Abstract
Purpose
Acuity measurement is a fundamental method to assess visual performance in the clinic. Little is known about how acuity measured in the presence of neighboring letters, as in the case of letter charts, changes with contrast and with non-foveal viewing. This information is crucial for acuity measurement using low-contrast charts and when patients cannot use their fovea. In this study, we evaluated how optotype acuity, with and without flankers, is affected by contrast and eccentricity.
Methods
Five young adults with normal vision identified the orientation of a Tumbling-E alone or in the presence of four flanking Tumbling-Es. Edge-to-edge letter spacing ranged from 1 to 20 bar widths. Stimuli were presented on a white background for 150 ms with Weber contrast ranging from −2.5% to −99%. Flankers had the same size and contrast as the target. Testings were performed at the fovea, 3, 5 and 10 degrees in the inferior visual field.
Results
When plotted as a function of letter spacing, acuity remains unaffected by the presence of flankers until the flankers are within the critical spacing, which averages an edge-to-edge spacing of 4.4 bar widths at the fovea, and approximately 16 bar widths at all three eccentricities. Critical spacing decreases with a reduction in contrast. When plotted as a function of contrast, acuity only worsens when the contrast falls below approximately 24% at the fovea and 17% in the periphery, for flanked and unflanked conditions alike.
Conclusions
The letter spacing on conventional letter charts exceeds the critical spacing for acuity measurement at the fovea, at all contrast levels. Thus these charts are appropriate for assessing foveal acuity. In the periphery, the critical spacing is larger than the letter spacing on conventional charts. Consequently, these charts may underestimate the acuity measured in the periphery due to the effects of crowding.
Keywords: acuity, contrast, eccentricity, crowding, periphery, critical spacing, critical contrast
Visual acuity measurement is one of the most fundamental methods to assess visual performance in the clinic, and the most common instrument for assessing acuity is the printed letter chart.1 Letter charts are utilized in a wide variety of situations, so it is important to understand the factors that affect acuity measurements under differing stimulus and observer conditions. The current best letter chart design is likely to be the Bailey-Lovie chart,2 or variants of it (e.g. the ETDRS chart3 or the Lea symbol chart4). A characteristic of these charts is that there are five optotypes on each line and the spacing between adjacent optotypes (one optotype width) is designed to minimize the effect of contour interaction. Contour interaction refers to the degrading effect on acuity due to the presence of nearby contours. Although acuity in the presence of this effect can be informative, for example to help detect amblyopia,4,5 the usual desire of clinicians is to avoid the deleterious influence that may introduce undesired variability to measurements of acuity.2 The adoption of a spacing of one full optotype width between adjacent optotypes on letter charts comes from the findings of Flom et al.6,7 Flom and colleagues measured the accuracy for identifying the orientation of small, high-contrast Landolt-C optotypes in the presence of flanking bars at a range of target-flanker spacings. When expressed in terms of multiples of the size of the gap of the Landolt-C, they found that flanking bars beyond 5 gap widths (equivalently ‘5 bar widths’, which equals one full letter width) had little detrimental effect on identification of the direction of the gap in the Landolt-C. However, these results, and the design of the Bailey-Lovie chart, assume foveal viewing and are based on high-contrast targets.
In the clinic, it is not uncommon to encounter patients who are unable to view a letter chart foveally, as in cases of people with central vision loss or even for patients with mild macular edema. It is necessary to understand how the measured acuity of these patients might be affected by contour interaction, or “crowding (Although Flom8 made a distinction between these two terms, we use them interchangeably9). Previous studies have shown that the deleterious effect of crowding on acuity extends over 5 bar widths in the periphery,10,11 but the maximum spatial extent of the interference, in terms of bar widths at resolution threshold, has not been quantified.8 There has been extensive study of the angular spatial extent of crowding in the periphery,12–14 and it is well known that isolated letter acuity changes with eccentricity.10,15 However, since the nominal critical spacing (the letter separation in terms of bar or letter widths necessary to overcome crowding) is dependent on both of these two variables, how it changes with eccentricity at resolution threshold remains an open question. An overview of the non-trivial issue of nominal versus angular critical spacing is discussed further in Appendix 1 (available at [LWW insert link]). The first goal of this study is to identify the nominal critical spacing in the periphery.
In addition to high contrast acuity, low contrast acuity is routinely assessed for some groups of patients in the clinic (e.g. low vision patients or patients with cataracts or corneal problems), since low contrast acuity may be more sensitive than traditional high contrast acuity in detecting certain abnormal ocular conditions.16–21 While it is now established that acuity measurements from low contrast charts viewed foveally will be less affected by crowding than their high contrast counterparts,22–27 it is unclear if this same reduction in the influence of crowding occurs peripherally. The second goal of this study is to determine the effect of contrast on the critical spacing in the periphery.
When using low contrast letter charts, how the contrast of the optotype affects the measured acuity, particularly in the presence of flanking letters in the periphery, is also an open question. For single-letter testing, it has been shown that at the fovea, above some critical contrast (the minimum contrast that still yields the maximal acuity) in the range of 20–40% Weber contrast, there is little change in letter or grating acuity with contrast.28–30 In the periphery, Thibos, et al.31 found a critical contrast of approximately 20% for resolution of gratings located at 30 degrees in the nasal visual field. The critical contrast for reading has also been identified, with values ranging from 2–5%,32 10%,33 to 20%.34 It is unknown exactly how the measurement of acuity in the presence of adjacent letters, as in the case of a letter chart, affects the critical contrast, and how the critical contrast for acuity measurement changes from foveal to peripheral viewing of a chart. If the critical contrast for a given condition (e.g. peripheral viewing of a chart) is below the contrast of the printed letters on a low contrast acuity chart, the usefulness and the effectiveness of this chart as a diagnostic aid may be affected.
Given all these considerations, the general goal of this paper is to examine the interplay of letter contrast, viewing eccentricity and letter spacing on acuity measurement. Specifically, the goals are: (1) to quantify the nominal critical spacing for high contrast optotypes in the periphery, (2) to evaluate how the nominal critical spacing changes with contrast in the periphery, and (3) to determine the critical contrast for acuity measurement in the fovea and periphery in the presence of crowding.
METHODS
Stimulus Characteristics
Acuity was measured using Tumbling E optotypes adhering to the recommended Sloan dimensions.35,36 The limbs (bars) and gaps of each character were one-fifth of the overall optotype size, which had equal width and height. For testing flanked acuity, four additional Tumbling Es appeared, located above, below, and to the left and right of the target letter. The orientation of the target and each of the four Tumbling E flankers (when present) was completely random, with the limbs of each letter pointing to the left, right, up, or down. The separation (in blank space) between the target and each of the flankers was specified as a multiple of the size of one limb of the ‘E’, occupying 1, 2, 4, 5, 10, or 20 bar widths. Eight different levels of contrast were evaluated: −2.5%, −3.4%, −6.7%, −12.5%, −22%, −44%, −70%, and −99% Weber contrast. Weber contrast is defined as (L−Lb)/Lb, where L indicates the luminance of the foreground optotypes and Lb denotes the luminance of the background. The contrast of the flankers was always the same as that of the target. The stimuli appeared in the fovea or one of three eccentricities in the lower visual field: 3°, 5°, or 10°. The stimuli were presented for 150 ms, a duration short enough to avoid voluntary saccadic eye movements to the stimulus once subjects fixated on the fixation target. As soon as the subjects responded the next stimulus appeared.
Testing Conditions
Testing took place in a dim room with less than 1 cd/m2 of ambient light. A 19″ NEC Accusync 120 CRT monitor at a resolution of 1280×1024 pixels was used. The luminance of the white background displayed on the monitor was 75 cd/m2. Luminance measurements were performed using a Minolta LS100 photometer. Subjects viewed the stimuli binocularly with their habitual correction. Distance from the monitor depended on the retinal eccentricity being tested. For the foveal condition, subjects were seated 2.4m from the monitor; for the 3° condition, at 1.8m; and for the remaining conditions (5° and 10°), 40cm from the monitor. At the farthest viewing distance (2.4m), one pixel on the monitor subtended 0.43 minutes of arc. For the eccentric conditions, a cross (which was present throughout a trial), served as the fixation target and the target E (flanked or unflanked) appeared at the appropriate eccentricity below the cross. The size of the fixation cross was 3.1mm, so the angular subtense of the cross varied with viewing distance, having a size of approximately 27′ at 40cm and 6′ at 1.8m. To avoid masking effects, no fixation cross appeared for the foveal targets. Stimuli were rendered and displayed with custom software written in the Python programming language using the PsychoPy psychophysics library.37
Subjects
Five subjects participated in this study. Table 1 lists the demographic information of the subjects. Written informed consent was obtained from each subject after the procedures of the experiment were explained, and before the commencement of data collection. The experimental protocol was approved by the Institutional Review Board at the University of California, Berkeley, and conformed to the Declaration of Helsinki.
Table 1.
Subject demographics.
| Subject | Gender | Age | Best Corrected Visual Acuity | Refractive Errors |
|---|---|---|---|---|
| S1 | M | 20 | OD: 20/20 OS: 20/20 |
OD: −5.57 DS OS: −4.75 DS |
| S2 (coauthor) | M | 27 | OD: 20/16 OS: 20/16 |
OD: −11.25 −0.50 × 003 OS: −11.75 −0.25 × 124 |
| S3 | F | 22 | OD: 20/20 OS: 20/16 |
OD: −4.00 −0.75 × 165 OS: −3.75 −0.75 × 170 |
| S4 | F | 19 | OS: 20/12.6 OS: 20/12.6 |
OD: −2.00 DS OS: −2.00 DS |
| S5 | F | 20 | OD: 20/20 OS: 20/20 |
OD: −2.50 −0.50 × 176 OS: −3.00 −0.50 × 013 |
Psychophysical Procedure
Threshold letter size (specified as the minimum angle of resolution, MAR, in units of minutes of arc) for each condition was determined using an adaptive 3-down, 1-up staircase procedure, which identified the threshold for 79% correct performance. For each staircase run, the target Tumbling E, with or without its four flankers, initially appeared at a size significantly above threshold for the selected testing eccentricity: 2° letters at the fovea, 2.5° letters at 3° eccentricity, 3° letters at 5° eccentricity, and 4° letters at 10° eccentricity. The stimulus size was reduced after three consecutive correct trials; and increased after a single incorrect trial. The amount by which the stimulus size changed after each of these reversals became progressively smaller, using the following sequence: three reversals of one log unit, four reversals of 0.2 log units, and five reversals of 0.1 log units. A single staircase ended when all 12 reversals were completed and took on average 78 trials, with 95% of the staircases taking between 50 and 200 trials. There was no systematic effect of retinal eccentricity, stimulus contrast, or letter spacing on the number of trials it took to estimate a threshold. The threshold was determined as the average of the sizes at which the reversals occurred, with the exclusion of the first two reversals, which were not used in the threshold calculation.
Testing Sequence
Every condition (eccentricity, contrast, spacing) was tested at least twice, with the order of execution determined as follows. For each subject, a random order of eccentricities was constructed from the set of eight eccentricities (four eccentricities, each appearing twice). To test an eccentricity, a random ordering of contrasts was generated. For each contrast, the order of flanker spacings (including unflanked) was randomized. For each of these conditions (eccentricity, contrast, and spacing), the software first displayed the parameters (eccentricity, contrast, and spacing) about to be tested, then commenced the staircase procedure defined in “Psychophysical Procedure to determine the threshold. Testing all conditions for one eccentricity (all contrasts and all spacings) took an hour to an hour and a half. This randomization of the sequence of trials was performed in order to minimize the effects of fatigue and practice.
Data Analysis: Fitting Individual Acuity vs. Letter Spacing
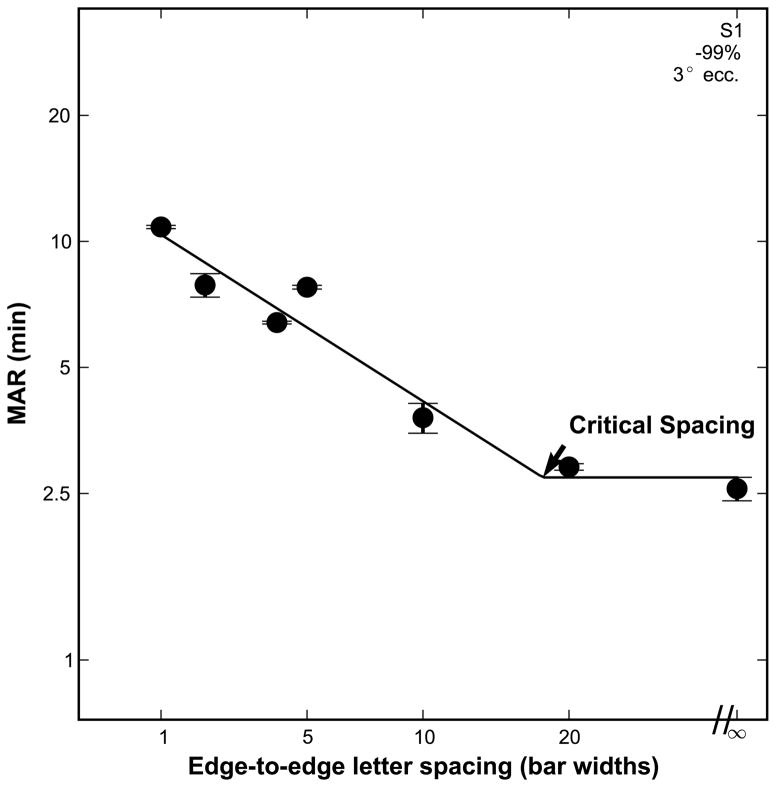
To evaluate how acuity is affected by the spacing between adjacent letters, and to derive the critical spacing for the different contrasts and eccentricities, thresholds are first analyzed as a function of letter spacing. The primary method of modeling the data is to adopt the formulation used by previous studies.38–41 This method has been used to model crowded acuity in the fovea and periphery of normal subjects, amblyopes and people with age-related macular degeneration. In this model, data for a given condition are fit using a two line function, with acuity plotted as threshold size against nominal spacing, on logarithmic axes. Figure 1 shows an example of this model with subject data collected at three degrees eccentricity in the lower visual field and with high contrast stimuli. When flankers are far from the target (or absent), acuity is unaffected by the spacing of the flankers, and the ordinate is a horizontal line. When the flankers are in close proximity to the target, acuity is affected by spacing. These data are well described by a line with a slope of negative one, which implies a complete trade-off between acuity and spacing. This complete trade-off between acuity and spacing is a direct consequence of the fixed angular size of the crowding zone at any given eccentricity.14 The intersection of the two lines is the critical spacing, by definition. The basis of this formulation is described in further detail in Appendix 2 (available at [LWW insert link]).. Although the fit is performed as described above, in this paper results are presented with the units of edge to edge letter spacing (in bar widths) on the abscissa, and MAR (in minutes) on the ordinate to better relate our findings to clinical practice. The effectiveness of this model in fitting the present data is demonstrated in the Results section. While more complex formulations have been used to fit data like ours, such as the rectangular parabola,42 the simplicity of the two-line fit, as well as the clear interpretation of its parameters, justify its use. With this fit, the dependent variable increases monotonically as flankers approach the target. Some researchers have identified a facilitation effect, whereby flankers very near the target may actually aid its identification,6,27,43,44 though with percent correct as the dependent variable. It is not clear that the same effect would be apparent when acuity is measured, nor has there been evidence of this effect in the periphery.
Figure 1.
Subject data with high contrast stimuli at 3 degrees in the lower vision field demonstrating the two line fit of acuity vs. letter spacing. The datum plotted at a letter spacing marked with “∞ represents unflanked acuity. The critical spacing is where the two lines intersect. Error bars indicate the standard deviation between the thresholds from the subject’s two separate staircase runs. To the right of the critical spacing acuity is flat, implying that it is unaffected by crowding. To the left of the critical spacing, adjacent characters are within the “crowding zone”, and thus crowding is evident. The slope in this portion is constrained to −1.
Data Analysis: Fitting Individual Acuity vs. Contrast Data
To evaluate how acuity is affected by stimulus contrast, the critical contrast for acuity measurements for the different eccentricities and letter spacings is derived. To do so, threshold is plotted as a function of contrast on log-log axes, for each eccentricity and letter spacing. The acuity versus contrast function can also be described by a two-line fit, but unlike the acuity versus spacing fit, the slope of the decreasing portion of the curve is allowed to vary. The critical contrast is defined as the contrast at which threshold begins to worsen from its optimal value, which is achieved at full contrast. This critical contrast is the value on the abscissa where the two lines intersect. A similar fit has been used previously by O’Brien et al.32 and Chung and Tjan,33 but with reading speed as the ordinate.
Curve Fitting
Curve fitting was accomplished using the scipy.opt optimization library in Python. Summed square error was minimized using the L-BFGS algorithm, an iterative fitting procedure capable of non-linear fitting. When the dependent variable was an acuity measurement, errors were minimized on a log axis, as suggested by Westheimer.45
RESULTS
Acuity vs. Letter Spacing
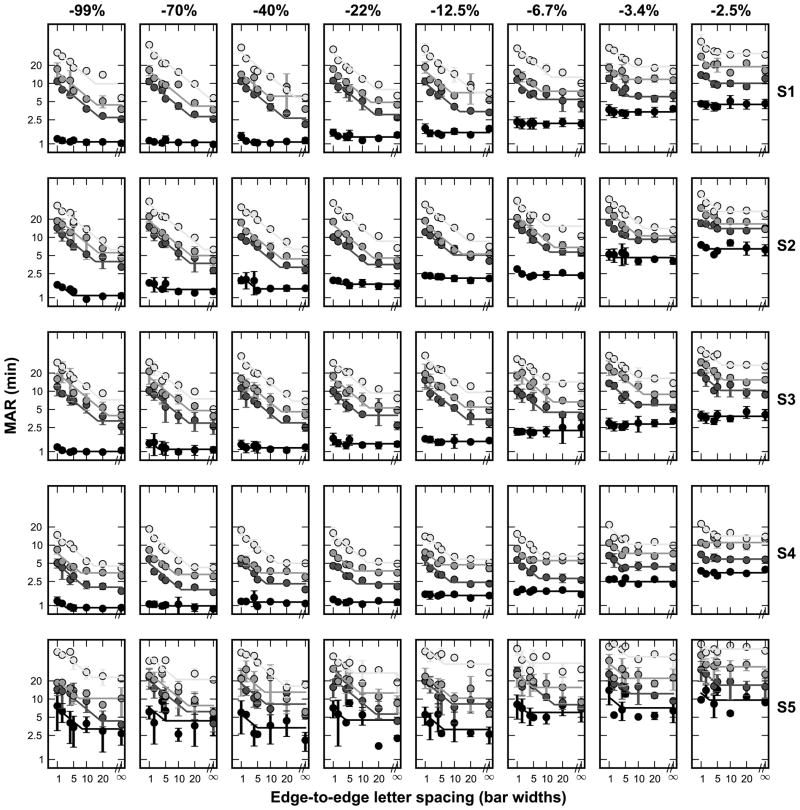
First, threshold size is analyzed as a function of nominal letter spacing. Figure 2 shows the individual subject data (S1–S5, separate rows) for all four eccentricities (different curves in each panel) at each stimulus contrast (each contrast in a column). As expected, acuity worsens as eccentricity increases, with the lowest curve (smallest threshold) representing data obtained at the fovea, and each curve above corresponding to data obtained at the more eccentric target locations. Acuity also worsens as contrast decreases (an upward shift in the family of four curves with the columns going from left to right). The unflanked foveal acuity measured at the highest contrast corresponds to a threshold of approximately one minute of arc for four of the subjects (1.08, 1.09, 1.0, and 0.92 min, respectively, for S1–S4), with much poorer acuity for S5 (3.19 min), who had higher overall variability. We suspect that location uncertainty for the foveal targets and short stimulation duration (150 ms) made the task difficult for our observers,46 which could account for why the high contrast unflanked foveal acuity was not better.
Figure 2.
Individual subject data showing acuity versus letter spacing at the four eccentricities tested (different shaded curves in each panel), at all stimulus contrasts. Each column is a given contrast and each row is a particular subject. Error bars indicate the standard deviation between the thresholds from the subject’s two separate staircase runs, and the colored lines show two-line fits. In each plot, the lowest curve comprises the foveal condition, with each successive eccentricity (3°, 5°, and 10°, respectively), stacked above.
To model the data, we use the constrained two-line fit as described above. R2 statistics for the fit to the peripheral data averaged 0.85 (+/−0.17) across all subjects and contrasts, implying that the two-line fit provides an excellent description of the peripheral data. However, in the fovea, the R2 values for the fit are typically low positive numbers, yielding an average of 0.33 (+/−0.3) across subjects and contrasts. This is due to the fact that the foveal crowding functions are relatively unaffected by the flankers for the range of spacings and contrasts tested, which is evident in Figure 2 by the flatness of the foveal curves. The two-line fit yields very small nominal critical spacings, meaning that a straight line would fit the data almost as well as the model, resulting in a low R2 despite a small sum of squared error. Regardless, it is parsimonious to have a single model that can describe the data accurately across all conditions.
The two-line fit summarizes the acuity at each condition with two parameters: the uncrowded acuity (the ordinate corresponding to the horizontal portion of the curve) and the nominal critical spacing (the abscissa corresponding to the intersection of the two lines). Table 2 lists the nominal critical spacings at −99% contrast for each subject as a function of eccentricity. To determine confidence intervals, 1000 individual Monte Carlo simulations based on the subject data were generated, and the model was fit for each simulation.47 The reported statistics indicate the mean of the fitted parameter values and the 95% confidence interval range. Table 3 shows fits at all contrasts that were tested. The foveal nominal critical spacing (averaged 4.4 bar widths) is generally much smaller than the peripheral values (15 – 20 bar widths), with the three peripheral values being very similar to each other. The average value of the foveal critical spacing (4.4 bar widths) agrees with previous reports.6 The novel contribution of this study is the finding that the nominal critical spacing in the periphery, known to be greater than 5 bar widths,8,10 is 15–20 bar widths at the eccentricities tested.
Table 2.
Nominal critical spacing at high contrast (−99%), in bar widths.
| Fovea | 3 deg | 5 deg | 10 deg | |
|---|---|---|---|---|
| S1 | 2 (0.88–3) | 18 (16–20) | 16 (8.4–26) | 15 (9.5–30) |
| S2 | 4.7 (3.6–6.6) | 15 (8.3–23) | 14 (3.2–38) | 27 (17–46) |
| S3 | 2.2 (0.95–3.1) | 16 (4.3–43) | 21 (7.5–41) | 19 (15–33) |
| S4 | 3 (−0.033−4.6) | 9.5 (3.6–15) | 7.7 (5–9.6) | 14 (9.6–17) |
| S5 | 8.2 (−0.95−35) | 17 (2–39) | 6.9 (3.4–51) | 13 (7.9–20) |
| AVG | 4.4+/−3 | 15+/−3 | 14+/−5.3 | 19+/−5.9 |
Mean of 1000 Monte Carlo simulations, with 95% confidence intervals in parentheses.
Table 3.
Nominal critical spacing (bar widths) at all contrasts, mean +/− standard deviation across subjects.
| Contrast | Fovea | 3 deg | 5 deg | 10 deg |
|---|---|---|---|---|
| −99.0% | 4.4+/−3 | 15+/−3 | 14+/−5.3 | 19+/−5.9 |
| −70.0% | 3.6+/−2.2 | 16+/−2.7 | 15+/−3.4 | 22+/−9.2 |
| −40.0% | 3.2+/−1.8 | 14+/−4.5 | 12+/−3.9 | 22+/−5.1 |
| −22.0% | 4+/−3.7 | 13+/−4 | 12+/−3.3 | 15+/−5.7 |
| −12.5% | 2.7+/−1.7 | 12+/−3.5 | 11+/−3.6 | 15+/−6.4 |
| −6.7% | 2+/−0.76 | 9.9+/−2.9 | 8.9+/−3.6 | 9.6+/−2.7 |
| −3.4% | 2.8+/−1.7 | 6.2+/−1.5 | 7.4+/−3.1 | 8.4+/−2.5 |
| −2.5% | 1.9+/−0.53 | 4.2+/−1.6 | 4.4+/−2 | 4.5+/−1.6 |
At −99% contrast, a repeated-measures ANOVA (using the software package R48) revealed a significant effect of eccentricity on critical spacing (F3,18 = 7.753, p = 0.002). Post hoc pair-wise comparison using the Tukey HSD test showed that the fovea was different from the non-foveal eccentricities (padj < 0.03 for the fovea versus each of the three eccentricities), while the non-foveal eccentricities were not different from each other (padj>0.5). As shown in Table 3, lower contrasts yielded smaller critical spacings at all eccentricities, with a larger decrease in the periphery. A repeated-measures ANOVA showed that contrast indeed had an effect on critical spacing (F7,128 = 18.351, p<0.001), although the interaction between contrast and eccentricity was not significant (F21,128=1.326, p=0.171). Furthermore, post-hoc pairwise comparison using the Tukey HSD test revealed which contrasts where significantly different from each other. Adjusted p-values from these comparisons are given in Table 4. In general, at 12.5% contrast and above, none of the corresponding critical spacings were significantly different from each other. Particularly at the lowest contrast (−2.5%), the nominal critical spacing was markedly reduced from the high contrast critical spacing, decreasing to less than 5 bar widths.
Table 4.
Pairwise significance testing of critical spacing as a function of stimulus contrast values from Table 3. Adjusted p-values are from Tukey HSD test. Bold cells are significant at 0.05 level.
| −2.5% | −3.4% | −6.7% | −12.5% | −22.0% | −40.0% | −70.0% | −99.0% | |
|---|---|---|---|---|---|---|---|---|
| −2.5% | n.s. | 0.002 | h.s. | h.s. | h.s. | h.s | h.s. | |
| −3.4% | n.s. | 0.04 | 0.003 | h.s. | h.s. | h.s. | ||
| −6.7% | n.s. | n.s. | 0.01 | 0.002 | h.s. | |||
| −12.5% | n.s. | n.s. | n.s. | n.s. | ||||
| −22.0% | n.s. | n.s. | n.s. | |||||
| −40.0% | n.s. | n.s. | ||||||
| −70.0% | n.s. |
h.s. = highly significant (adj. p<0.001)
n.s. = not significant at 0.05 level
Acuity vs. Contrast
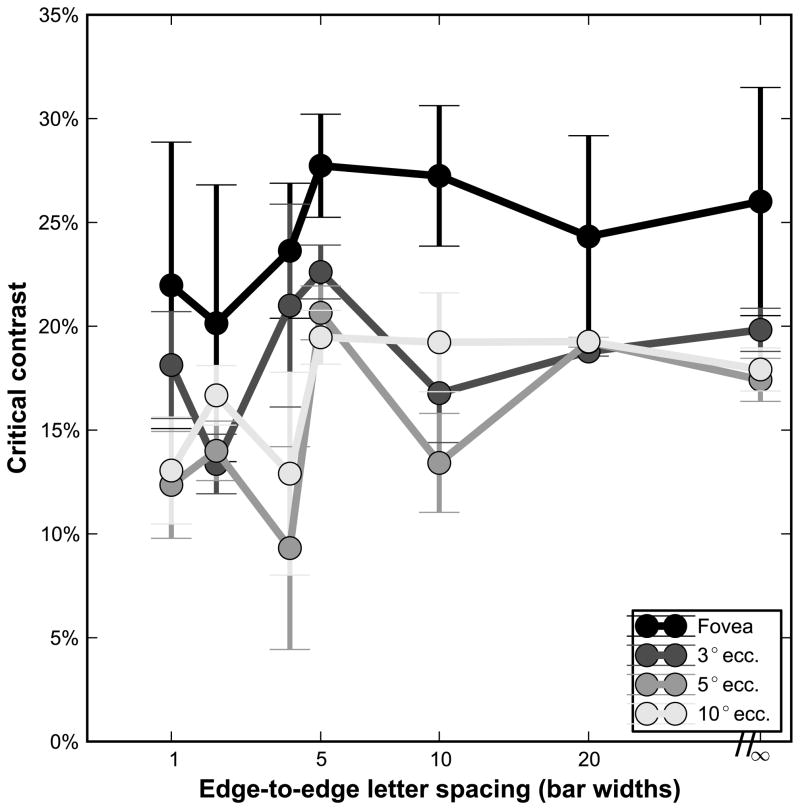
In addition to the effect of letter spacing at each eccentricity and contrast level, the effect of contrast on acuity for a given condition (eccentricity and letter spacing) was analyzed, using the unconstrained two-line fit described earlier. The main parameter of interest in this analysis is the critical contrast, the contrast value at which acuity begins to worsen with decreasing contrast. Figure 3 shows a summary of the critical contrasts at each eccentricity for all letter spacings, averaged across subjects, and Table 5 lists all the critical contrasts, averaged across subjects. Critical contrasts were on average lower in the periphery (14.5% (flanked) to 18% (unflanked)) than at the fovea (22% (flanked) to 26% (unflanked)), with similar values at the three non-foveal eccentricities. Repeated-measures ANOVA with both eccentricity and spacing as factors revealed a significant effect of eccentricity on critical contrast (F3,112 = 9.635, p<0.001), a nearly significant effect of spacing (F6,112 = 2.128, p=0.056), and no interaction (F18,112 = 0.472, p=0.97). Post-hoc pair-wise comparison using the Tukey HSD test showed that the fovea was different from the non-foveal eccentricities (padj<0.01 for the fovea versus each of the three eccentricities), while the peripheral eccentricities were not different from each other (padj>0.24).
Figure 3.
Critical contrasts for each eccentricity at the various letter spacings, averaged over all subjects. Error bars represent the standard deviation between the five subjects on the given condition.
Table 5.
Critical contrast (absolute value %) at all letter spacings, mean +/− standard deviation across subjects.
| Spacing (bar widths) | Fovea | 3 deg | 5 deg | 10 deg |
|---|---|---|---|---|
| 1 | 22+/−6.9 | 18+/−5.3 | 12+/−9.4 | 13+/−7.7 |
| 2 | 20+/−6.7 | 13+/−10 | 14+/−8.3 | 17+/−4.7 |
| 4 | 24+/−3.3 | 21+/−7.5 | 9.3+/−3.8 | 13+/−9.3 |
| 5 | 28+/−2.5 | 23+/−7.6 | 21+/−9.7 | 19+/−8.5 |
| 10 | 27+/−3.4 | 17+/−6.6 | 13+/−5.6 | 19+/−7.8 |
| 20 | 24+/−4.9 | 19+/−7 | 19+/−7.4 | 19+/−7.3 |
| Unflanked | 26+/−5.5 | 20+/−5.2 | 17+/−6 | 18+/−5.6 |
| AVG | 24+/−5.6 | 19+/−7.7 | 15+/−8.3 | 17+/−7.9 |
DISCUSSION
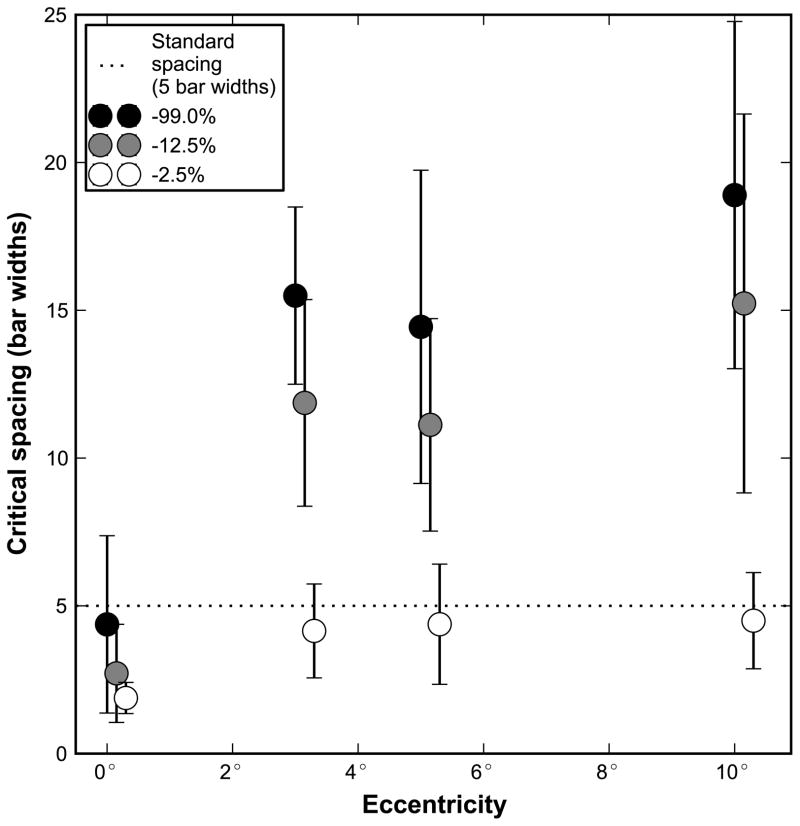
In his classic 1991 review of contour interaction and crowding,8 Flom noted that the critical spacing value of “5 gap widths in the fovea had not yet been extended to the retinal periphery. This extent has now been quantified as approximately 15–20 bar widths between 3 and 10 degrees eccentricity, as shown by the black points in Figure 4. The critical spacing is relatively invariant to changes in retinal location over this range of eccentricities. For comparison, the horizontal dotted line in Figure 4 indicates the character spacing on a modern chart designed with the principles to avoid foveal crowding.2 Note that the characters on such a chart are outside the critical spacing at the fovea (the dotted line is above the critical spacing we measured), meaning acuity is unaffected by crowding for this letter spacing. Outside the fovea, however, because the critical spacing is much larger, adjacent characters on such a chart are within the critical spacing. Thus, non-foveal acuity measurements using a traditional letter chart may not be optimal as they are limited by crowding. Figure 5 illustrates how a traditional letter chart (left side) could be modified to yield optimal acuity for peripheral viewing up to about 10 degrees (right side). Alternately, optotypes may be presented in isolation, if isolated letter cards are available. However, a clinician may be interested in assessing additional information with a letter chart, such as the search ability of patients. This is especially important for patients with central vision loss who often lose their place during reading of text or when viewing letters on an acuity chart. Even if isolated letters are used, it is important to know how much whitespace is necessary to surround a single letter, since any edges in the visual environment may cause lateral interference. Finally, if there is no alternative to using a traditional letter chart to assess peripheral acuity, in Appendix 2 (available at [LWW insert link]) we describe a simple way to predict the optimal (isolated letter) acuity based on the crowded acuity. This is possible for two reasons: 1) the crowded thresholds fall on the line with a slope of negative one as described earlier, and 2) the nominal critical spacing is roughly invariant to retinal location within 3–10 degrees eccentricity.
Figure 4.
Critical spacing plotted as a function of eccentricity for contrasts of −99% (black dots), −12.5% (gray dots), and −2.5% (white dots). Each point represents the average of the five subjects, and error bars indicate the standard deviation. The dotted line shows the spacing of standard chart designs following Bailey-Lovie guidelines, which have 1 letter width (5 bar widths) between each character. Values that fall below the dotted line indicate acuity measurements not limited by crowding based on the letter spacing of a standard letter chart; acuity measurements that fall above the line will be limited by crowding with the letter spacing recommended by Bailey-Lovie chart design. We chose to show the critical spacing for −12.5% contrast to illustrate that for the commercially available low contrast versions of the Bailey-Lovie or ETDRS charts, which have a contrast close to −12.5%, the letter spacing is smaller than the critical spacing in the periphery. Hence, acuity measured using these low contrast charts for patients who cannot view foveally may underestimate the peripheral acuity.
Figure 5.
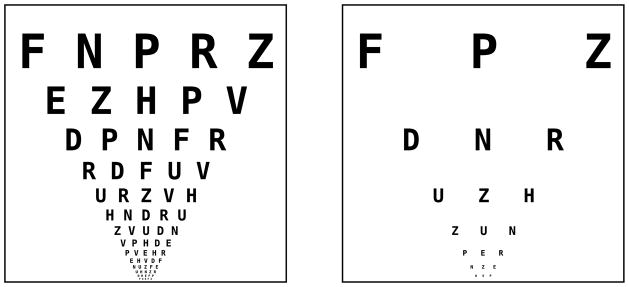
Schematic demonstration of a modified Bailey-Lovie/ETDRS chart with 3 letter widths (15 bar widths) critical spacing. Every other line was removed, and every other character of the remaining lines was removed.
Since the first groundbreaking studies of Flom et al.,6,7 there have been many explorations of crowding, but all with different aims from the present study. For high contrast targets in the periphery, critical spacing has primarily been analyzed in terms of absolute angular distance.9,12–14,42,49–51 The now well-established finding that absolute critical spacing changes linearly with eccentricity, and is independent of stimulus variables such as size is useful to researchers, but is of less interest when considering performance on letter charts, for which the character-to-character spacing is fixed physically, and the whole chart scales with distance. Furthermore, previous studies have measured thresholds in various ways that introduce confounding factors. First, some studies have measured threshold as a reduction in percent correct with stimuli of fixed size,12,49,52 which may not directly translate to results in a threshold acuity paradigm where target and flankers are size-scaled together. Others used threshold contrast for identifying fixed-size stimuli,9,14,50,53–55 which is potentially a confound for crowding in general,56 and definitely cannot be utilized if evaluating the effect of contrast on critical spacing. There are several studies that have considered high-contrast, peripheral crowding with flanker spacing measured in terms of bar-widths at resolution threshold.10,11,42,51 Jacobs10 and Leat, et al.11 did measure threshold acuity and showed that the critical spacing for crowding in the periphery exceeded five bar widths and was potentially much greater, but the maximal spatial extent was not identified. Latham and Whitaker51 and Gurnsey et al.,42 scaled target, flankers, and spacing as in the present study, and fit their data with complex mathematical functions, but did not determine the critical spacing in terms of bar widths that would be useful to peripheral letter chart design, nor did they examine the effects of contrast. Lastly, while Tripathy et al.,49 did measure the angular critical spacing in the periphery using low contrast letters, they utilized contrast to equate the effective visibility of stimuli of various sizes, whereas we systematically varied contrast and measured acuity.
We have shown that in the periphery, the nominal critical spacing is smaller when acuity was assessed using low contrast letters than with high contrast letters. The weaker effect of crowding on acuity measurement with low contrast letters has previously been shown in the fovea,22–27 and here we report a similar effect in the periphery. The effect of crowding on acuity is even weaker in the periphery, where the low contrast critical spacing is a third of the high contrast critical spacing for the lowest contrast (−2.5%), reducing from 15–20 bar widths down to 4–5 bar widths (see Table 3). At this low contrast (−2.5%), nominal edge-to-edge critical spacing in the periphery was as small as the extent of high contrast letters in the periphery (4.4 bar widths). Besides determining the critical spacing required for optimal acuity measurement using letter charts with multiple letters, we were also interested in determining the critical contrast for acuity measurement that would make the assessment of low contrast acuity useful. At the fovea, acuity is independent of contrast above a letter contrast of approximately 24%. In the periphery, this critical contrast is about 17%. These findings imply that if using a letter chart printed in a letter contrast of, for example, 20%, there will be little difference in peripheral acuity between this letter chart and the high contrast version of the chart, whereas the foveal acuity (the condition which the chart may have been designed for), would exhibit a measurable difference in acuity. In other words, the additional information that could be obtained by measuring low contrast acuities will be lost. Low contrast acuity has been shown to be more sensitive in picking up diseases,16–21 but to benefit from the measurement, the contrast should be low enough to affect acuity, particularly for the specific condition in which it is utilized, such as in the periphery. Here we show that the letters should be printed at a (Weber) contrast of 17% or lower in order for the chart to be useful in helping the diagnosis of diseases or to evaluate how contrast affects acuity. In sum, greater care should be used when employing tests based on contrast for measuring acuity in the periphery.
CONCLUSIONS
This study identified the nominal critical spacing for high contrast letters in the periphery, finding a critical spacing of approximately 15–20 bar widths from 3 to 10 degrees eccentricity in the lower vision field. This translates to a required increase in letter spacing from a one character gap (5 bars widths) to a 3–4 character gap (15–20 bars widths) if a chart is intended for use in the periphery such that the acuity measurement will not be affected by crowding. Thus modern letter charts, designed to avoid the effects of high contrast foveal crowding, will exhibit effects of crowding when used in the periphery. Two solutions to this problem were offered: the reduction in acuity due to crowding can be predicted mathematically, or optotypes should be given greater isolation when charts are used peripherally, such as illustrated in the right panel of Figure 5.
Decrease in contrast leads to reduced critical spacing (less influence of crowding) for a wide range of contrasts and eccentricities, with a greater reduction in the periphery than in the fovea. Low contrast charts used in the fovea will yield acuity measurements unaffected by crowding, as noted by numerous previous reports. In the periphery, the decrease in critical spacing is more marked (even less crowding), but the low contrast peripheral critical spacing may still exhibit more crowding than the 5 bar width spacing of traditional letter charts. The finding that there is a small (but significant) difference between the critical contrast in the fovea versus the periphery implies that care should be taken when comparing contrast-dependent effects based on peripheral acuity measurements.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health research grant R01-EY012810 and training grant T32-EY007043, and the UC Berkeley Undergraduate Research Apprentice Program. We sincerely thank the detailed helpful comments of Harold Bedell and two anonymous reviewers.
APPENDIX
The appendices are available at [LWW insert links].
References
- 1.Bailey IL. Perspective: visual acuity-keeping it clear. Optom Vis Sci. 2012;89:1247–8. doi: 10.1097/OPX.0b013e318269926f. [DOI] [PubMed] [Google Scholar]
- 2.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–5. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 4.Hyvärinen L, Näsänen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol (Copenh) 1980;58:507–11. doi: 10.1111/j.1755-3768.1980.tb08291.x. [DOI] [PubMed] [Google Scholar]
- 5.McGraw PV, Winn B, Gray LS, Elliott DB. Improving the reliability of visual acuity measures in young children. Ophthalmic Physiol Opt. 2000;20:173–84. [PubMed] [Google Scholar]
- 6.Flom MC, Weymouth FW, Kahneman D. Visual resolution and contour interaction. J Opt Soc Am. 1963;53:1026–32. doi: 10.1364/josa.53.001026. [DOI] [PubMed] [Google Scholar]
- 7.Flom MC, Heath GG, Takahashi E. Contour interaction and visual resolution: contralateral effects. Science. 1963;142:979–80. doi: 10.1126/science.142.3594.979. [DOI] [PubMed] [Google Scholar]
- 8.Flom MC. Contour interaction and the crowding effect. Probl Optom. 1991;3:237–57. [Google Scholar]
- 9.Chung STL, Levi DM, Legge GE. Spatial-frequency and contrast properties of crowding. Vision Res. 2001;41:1833–50. doi: 10.1016/s0042-6989(01)00071-2. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs RJ. Visual resolution and contour interaction in the fovea and periphery. Vision Res. 1979;19:1187–95. doi: 10.1016/0042-6989(79)90183-4. [DOI] [PubMed] [Google Scholar]
- 11.Leat SJ, Li W, Epp K. Crowding in central and eccentric vision: the effects of contour interaction and attention. Invest Ophthalmol Vis Sci. 1999;40:504–12. [PubMed] [Google Scholar]
- 12.Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226:177–8. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- 13.Toet A, Levi DM. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Res. 1992;32:1349–57. doi: 10.1016/0042-6989(92)90227-a. [DOI] [PubMed] [Google Scholar]
- 14.Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J Vis. 2004;4:1136–69. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- 15.Weymouth FW. Visual sensory units and the minimal angle of resolution. Am J Ophthalmol. 1958;46:102–13. doi: 10.1016/0002-9394(58)90042-4. [DOI] [PubMed] [Google Scholar]
- 16.Regan D, Neima D. Low-contrast letter charts in early diabetic retinopathy, ocular hypertension, glaucoma, and Parkinson’s disease. Br J Ophthalmol. 1984;68:885–9. doi: 10.1136/bjo.68.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods RL, Tregear SJ, Mitchell RA. Screening for ophthalmic disease in older subjects using visual acuity and contrast sensitivity. Ophthalmology. 1998;105:2318–26. doi: 10.1016/S0161-6420(98)91235-0. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner RC, Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106:55–7. doi: 10.1001/archopht.1988.01060130061028. [DOI] [PubMed] [Google Scholar]
- 19.Regan D, Neima D. Low-contrast letter charts as a test of visual function. Ophthalmology. 1983;90:1192–200. doi: 10.1016/s0161-6420(83)34407-9. [DOI] [PubMed] [Google Scholar]
- 20.Schneck ME, Haegerstrom-Portnoy G, Lott LA, Brabyn JA, Gildengorin G. Low contrast vision function predicts subsequent acuity loss in an aged population: the SKI study. Vision Res. 2004;44:2317–25. doi: 10.1016/j.visres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Haegerstrom-Portnoy G. The Glenn A. Fry Award Lecture 2003: Vision in elders--summary of findings of the SKI study. Optom Vis Sci. 2005;82:87–93. doi: 10.1097/01.opx.0000153162.05903.4c. [DOI] [PubMed] [Google Scholar]
- 22.Kothe AC, Regan D. Crowding depends on contrast. Optom Vis Sci. 1990;67:283–6. doi: 10.1097/00006324-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Bailey IL, Raasch TW, Koh P, Hetland M, Park A. Non-invasive Assessment of the Visual System, OSA Technical Digest Series. Vol. 3. Washington, DC: Optical Society of America; 1993. Contour interaction with high and low contrast charts; pp. 228–31. [Google Scholar]
- 24.Giaschi DE, Regan D, Kraft SP, Kothe AC. Crowding and contrast in amblyopia. Optom Vis Sci. 1993;70:192–7. doi: 10.1097/00006324-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Pascal E, Abadi RV. Contour interaction in the presence of congenital nystagmus. Vision Res. 1995;35:1785–9. doi: 10.1016/0042-6989(94)00277-s. [DOI] [PubMed] [Google Scholar]
- 26.Simmers AJ, Gray LS, McGraw PV, Winn B. Contour interaction for high and low contrast optotypes in normal and amblyopic observers. Ophthalmic Physiol Opt. 1999;19:253–60. [PubMed] [Google Scholar]
- 27.Siderov J, Waugh SJ, Bedell HE. Foveal contour interaction for low contrast acuity targets. Vision Res. 2013;77:10–3. doi: 10.1016/j.visres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Herse PR, Bedell HE. Contrast sensitivity for letter and grating targets under various stimulus conditions. Optom Vis Sci. 1989;66:774–81. doi: 10.1097/00006324-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 29.van Nes FL, Jacobs JC. The effect of contrast on letter and word recognition. IPO Ann Prog Rep. 1981;16:72–80. [Google Scholar]
- 30.Ludvigh E. Effect of reduced contrast on visual acuity as measured with Snellen test letters. Arch Opthlalmol. 1941;25:469–74. [Google Scholar]
- 31.Thibos LN, Still DL, Bradley A. Characterization of spatial aliasing and contrast sensitivity in peripheral vision. Vision Res. 1996;36:249–58. doi: 10.1016/0042-6989(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien BA, Mansfield JS, Legge GE. The effect of contrast on reading speed in dyslexia. Vision Res. 2000;40:1921–35. doi: 10.1016/s0042-6989(00)00041-9. [DOI] [PubMed] [Google Scholar]
- 33.Chung STL, Tjan BS. Spatial-frequency and contrast properties of reading in central and peripheral vision. J Vis. 2009;9:16, 1–9. doi: 10.1167/9.9.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legge GE, Rubin GS, Luebker A. Psychophysics of reading. V. The role of contrast in normal vision. Vision Res. 1987;27:1165–77. doi: 10.1016/0042-6989(87)90028-9. [DOI] [PubMed] [Google Scholar]
- 35.Sloan LL. New test charts for the measurement of visual acuity at far and near distances. Am J Ophthalmol. 1959;48:807–13. doi: 10.1016/0002-9394(59)90626-9. [DOI] [PubMed] [Google Scholar]
- 36.Committee on Vision. Recommended standard procedures for the clinical measurement and specification of visual acuity. Report of working group 39. Assembly of Behavioral and Social Sciences, National Research Council, National Academy of Sciences, Washington, DC. Adv Ophthalmol. 1980;41:103–48. [PubMed] [Google Scholar]
- 37.Peirce JW. Generating stimuli for neuroscience using PsychoPy. Front Neuroinform. 2008;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song S, Levi DM, Pelli DG. Size and spacing limit letter identification, with promise of improved visual screening for amblyopia. J Vis. doi: 10.1167/14.5.3. (in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S. PhD dissertation. University of California; Berkeley: 2009. Acuity, Crowding, Feature Detection, and Fixation in Normal and Amblyopic Vision. [Google Scholar]
- 40.Pelli D, Song S, Levi D. Improving the screening of children for amblyopia. J Vis. 2011;11(11):411. [Google Scholar]
- 41.Chung STL. Size or spacing: which limits letter identification in people with AMD? Invest Ophthalmol Vis Sci. 2011;52:E-Abstract 1194. [Google Scholar]
- 42.Gurnsey R, Roddy G, Chanab W. Crowding is size and eccentricity dependent. J Vis. 2011;11:15. doi: 10.1167/11.7.15. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi E. PhD dissertation. University of California; Berkeley: 1968. Effects of Flanking Contours on Visual Resolution at Foveal and Near-foveal Loci. [Google Scholar]
- 44.Danilova MV, Bondarko VM. Foveal contour interactions and crowding effects at the resolution limit of the visual system. J Vis. 2007;7:25:1–18. doi: 10.1167/7.2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westheimer G. Scaling of visual acuity measurements. Arch Ophthalmol. 1979;97:327–30. doi: 10.1001/archopht.1979.01020010173020. [DOI] [PubMed] [Google Scholar]
- 46.Baron WS, Westheimer G. Visual acuity as a function of exposure duration. J Opt Soc Am. 1973;63:212–9. doi: 10.1364/josa.63.000212. [DOI] [PubMed] [Google Scholar]
- 47.Kingdom FAA, Prins N. Psychophysics: A Practical Introduction. London: Elsevier/Academic Press; 2009. [Google Scholar]
- 48.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 49.Tripathy SP, Cavanagh P. The extent of crowding in peripheral vision does not scale with target size. Vision Res. 2002;42:2357–69. doi: 10.1016/s0042-6989(02)00197-9. [DOI] [PubMed] [Google Scholar]
- 50.Strasburger H, Harvey LO, Jr, Rentschler I. Contrast thresholds for identification of numeric characters in direct and eccentric view. Percept Psychophys. 1991;49:495–508. doi: 10.3758/bf03212183. [DOI] [PubMed] [Google Scholar]
- 51.Latham K, Whitaker D. Relative roles of resolution and spatial interference in foveal and peripheral vision. Ophthalmic Physiol Opt. 1996;16:49–57. [PubMed] [Google Scholar]
- 52.Hess RF, Dakin SC, Kapoor N, Tewfik M. Contour interaction in fovea and periphery. J Opt Soc Am (A) 2000;17:1516–24. doi: 10.1364/josaa.17.001516. [DOI] [PubMed] [Google Scholar]
- 53.Hariharan S, Levi DM, Klein SA. “Crowding” in normal and amblyopic vision assessed with Gaussian and Gabor C’s. Vision Res. 2005;45:617–33. doi: 10.1016/j.visres.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 54.Levi DM, Klein SA, Hariharan S. Suppressive and facilitatory spatial interactions in foveal vision: foveal crowding is simple contrast masking. J Vis. 2002;2:140–66. doi: 10.1167/2.2.2. [DOI] [PubMed] [Google Scholar]
- 55.Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in peripheral vision: peripheral crowding is neither size invariant nor simple contrast masking. J Vis. 2002;2:167–77. doi: 10.1167/2.2.3. [DOI] [PubMed] [Google Scholar]
- 56.Petrov Y, Popple AV, McKee SP. Crowding and surround suppression: not to be confused. J Vis. 2007;7:12:1–9. doi: 10.1167/7.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.