit pair (i.e., baseline visit) immediately prior to infection outcome assessment. Viral load measures were normalized using log transformation. The absolute measure of viral load at the baseline and index visit was analyzed as a continuous value or categorized as <2000 vs. ≥2000 copies/104 cells equating to ~1 pg/ml HPV 16 DNA as previously described.34
Contingency tables comparing covariates across infection outcome status were evaluated and the Chi-squared test was used to determine statistical significance. Wilcoxon ranksum test was used to assess differences in median values of viral load across infection outcome status and covariates. Logistic regression using the generalized estimating equation (GEE) approach was used to estimate adjusted odds ratios (AOR) to assess the strength of the association of HPV 16 viral load measures and infection outcome. A p-value of <0.05 was considered statistically significant.
3. Results
This analysis included 50 women with either incident (n = 16) or prevalent (n = 34) HPV 16 infections detectable over at least two study visits. These women contributed a total of 303 person visits (mean/participant: 6 (SD: 3)) and 253 visit-pairs (mean/participant: 5 (SD: 3.1)) for HPV 16 viral load measures, equating to a total 767 months of follow-up (mean: 15 months (SD: 7.8)). The average time between visits was 2.6 months (SD: 0.9). Twenty-one (n = 21) infections cleared during follow-up with an average duration of detection of 11 months (SD: 5.5).
At enrollment the mean age of the sample was 26.8 years (SD: 4.6) and the majority were cytologically normal with only 4 (8%) having an enrollment pap diagnosis of ASC-US (Table 1). Women who reported use of COC were less likely to clear their infection as compared to non-hormonal contraceptive users and DMPA users (p = 0.024). Conversely, women who reported use of DMPA for >4 years prior to enrollment were more likely to clear their infections as compared to women who reported use of <1 year (p = 0.036).
Table 1.
Distribution of the demographic, reproductive, clinical, and STI factors of women with viral load measures at study enrollment.
| Variable | Sample (N = 50) | Cleared (n = 21) n (%) | Persisted (n = 29) n (%) | p-value |
|---|---|---|---|---|
| Mean age (SD) | 26.8 (4.6) | 37 (3.8) | 26.7 (5.2) | p = 0.75 |
| Study site | ||||
| N (Chiang-Mai) | 13 | 2 (15.4) | 11 (84.6) | |
| NE (Khon Kaen) | 9 | 6 (66.7) | 3 (33.3) | |
| S (Songhkla-Hat Yai) | 14 | 5 (35.7) | 9 (64.3) | |
| C (Bangkok) | 14 | 8 (57.1) | 6 (42.9) | p = 0.056 |
| Current contraceptive use | ||||
| NHC | 13 | 9 (69.2) | 4 (30.8) | |
| COC | 22 | 5 (22.7) | 17 (77.3) | |
| DMPA | 15 | 7 (46.7) | 8 (53.3) | p = 0.024 |
| Duration of COC usea | ||||
| <1 year | 4 | 1 (25) | 3 (75) | |
| 4–5 years | 8 | 3 (37.5) | 5 (62.5) | |
| >5 years | 10 | 1 (10) | 9 (90) | p = 0.381 |
| Duration of DMPA usea | ||||
| <1 year | 5 | 0 (0) | 5 (100) | |
| 4–5 years | 6 | 4 (66.7) | 2 (33.3) | |
| >5 years | 4 | 3 (75) | 1 (25) | p = 0.036 |
| Lifetime number of partners | ||||
| 1 | 24 | 13 (54.2) | 11 (45.8) | |
| 2 | 12 | 6 (50) | 6 (50) | |
| 3 | 2 | 1 (50) | 1 (50) | |
| ≥4 | 12 | 9 (75) | 3 (25) | p = 0.586 |
| Number of recent sexual partnersb | ||||
| 1 | 38 | 21 (55.3) | 17 (44.7) | |
| >1 | 12 | 8 (66.7) | 4 (33.3) | p = 0.485 |
| Cytological diagnosisc | ||||
| Normal | 28 | 14 (50) | 14 (50) | |
| Inflammation | 18 | 6 (33.3) | 12(66.7) | |
| AS-CUS | 4 | 1 (25) | 3 (75) | |
| ≥LSIL | 0 | 0 (0) | 0 (0) | p = 0.414 |
| Worst pap diagnosis during follow-up | ||||
| Normal | 17 | 10 (58.8) | 7 (41.2) | |
| Inflammation | 26 | 9 (34) | 17 (65.4) | |
| AS-CUS | 6 | 2 (33) | 4 (66.7) | |
| LSIL | 0 | 0 (0) | 0 (0) | |
| HSIL | 1 | 0 (0) | 1 (100) | p = 0.529 |
| STI infection status | ||||
| Gonorrhea | 1 | 1 (100) | 0 (0) | p = 0.235 |
| Chlamydia | 9 | 7 (77.8) | 2 (22.2) | p = 0.735 |
| HPV 16 infection type | ||||
| Prevalent | 34 | 15 (44.1) | 19 (55.9) | |
| Incident | 16 | 6 (37.5) | 10 (62.5) | p = 0.574 |
Among COC/DMPA users.
In the last 3 months.
At study enrollment.
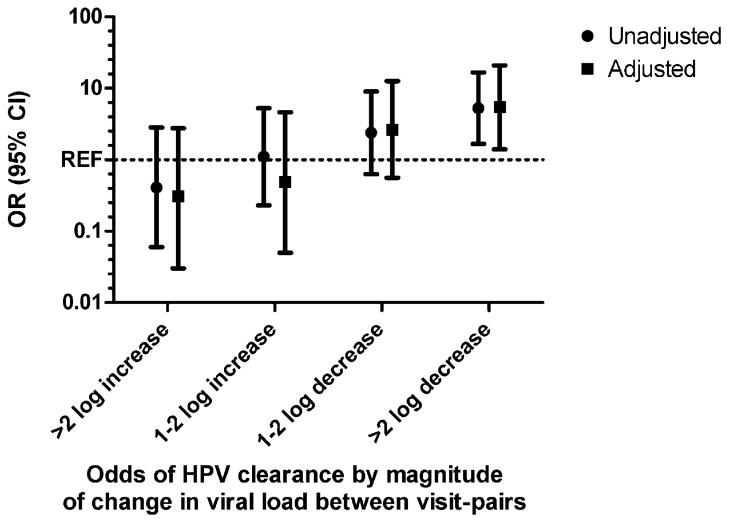
The median change in viral load between two consecutive visit pairs was −6.9 copies/10,000 cells (IQR: −4672.4, 615.9) (Table 2). Among visit-pairs preceding a cleared vs. a persistent infection, the median change was −612.2 copies/10,000 cells (IQR: −7679.2, 0.07) vs. 18.2 copies/10,000 cells (IQR: −3668.9, 5660.3), respectively (p < 0.05). A ≥2 log fold decline in HPV 16 viral load was found to be significantly associated with viral clearance (Table 3 and Fig. 2) which remained significant after controlling for age, cytological diagnosis at enrollment, contraceptive group, concurrent GC/CT infection and infection type. Conversely, increasing viral loads of ≥2 log fold across a given study visit pair was observed to be non-significantly protective against clearance (i.e., associated with an increased risk of viral persistence; p = 0.291).
Table 2.
Differences in median HPV 16 viral load measured at study entry and baseline visit across infection outcome.
| Viral load measure | Sample (N = 50) | Cleared (n = 21) | Persistent (n = 29) | p-Value |
|---|---|---|---|---|
| Median at study entry (IQR)a | 262.2 (15.9, 5414.6) | 576.8 (9.9, 18,465.7) | 175.4 (39.5, 2,056.6) | p = 0.78 |
| ≤2000 copiesb | 33 | 12 (36.4) | 21 (63.6) | |
| >2000 copies | 17 | 9 (52.9) | 8 (47.1) | p = 0.31 |
| Median at baseline visit (IQR)a | 1757 (53.4, 14,832.7) | 954.2 (36, 24,843) | 2135.3 (65.5, 13,971) | p = 0.56 |
| ≤2000 copiesb | 26 | 12 (46.2) | 14 (53.8) | |
| >2000 copies | 24 | 9 (37.5) | 15 (62.5) | p = 0.66 |
Study entry, visit of first detection of HPV 16; baseline visit, first visit of a visit-pair.
Per 104 human cells.
Table 3.
Association of viral load measures on HPV 16 viral clearance.
| Variable | Unadjusted (OR (95%CI)) | Adjusteda (AOR (95% CI)) |
|---|---|---|
| Higher study entry viral loada | 1.00 (0.88, 1.15) | 1.00 (0.84, 1.19) |
| Index visit viral load | ||
| ≤2000 copies/104 cells | 1.0 | 1.0 |
| >2000 copies/104 cells | 1.59 (0.61, 4.1) | 1.29 (0.39, 4.22) |
| Higher baseline visit viral load | 0.99 (0.87, 1.1) | 0.87 (0.70, 1.1) |
| Baseline visit viral load | ||
| ≤2000 copies/104 cells | 1.0 | 1.0 |
| >2000 copies/104 cells | 0.95 (0.4, 2.3) | 0.42 (0.12, 1.4) |
| Viral load change across visit-pair | ||
| Increase | 1.0 | 1.0 |
| Decrease | 3.16 (1.31, 7.65) | 5.62 (1.75, 17.9) |
| >2 log increase | 0.41 (0.06,2.83) | 0.31 (0.03, 2.77) |
| 1–2 log increase | 1.11 (0.23,5.26) | 0.49 (0.05, 4.62) |
| ≤1 log change (+/−) | 1.0 | 1.0 |
| 1–2 log decrease | 2.39 (0.63, 9.02) | 2.61 (0.56, 12.6) |
| >2 log decrease | 5.26 (1.66, 16.61) | 5.49 (1.40, 20.9) |
Adjusted for age, study site, contraceptive use, STI diagnosis during follow-up, cytology during follow-up, infection type.
Fig. 2.
The association of change in viral load and risk of HPV 16 viral clearance. Unadjusted and adjusted odds ratios (ORs) are presented. The reference group is a change in HPV 16 viral load <1.0 log in either direction between visits. OR > 1.0 denotes an increased risk of clearance; OR < 1.0 denotes a reduced risk of clearance. ORs were adjusted for age at enrollment, study site, contraceptive use and STI diagnosis at follow-up, and infection type. REF= <1.0 log-fold increase or decrease in viral load.
A second phase of the initial prospective study was initiated within six to twelve months after the final 24-month study visit for longer term assessment of cervical disease outcomes. A total of 36 out of 50 women with an HPV16 positive test result at the final HC-HIV visit (72%) were re-consented and enrolled into this study (mean time between last virologic measure and second phase study enrollment was 10 months, SD: 7.5) (Table 4). All women who re-enrolled into the extended follow-up were given a Pap smear at the baseline re-enrollment visit. Among these women, 25 (69%) remained detectable for HPV 16 at the enrollment visit of the follow-up study. Additionally, 24 (66.7%) women with follow-up were cytologically normal or had inflammation, 4 (11.1%) were diagnosed with AS-CUS, 3 (8.3%) LSIL, and 5 (13.9%) HSIL. Women with HSIL as compared to women remaining cytologically normal had a higher median HPV 16 viral load at the visit of first detection (3250.8 copies/104 cells vs. 87.5 copies/104 cells, p = 0.564) but this difference did not reach statistical significance.
Table 4.
Differences in HPV 16 viral load assessed at the visit of first detection (study entry) by cytological outcomes at six-month post-study follow-up.
| Cytological diagnosis at follow-up: | N | Median HPV 16 VL (IQR) | p-Value |
|---|---|---|---|
| Normal/inflammation | 24 | 87.5 (14.9, 1,749.2) | |
| AS-CUS | 4 | 107.9 (22.9, 426.9) | |
| LSIL | 3 | 144.1 (0.43,134.2) | |
| HSIL | 5 | 3,250 (235.2, 11,156.6) | p = 0.564 |
4. Discussion
This study demonstrates that in a cohort of women aged 18–35 years from Thailand, a >2 log fold decline in HPV 16 viral load across two consecutive visits is predictive of viral clearance. While a previous study among women in Colombia observed that high HPV viral load was associated with an increased risk of viral clearance,33 their analysis was based on peak viral loads over the course of incident infection. In our analysis, a higher peak viral load was non-significantly associated with a reduced risk of viral clearance (OR: 0.29, 95% CI [0.7, 1.13] for a 1 log increase in peak viral load). Replication of this effect is retrospectively possible for epidemiological analysis. However, our observation that the magnitude of change between 2 viral load measures predicts viral clearance offers potential utility of viral load measures in real time. For example, one study of hospitalized women from France did evaluate the impact of viral load changes on disease outcomes. Consistent with our results, they found that declines in HPV 16 viral load were associated with the maintenance of normal cytological outcomes.3
The association between declining HPV 16 viral load and HPV 16 clearance may reflect recognition of viral infection by the host immune response. Clearance of HPV is facilitated by a robust CD8+ cytotoxic T-cell response39 and is characterized by increases in immunologic markers such as IFN-γ and IL-2 in both the periphery and cervical immune environments.40–42 If it can be confirmed that decreasing viral load correlates with cellular immune responses, serial viral load measurements may have potential utility as early biomarkers of effect in the evaluation of response to therapeutic interventions on HPV infection.
This study has several strengths. The high density of sampling of study participants allows for more precise evaluation of the kinetics of HPV viral load. Second, this study utilized a highly sensitive and specific real-time PCR assay for viral load quantitation normalized to β-globin to minimize differences due to sampling heterogeneity.
This study has some limitations. First, we did not detect and measure viral load of other oncogenic and non-oncogenic HPV types that may be co-infecting women along with HPV 16. Second, the high sampling density in this study made the presence of intercurrent negative visits fairly common with eleven women (22%) having a single HPV 16 DNA negative visit flanked by two positive visits. This could have been the result of laboratory error leading to false negativity or true loss of detection either through suppression of viral replication or complete viral clearance by the host. Women with intercurrent negative visits did not significantly differ by virological (i.e., clearance) and clinical outcomes (i.e., pap status) or demographic factors (i.e., age, study site, hormonal contraceptive group) from those with no intercurrent negative results, and the significance and direction of the association of viral clearance with viral load were not altered after intercurrent negative visits were excluded from the analysis (data not shown). In addition, the sample size was too small to examine the viral load effects among prevalent and incidentally detected infections. The women enrolled in this study were part of a trial assessing the effects of hormonal contraceptive use on HIV acquisition which may limit the generalizablility of study findings to other women in Thailand or other settings worldwide.
Application of HPV 16 viral load as a surrogate endpoint may be valuable in evaluating immunologic response to infection, which may especially have utility in immunotherapeutic studies where it is not possible to wait for lesion regression. Studies are ongoing to determine if viral load changes are correlated with a cellular immune response. If this can be demonstrated, viral load declines in response to therapy may provide an ethically acceptable alternative endpoint for decisions to pursue further clinical trials.
Acknowledgments
This work was funded in part by the NIAID pre-doctoral training fellowship in sexually transmitted infections (5T32AI050056-09). This study was funded by Merck & Co. Inc.
Abbreviations
- HPV
human papillomavirus
- HR-HPV
high risk human papillomavirus
- COC
combined oral contraception
- DMPA
depomedroxyprogesterone acetate
- GEE
generalized estimating equation
Footnotes
5. Conflict of interest statement
SG – employee of Merck Research Laboratories who funded research; KLL – employee of Merck, Sharp and Dohme, which manufactures the quadrivalent HPV vaccine, owns Merck stocks and options; AT – employee of Merck.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006 Aug;24S3:S1–10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 3.Castle PE, Wacholder S, Sherman ME, Lorincz AT, Glass AG, Scott DR, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002 Nov;95(10):2145–51. doi: 10.1002/cncr.10927. [DOI] [PubMed] [Google Scholar]
- 4.Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003 Sep;106(3):396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 5.Healey SM, Aronson KJ, Mao Y, Schlecht NF, Mery LS, Ferenczy A, et al. Oncogenic human papillomavirus infection and cervical lesions in aboriginal women of Nunavut, Canada. Sex Transm Dis. 2001 Dec;28(12):694–700. doi: 10.1097/00007435-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Hernandez DM, Ornelas-Bernal L, Guido-Jimenez M, Apresa-Garcia T, Alvarado-Cabrero I, Salcedo-Vargas M, et al. Association between high-risk human papillomavirus DNA load and precursor lesions of cervical cancer in Mexican women. Gynecol Oncol. 2003 Aug;90(2):310–7. doi: 10.1016/s0090-8258(03)00320-2. [DOI] [PubMed] [Google Scholar]
- 7.Hesselink AT, van den Brule AJ, Brink AA, Berkhof J, van Kemenade FJ, Verheijen RH, et al. Comparison of hybrid capture 2 with in situ hybridization for the detection of high-risk human papillomavirus in liquid-based cervical samples. Cancer. 2004 Feb;102(1):11–8. doi: 10.1002/cncr.11904. [DOI] [PubMed] [Google Scholar]
- 8.Ho GY, Palan PR, Basu J, Romney SL, Kadish AS, Mikhail M, et al. Viral characteristics of human papillomavirus infection and antioxidant levels as risk factors for cervical dysplasia. Int J Cancer. 1998 Nov;78(5):594–9. doi: 10.1002/(sici)1097-0215(19981123)78:5<594::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Ikenberg H, Goppinger A, Bauer H, Schmitt B. Semiquantitative analysis of human papillomavirus DNA in cervical intraepithelial neoplasia by a differential polymerase chain reaction. J Obstet Gynaecol. 1997 Mar;17(2):176–9. doi: 10.1080/01443619750113816. [DOI] [PubMed] [Google Scholar]
- 10.Lillo FB, Lodini S, Ferrari D, Stayton C, Taccagni G, Galli L, et al. Determination of human papillomavirus (HPV) load and type in high-grade cervical lesions surgically resected from HIV-infected women during follow-up of HPV infection. Clin Infect Dis. 2005 Feb;40(3):451–7. doi: 10.1086/427032. [DOI] [PubMed] [Google Scholar]
- 11.Santos AL, Derchain SF, Martins MR, Sarian LO, Martinez EZ, Syrjanen KJ. Human papillomavirus viral load in predicting high-grade CIN in women with cervical smears showing only atypical squamous cells or low-grade squamous intraepithelial lesion. Sao Paulo Med J. 2003 Nov;121(6):238–43. doi: 10.1590/S1516-31802003000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun CA, Lai HC, Chang CC, Neih S, Yu CP, Chu TY. The significance of human papillomavirus viral load in prediction of histologic severity and size of squamous intraepithelial lesions of uterine cervix. Gynecol Oncol. 2001 Oct;83(1):95–9. doi: 10.1006/gyno.2001.6336. [DOI] [PubMed] [Google Scholar]
- 13.Sun CA, Liu JF, Wu DM, Nieh S, Yu CP, Chu TY. Viral load of high-risk human papillomavirus in cervical squamous intraepithelial lesions. Int J Gynaecol Obstet. 2002 Jan;76(1):41–7. doi: 10.1016/s0020-7292(01)00529-x. [DOI] [PubMed] [Google Scholar]
- 14.Tsai HT, Wu CH, Lai HL, Li RN, Tung YC, Chuang HY, et al. Association between quantitative high-risk human papillomavirus DNA load and cervical intraepithelial neoplasm risk. Cancer Epidemiol Biomarkers Prev. 2005 Nov;14(11 Pt 1):2544–9. doi: 10.1158/1055-9965.EPI-05-0240. [DOI] [PubMed] [Google Scholar]
- 15.Carcopino X, Henry M, Benmoura D, Fallabregues AS, Richet H, Boubli L, et al. Determination of HPV type 16 and 18 viral load in cervical smears of women referred to colposcopy. J Med Virol. 2006 Aug;78(8):1131–40. doi: 10.1002/jmv.20673. [DOI] [PubMed] [Google Scholar]
- 16.Cricca M, Morselli-Labate AM, Venturoli S, Ambretti S, Gentilomi GA, Gallinella G, et al. Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions. Gynecol Oncol. 2007 Sep;106(3):549–57. doi: 10.1016/j.ygyno.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Fiander AN, Hart KW, Hibbitts SJ, Rieck GC, Tristram AJ, Beukenholdt RW, et al. Variation in human papillomavirus type-16 viral load within different histological grades of cervical neoplasia. J Med Virol. 2007 Sep;79(9):1366–9. doi: 10.1002/jmv.20875. [DOI] [PubMed] [Google Scholar]
- 18.Forslund O, Lindqvist P, Haadem K, Czegledy J, Hansson BG. HPV 16 DNA and mRNA in cervical brush samples quantified by PCR and microwell hybridization. J Virol Methods. 1997 Dec;69(1–2):209–22. doi: 10.1016/s0166-0934(97)00161-4. [DOI] [PubMed] [Google Scholar]
- 19.Gravitt PE, Burk RD, Lorincz A, Herrero R, Hildesheim A, Sherman ME, et al. A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiol Biomarkers Prev. 2003 Jun;12(6):477–84. [PubMed] [Google Scholar]
- 20.Ho CM, Chien TY, Huang SH, Lee BH, Chang SF. Integrated human papillomavirus types 52 and 58 are infrequently found in cervical cancer, and high viral loads predict risk of cervical cancer. Gynecol Oncol. 2006 Jul;102(1):54–60. doi: 10.1016/j.ygyno.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Lai HC, Peng MY, Nieh S, Yu CP, Chang CC, Lin YW, et al. Differential viral loads of human papillomavirus 16 and 58 infections in the spectrum of cervical carcinogenesis. Int J Gynecol Cancer. 2006 Mar-Apr;16(2):730–5. doi: 10.1111/j.1525-1438.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 22.Lo KW, Yeung SW, Cheung TH, Siu NS, Kahn T, Wong YF. Quantitative analysis of human papillomavirus type 16 in cervical neoplasm: a study in Chinese population. J Clin Virol. 2005 Sep;34(1):76–80. doi: 10.1016/j.jcv.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Oikonomou P, Mademtzis I, Messinis I, Tsezou A. Quantitative determination of human telomerase reverse transcriptase messenger RNA expression in premalignant cervical lesions and correlation with human papillomavirus load. Hum Pathol. 2006 Feb;37(2):135–42. doi: 10.1016/j.humpath.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Rajeevan MS, Swan DC, Nisenbaum R, Lee DR, Vernon SD, Ruffin MT, et al. Epidemiologic and viral factors associated with cervical neoplasia in HPV-16-positive women. Int J Cancer. 2005 May;115(1):114–20. doi: 10.1002/ijc.20894. [DOI] [PubMed] [Google Scholar]
- 25.Snijders PJ, Hogewoning CJ, Hesselink AT, Berkhof J, Voorhorst FJ, Bleeker MC, et al. Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16 18, 31 and 33-positive women with normal cytology. Int J Cancer. 2006 Sep;119(5):1102–7. doi: 10.1002/ijc.21956. [DOI] [PubMed] [Google Scholar]
- 26.Swan DC, Tucker RA, Tortolero-Luna G, Mitchell MF, Wideroff L, Unger ER, et al. Human papillomavirus (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J Clin Microbiol. 1999 Apr;37(4):1030–4. doi: 10.1128/jcm.37.4.1030-1034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang-Johanning F, Lu DW, Wang Y, Johnson MR, Johanning GL. Quantitation of human papillomavirus 16 E6 and E7 DNA and RNA in residual material from ThinPrep Papanicolaou tests using real-time polymerase chain reaction analysis. Cancer. 2002 Apr;94(8): 2199–210. doi: 10.1002/cncr.10439. [DOI] [PubMed] [Google Scholar]
- 28.van Duin M, Snijders PJ, Schrijnemakers HF, Voorhorst FJ, Rozendaal L, Nobben-huis MA, et al. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int J Cancer. 2002 Apr;98(4):590–5. doi: 10.1002/ijc.10232. [DOI] [PubMed] [Google Scholar]
- 29.Josefsson AM, Magnusson PK, Ylitalo N, Sorensen P, Qwarforth-Tubbin P, Andersen PK, et al. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case–control study. Lancet. 2000 Jun;355(9222):2189–93. doi: 10.1016/S0140-6736(00)02401-6. [DOI] [PubMed] [Google Scholar]
- 30.Ylitalo N, Sorensen P, Josefsson AM, Magnusson PK, Andersen PK, Ponten J, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case–control study. Lancet. 2000 Jun;355(9222):2194–8. doi: 10.1016/S0140-6736(00)02402-8. [DOI] [PubMed] [Google Scholar]
- 31.Moberg M, Gustavsson I, Gyllensten U. Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. Int J Cancer. 2004 Dec;112(5):854–9. doi: 10.1002/ijc.20480. [DOI] [PubMed] [Google Scholar]
- 32.Moberg M, Gustavsson I, Wilander E, Gyllensten U. High viral loads of human papillomavirus predict risk of invasive cervical carcinoma. Br J Cancer. 2005 Mar;92(5):891–4. doi: 10.1038/sj.bjc.6602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz N, Hernandez-Suarez G, Mendez F, Molano M, Posso H, Moreno V, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009 Apr;100(7):1184–90. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monnier-Benoit S, Dalstein V, Riethmuller D, Lalaoui N, Mougin C, Pretet JL. Dynamics of HPV16 DNA load reflect the natural history of cervical HPV-associated lesions. J Clin Virol. 2006 Mar;35(3):270–7. doi: 10.1016/j.jcv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Morrison CS, Richardson BA, Mmiro F, Chipato T, Celentano DD, Luoto J, et al. Hormonal contraception and the risk of HIV acquisition. Aids. 2007 Jan;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 36.Else EA, Swoyer R, Zhang Y, Taddeo FJ, Bryan JT, Lawson J, et al. Comparison of real time multiplex HPV PCR assays with Inno-Lipa HPV genotyping extra assay. J Clin Microbiol. 2010 Nov; doi: 10.1128/JCM.00236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iftner T, Germ L, Swoyer R, Kjaer SK, Breugelmans JG, Munk C, et al. Study comparing human papillomavirus (HPV) real-time multiplex PCR and Hybrid Capture II INNO-LiPA v2 HPV genotyping PCR assays. J Clin Microbiol. 2009 Jul;47(7):2106–13. doi: 10.1128/JCM.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gravitt PE, Peyton C, Wheeler C, Apple R, Higuchi R, Shah KV. Reproducibility of HPV 16 and HPV 18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003 Sep;112(1–2):23–33. doi: 10.1016/s0166-0934(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 39.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006 Mar;1(24 Suppl):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Scott M, Stites DP, Moscicki AB. Th1 cytokine patterns in cervical human papillomavirus infection. Clin Diagn Lab Immunol. 1999 Sep;6(5):751–5. doi: 10.1128/cdli.6.5.751-755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jong A, van der Burg SH, Kwappenberg KM, van der Hulst JM, Franken KL, Geluk A, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002 Jan;62(2):472–9. [PubMed] [Google Scholar]
- 42.Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003 Feb;63(3):636–41. [PubMed] [Google Scholar]