Abstract
The specific locations of double bonds in mammalian lipids have profound effects on biological membrane structure, dynamics and lipid second messenger production. Herein, we describe a shotgun lipidomics approach that exploits charge-switch derivatization with N-(4-aminomethylphenyl) pyridinium (AMPP) and tandem mass spectrometry for identification and quantification of fatty acid double bond positional isomers. Through charge-switch derivatization of fatty acids followed by positive-ion mode ionization and fragmentation analysis, a marked increase in analytic sensitivity (low fmol/μL) and the identification of double bond positional isomers can be obtained. Specifically, the locations of proximal double bonds in AMPP-derivatized fatty acids are identified by diagnostic fragment ions resulting from the markedly reduced 1,4-hydrogen elimination from the proximal olefinic carbons. Additional fragmentation patterns resulting from allylic cleavages further substantiated the double bond position assignments. Moreover, quantification of fatty acid double bond positional isomers is achieved by the linear relationship of the normalized intensities of characteristic fragment ions vs. the isomeric compositions of discrete fatty acid positional isomers. The application of this approach for the analysis of fatty acids in human serum demonstrated the existence of two double bond isomers of linolenic acid (i.e., Δ6,9,12 18:3, γ-linolenic acid (GLA), and Δ9,12,15 18:3, α-linolenic acid (ALA)). Remarkably, the isomeric ratio of GLA vs. ALA esterified in triglycerides was three-fold higher than the ratio of their non-esterified moieties. Through this developed method, previously underestimated or unidentified alterations in fatty acid structural isomers can be determined facilitating the identification of novel biomarkers and maladaptive alterations in lipid metabolism during disease.
Keywords: Mass spectrometry, shotgun lipidomics, unsaturated fatty acids, double bond position, charge-switch derivatization
INTRODUCTION
The diversity of fatty acids (FAs) in mammalian organisms fulfills multiple critical roles in cellular functions through: 1) covalent tailoring of aliphatic chains to create a wide variety of discrete lipid molecular species in biological membranes; 2) modulation of membrane curvature, stereoelectronic interactions and membrane dynamics; and 3) provision of a diverse repertoire of precursors for the generation of lipid signaling molecules.1–6 The diversified structures of FAs include varied chain lengths, varied degrees of unsaturation and locations of double bonds in aliphatic chains, oxidized, branched-chain and other modified structures.1 Alterations in FA composition and structure have been demonstrated to have potent effects on membrane function, bioenergetic efficiency and cellular signaling cascades that efficiently regulate integrated biologic functions in health but adversely impact the progression of multiple disease processes.1,3,7–13
Mass spectrometry (MS) in conjunction with chromatography has been a major tool for the analysis of FAs in biological samples. Electrospray ionization (ESI) MS coupled with liquid chromatography (LC) has dramatically improved the analysis of FAs in comparison to traditional electron ionization GC-MS.14–19 Typically, the LC/MS approaches target the carboxylate anions ionized in the negative-ion mode which limit not only the analytical sensitivity but also the unambiguous structural identification of FAs. This is largely due to the diminutive amounts of informative fragments generated particularly from polyunsaturated FA anions which readily lose CO2.14,19 Thus, the precise identification of FA structural isomers has been challenging in lipidomics. Recently, charge-remote fragmentation in mass spectrometry has facilitated the identification of FA double bond locations using high-energy collisional induced dissociation (CID)20–22 and more recently using low-energy CID19,23–25 of precursor ions ionized either in the negative-ion mode (i.e., as carboxylate anions14,19) or in the positive-ion mode (e.g., as metal adduct ions23,24). However, the broader application of these approaches is limited by low ionization efficiency and excessive fragmentation leading to difficulties in spectral interpretation. Ozonolysis has been utilized to locate double bond positions through the injection of ozone into either the ion source (cleaving all precursor ions) or an ion trap or collision chamber after precursor ion selection. 26–31 More recently, additional techniques have been developed, including low temperature plasma (LTP) ionization MS,32 radical-directed dissociation (RDD) MS,15,33 metal-adduction and electron-induced dissociation (EID) MS,34 and dual stage CID coupled with ion mobility MS.35 Each of the techniques has its own strengths, but the necessity of specialized instrumentation and/or the generation of complex fragmentation patterns have precluded their widespread use.
Previously, we developed a method for the analysis of FA double bond positions through direct infusion using ESI/MS in the negative-ion mode by determination of the collision energy-induced intensity distribution of the specific fragment ion after loss of CO2 or H2O from carboxylate anions.36 However, this approach suffers from the relatively low sensitivity present in the negative-ion mode and the less sensitive loss of CO2 or H2O from monounsaturated FA precursors. To overcome the low ionization efficiency in the negative-ion mode, charge-switch derivatization has been previously used to increase the sensitivity of carboxylic acid analysis by LC/MS approaches.17,37–40 Recently, Bollinger et al reported the utilization of N-(4-aminomethylphenyl) pyridinium (AMPP) derivatization for eicosanoid analysis by LC-MS/MS with a dramatic improvement in sensitivity and the production of structurally informative fragment ions during CID.41
Herein, using a shotgun lipidomics approach we demonstrate that tandem mass spectrometry of AMPP-derivatized unsaturated FAs provides multiple diagnostic fragment ions that unambiguously identify the locations of double bonds. Using this strategy we identified substantial differences in double bond positional isomers of non-esterified vs. esterified polyunsaturated FAs in human serum. Collectively, this methodology concurrently identifies and quantifies FA double bond isomers with high sensitivity and specificity thus providing insights in the alterations of FA metabolism in health and disease.
MATERIALS AND METHODS
All reagents, fatty acids and a triglyceride standard were purchased commercially and utilized without further purification. Full details are provided in Supporting Information.
Lipid extracts were prepared from 100 GL of pooled normal human serum by a modified Bligh and Dyer procedure as previously described. 42 An aliquot of the lipid extract was then extracted with hexane to isolate triglycerides (TGs) from the total chloroform extract, followed by base hydrolysis to release FAs from TGs. Detailed experimental procedures are provided in Supporting Information. Individual FA stock solutions, non-esterified (free) FAs present in the total lipid extract or esterified FAs released from TGs were derivatized with AMPP essentially by the method of Bollinger et al,41 followed by extraction with butanol/water to remove excess water-soluble reagents as described in Supporting Information. Mass spectrometric analysis of AMPP-derivatized FAs was performed using a TSQ Quantum Ultra mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with an automated nanospray apparatus (i.e., Nanomate HD, Advion BioSciences, Ithaca, NY) as previously described.42,43 Product ion scanning or precursor ion scanning was performed in the positive-ion mode. Detailed instrument operation conditions are provided in Supporting Information.
RESULTS AND DISCUSSION
Tandem Mass Spectrometric Analysis of AMPP-derivatized Fatty Acids to Specifically Profile Fatty Acids with Exquisitely High Sensitivity
Tandem mass spectra of an AMPP-derivatized FA internal standard (i.e., d4-16:0 FA) demonstrated two major fragment ions present at m/z 169 and 183 arising from cleavage of the AMPP tag41 (Scheme 1 and Fig. 1B inset). Tandem mass spectra of a variety of AMPP-derivatized FAs differing in chain length, degree of unsaturation and double bond position(s) also consistently demonstrated the fragment ion at m/z 183 in high abundance (Figs. 2–5 and S1–S5). Accordingly, precursor ion scanning (PIS) of m/z 183 of AMPP-derivatized FAs can globally profile the wide diversity of FAs present in biological samples.
Scheme 1. Proposed fragmentation routes for AMPP-derivatized unsaturated FAs.
The fragmentation pathway for AMPP-derivatized Δ9,12,15 18:3 FA is presented. The C-C bond highlighted in red in the fragmentation intermediates indicates the site of cleavage.
Figure 1. Mass spectrometric analysis of FA with or without AMPP derivatization at varied concentrations.

AMPP-derivatized FA internal standard (I.S.), d4-16:0 FA, was analyzed by full MS scan (A) and precursor ion scan (PIS) of m/z 183 (B) in the positive-ion mode. A representative spectrum of product ion scan of AMPP-derivatized d4-16:0 FA is presented in red inset. The FA anion was analyzed by full MS scan (C) in the negative-ion mode. The mass spectra in panels (a)–(d) are from FA solutions having sequentially decreasing concentrations and all are presented by the same y-axis scale as demonstrated in panels (a). The peak at m/z 255 in (C) represents a contaminant having the mass of 16:0 FA that is absent in (A) and (B).
Figure 2. Tandem mass spectra of AMPP-derivatized FAs having identical chain length and identical proximal double bond positions but different degrees of unsaturation.
The tandem mass spectra for 18 carbon-containing saturated, mono-, di- or tri-unsaturated FAs with a proximal double bond at C9 are shown in panels (a)–(d), respectively. “*” indicates the diagnostic fragment ions that demonstrate a dramatic reduction in intensity in comparisons to the corresponding saturated FA and to the intensities of their preceding smaller fragment ions. “#” indicates the 40 Th series of fragment ions that are separated by 40 Th (as described in text). The red insets are vertically enlarged spectra.
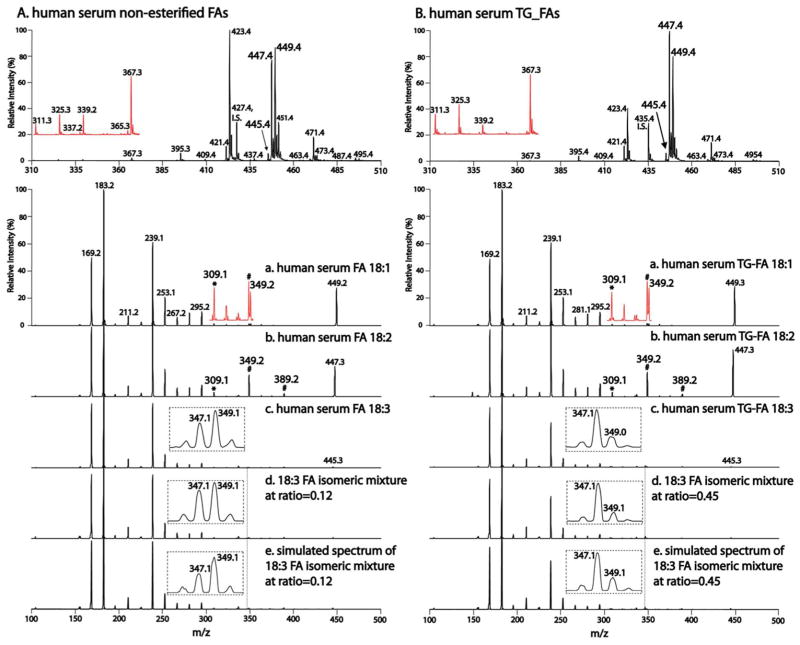
Figure 5. Analysis of FA double bond positional isomers present in human serum.
AMPP-derivatized non-esterified FAs and esterified FAs in triglycerides (TG-FA) in pooled normal human serum samples were prepared as described in Materials and Methods. The top panels are PIS of m/z 183 spectra for non-esterified FAs (A) and TG-FAs (B), where I.S. stands for the internal standards of non-esterified FA (i.e., d4-16:0 FA) in (A) or esterified FA released from the TG internal standard (i.e., 17:1 TG) in (B). The red insets are vertically enlarged spectra. Panels (a)–(c) are tandem mass spectra of 18:1, 18:2 and 18:3 FAs in human serum. “*” and “#”are as indicated in Fig. 2. Panel (d) represents the tandem mass spectra of the 18:3 FA isomeric mixtures prepared from the authentic isomers at the calculated compositions from (c). Panel (e) represents the simulated in silico spectra of the same 18:3 FA isomeric mixtures.
To examine the sensitivity and specificity of FA profiling through PIS of m/z 183, the full MS scan and PIS of m/z 183 of AMPP-derivatized d4-16:0 FA in the positive-ion mode were compared to the full MS scan of d4-16:0 FA anion in the negative ion mode (Fig. 1). The results demonstrated a 25-fold increase in ionization efficiency resulting from the charge switch (Figs. 1A_a vs. 1C_a). In addition, the utility of PIS of m/z 183 of AMPP-derivatized FAs for FA profiling has multiple advantages over direct profiling of FAs in the negative-ion mode. These include: (1) a reduction in background noise and the resultant increase in sensitivity (e.g., an S/N of > 200 in the PIS of m/z 183 of AMPP-derivatized d4-16:0 FA vs. an S/N of < 2 in the full MS scan of d4-16:0 FA anion at 5 fmol/μL (Figs. 1B_d vs. 1C_d)); and (2) an improvement in analytical specificity through elimination of interferences from background ions, contaminants, or low mass fragment ions emanating from a variety of mechanisms that only become evident in the low abundance regime (Fig. 1C).
Identification of Diagnostic Fragment Ions of AMPP-derivatized Fatty Acids to Localize Double Bond Positions
In addition to the two major fragment ions present at m/z 169 and m/z 183, tandem mass spectra of AMPP-derivatized saturated FA (18:0) demonstrated a well-defined series of fragment ions differing by 14 Th (Fig. 2a). These ions likely result from successive cleavages of methylene (CH2) groups from the saturated aliphatic chain23 (Fig. 2 inserted structure). In contrast, tandem mass spectra of AMPP-derivatized unsaturated FAs revealed substantially different fragmentation patterns (Fig. 2b–d). As anticipated, the presence of double bond(s) disrupts the continuity of ions corresponding to the successive cleavages of methylene groups. Specifically, three AMPP-derivatized unsaturated FAs (i.e., Δ9 18:1, Δ9,12 18:2 and Δ9,12,15 18:3 FAs) were analyzed, each having identical carbon numbers (18 carbons), identical positions of the proximal double bond (at C9) but containing different numbers (one, two or three) of double bonds. Tandem mass spectra of these three unsaturated FAs were remarkable for a dramatic decrease in the intensity of the fragment ion at m/z 309 while the pattern of fragment ions smaller than m/z 309 was strikingly similar to the tandem mass spectrum of 18:0 FA (Fig. 2). For example, the intensity ratio of the fragment ion at m/z 309 vs. its closest preceding fragment ion at m/z 295 is 0.9 in the tandem mass spectrum of 18:0 FA (Fig. 2a). This intensity ratio is reduced to 0.06, 0.3 and 0.2 (see discussion below) in the tandem mass spectra of Δ9 18:1 FA, Δ9,12 18:2 FA and Δ9,12,15 18:3 FA, respectively (Fig. 2b–d). This specific loss of the m/z 309 fragment ion (formed by cleavage of C8–C9 bond in saturated aliphatic chains) is diagnostic and can be used to assign the position of proximal double bond at C9 for each of these three unsaturated FAs. Since the double bonds within an aliphatic chain are typically separated by a single methylene group (i.e., the divinylmethane pattern) in most naturally-occurring polyunsaturated FAs,44 the identification of the proximal double bond position provides important information on the positions of the other double bonds that was verified by independent analysis of additional informative fragment ions as described below.
Tandem mass spectra of the three AMPP-derivatized unsaturated FAs exhibited an additional fragment ion at m/z 349 that was separated from the m/z 309 fragment ion by 40 Th (Fig. 2 red insets) and was 2 Th less than the corresponding m/z 351 fragment ion in the mass spectrum of 18:0 FA (Fig. 2a). This 2 Th mass shift indicated the presence of an additional double bond in the m/z 349 fragment ion in comparison to the m/z 351 fragment ion (formed by the cleavage of C11–C12 bond), resulting from the presence of the proximal double bond at C9 prior to the cleavage site. Similarly, a fragment ion at m/z 389 (separated from m/z 349 by 40 Th) was also present in the tandem mass spectra of 18:2 and 18:3 FAs, as well as a fragment ion at m/z 429 (separated from m/z 389 by 40 Th) in the tandem mass spectrum of 18:3 FA. This series of fragment ions, with members separated by 40 Th (hereafter termed the 40 Th series of fragment ions), substantiate the presence of the divinylmethane pattern thereby confirming the assignments of the other double bond locations (i.e., at C12, C15) in polyunsaturated FAs containing the identified proximal double bond position (i.e., at C9).
Although much less common, there are some polyunsaturated FAs that either have double bonds separated by more than one methylene group or possess conjugated double bonds.44,45 The utilization of the 40 Th series of fragment ions can discriminate these positional isomers from the more commonly occurring bis-allylic structures. Tandem mass spectra of conjugated Δ9,11 18:2 FA demonstrated the presence of a diagnostic fragment ion at m/z 309 indicating that the proximal double bond was present at C9. However, the spectrum lacked the 40 Th series of fragment ions indicating the absence of methylene group after the proximal double bond thus demonstrating the presence of conjugated double bonds (Fig. S1).
The formation of the 40 Th series of fragment ions was previously observed for dilithiated unsaturated FA adducts under low energy CID and was proposed to result from allylic cleavages between double bonds.23,24 Similarly, the tandem mass spectrometric analysis of AMPP-derivatized unsaturated FAs generated the 40 Th series of fragment ions separated from each other by a –CH2CH=CH– (40 Da) unit (Scheme 1). In contrast to the 40 Th series of fragment ions whose m/z values are larger than the diagnostic fragment ion at m/z 309, the fragment ions of m/z values smaller than m/z 309 were present in a similar pattern in tandem mass spectra of unsaturated FAs compared to the corresponding saturated FA (Fig. 2). Possible fragmentation mechanisms leading to formation of those smaller fragment ions include 1,4-hydrogen elimination followed by homolytic scission and subsequent radical elimination23,46 (Scheme 1). This mechanism was substantiated by examination of tandem mass spectra of AMPP-derivatized stable isotope labeled FA (i.e., 7,7,8,8-d4-16:0 FA; see Scheme S1 and Fig. S2). Notably, the fragment ions at m/z 295 and 309 would be difficult to form through 1,4-hydrogen elimination due to the energetically unfavorable extraction of the olefinic hydrogen (i.e., hydrogen from C9 or C10). However, the m/z 295 fragment ion was likely formed through the cleavage of C7–C8 bond resulting from β-cleavage by a McLaffery rearrangement24 (Scheme 1). Thus, the formation of the fragment ion at m/z 309 was dramatically reduced due to the lack of alternative routes of generation, leading to a signature fragment ion that enables the unambiguous assignment of the proximal double bond position.
Utilization of Diagnostic Fragment Ions of AMPP-derivatized Fatty Acids to Identify Double Bond Positional Isomers
Three AMPP-derivatized 18:1 FA isomers were each analyzed individually (Fig. 3). The double bond positions (i.e., at C6, C9 or C11) were identified utilizing the diagnostic fragment ions at m/z 267, m/z 309 or m/z 337 that were dramatically reduced in intensity. For example, the intensity ratio of the fragment ion at m/z 267 to its closest preceding fragment ion at m/z 253 is 0.7 vs. 0.07 in the tandem mass spectra of 18:0 FA (Fig. 2a) and Δ6 18:1 FA (Fig. 3a). The intensity ratio of the fragment ion at m/z 337 to its closest preceding fragment ion at m/z 323 is 0.9 vs. 0.06 in the tandem mass spectra of 18:0 FA (Fig. 2a) and Δ11 18:1 FA (Fig. 3c). The 40 Th fragment ions at m/z 307, m/z 349 or m/z 377 (reflecting 40 Th separations from m/z 267, m/z 309 or m/z 337, respectively) were also present (Fig. 3 red insets), thereby validating the assignment of the double bond positions. As anticipated, these diagnostic fragment ions are specific to the locations of double bonds and are therefore least affected by the length of the saturated aliphatic portion after the last double bond. This conclusion was confirmed by the nearly identical tandem mass spectra of Δ11 18:1 FA (Fig. 3c) and Δ11 20:1 FA (Fig. 3d).
Figure 3. Tandem mass spectra of AMPP-derivatized monounsaturated FAs having different double bond positions.
The tandem mass spectra presented in panels (a)–(c) are from three double bond positional isomers of 18:1 FA with double bond located at C6, C9 or C11, respectively. The tandem mass spectrum in panel (d) is from Δ11 20:1 FA. “*” and “#” are as indicated in Fig. 2. The red insets are vertically enlarged spectra.
To further demonstrate the potential widespread application of the unique diagnostic fragment ions to a broad range of double bond locations, a series of monounsaturated FAs possessing double bonds located at different positions from C5 to C15 were analyzed (Fig. S3). The diagnostic fragment ions defining the location of the proximal double bonds were determined by the signature reduction in their intensities (Fig. S3, dotted line). In parallel to these diagnostic fragment ions, the 40 Th informative fragment ions were also present (Fig. S3 red insets), further substantiating the assigned double bond positions. Tandem mass spectrometric analysis of AMPP-derivatized polyunsaturated FA isomers (e.g., Δ5,8,11,14 and Δ8,11,14,17 20:4 FAs) were also performed (Fig. S4). The proximal double bond positions (i.e., C5 or C8) were identified through the diagnostic fragment ions present at m/z 253 or m/z 295, respectively. The presence of the 40 Th series of fragment ions substantiated the proximal double bond assignment and the presence of the divinylmethane pattern. Additional examples of the utility of this method to distinguish a wide variety of FA double bond positional isomers are presented in Fig. S5.
Quantitation of Fatty Acid Double Bond Positional Isomers
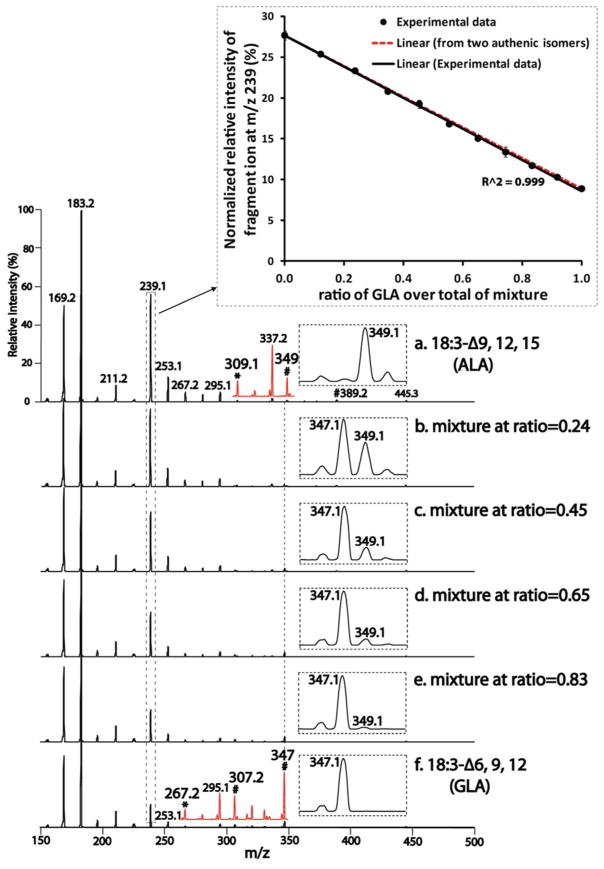
As described above, individual FA double bond positional isomers can be unambiguously identified using the diagnostic fragment ions that define proximal double bond positions in conjunction with the 40 Th series of fragment ions. In biological samples, mixtures of FA double bond isomers are typically present.47 Accordingly, the precise quantification of the relative amounts of distinct FA isomers is of substantial importance in identifying the specific roles that each isomer plays in biologic processes. We examined tandem mass spectra of the mixtures of 18:3 FA isomers (i.e., Δ6,9,12 18:3, γ-linolenic acid (GLA), and Δ9,12,15 18:3, α-linolenic acid (ALA)). The results demonstrated diagnostic fragmentation patterns containing spectral features corresponding to each isomer (Fig. 4). For example, the spectra of these mixtures displayed two informative fragment ions at m/z 349 and m/z 347 that are indicative of the presence of ALA and GLA, respectively (Fig. 4 insets). Those features, although specific and unambiguous in determining the coexistence of these isomers in a mixture, are in relatively low abundance and thus render precise quantitation difficult. Another informative mass spectral feature of these FA isomers is the marked (over 4-fold) decrease in the relative intensity of the fragment ion at m/z 239 in the tandem mass spectra of GLA (Fig. 4f) vs. ALA (Fig. 4a). As demonstrated in Scheme 1, this fragment ion (i.e., AMPP-derivatized prop-2-enoic acid) arises from the cleavage of C3–C4 bond through 1,4-hydrogen elimination (i.e., from C2 and C5), and is stabilized by the resultant α,β-unsaturated amide leading to its relatively high abundance. The decreased intensity of this fragment ion in tandem mass spectrum of GLA results from a decreased rate of hydrogen elimination from C2 and C5. This is likely due to the presence of unsaturation at both C1 and C6 (i.e., C=O carbonyl and C6=C7 olefin) leading to stereoelectronic alterations that impede 1,4-hydrogen elimination (Scheme S1). Accordingly, this fragment ion is also present in reduced intensity in tandem mass spectra of unsaturated FAs containing double bonds at C4 or C5 due to the difficulty in extraction of olefinic hydrogen. Examples supporting this conclusion include Δ6 18:1 FA (Fig. 3a), Δ5 20:1 FA (Fig. S3a), Δ5,8,11,14 20:4 FA (Fig. S4a), Δ5,8,11 20:3 FA (Fig. S5B_b), and Δ4,7,10,13,16 22:5 FA (Fig. S5C_b).
Figure 4. Quantitation of AMPP-derivatized FA double bond positional isomers.
The tandem mass spectra in panels (a) and (f) are from 18:3 FA isomers, Δ9,12,15 (i.e., ALA) and Δ6,9,12 (i.e., GLA), respectively. “*” and “#”are as indicated in Fig. 2. The red insets are vertically enlarged spectra. The tandem mass spectra in panels (b)–(e) are from mixtures of GLA and ALA at different isomeric compositions (represented by the percentages of GLA in the mixtures containing different molar ratios of GLA and ALA). The framed small insets are expanded spectra. The framed large inset represents the plot of normalized intensity of the m/z 239 fragment ion (as described in text) vs. the isomeric compositions of mixtures. Data points represent the mean ± SD of three measurements. Two linear relationships are displayed: the one established from the experimental data (solid line), and the one from the two data points (red dashed line) of authentic isomers (i.e., ALA and GLA).
The marked (over 4-fold) difference in intensity of this relatively high abundance m/z 239 fragment ion was utilized to accurately quantify the 18:3 FA isomers. A sequential decrease in relative intensity of the m/z 239 fragment ion was observed in mixtures containing a sequential increase in the ratio of GLA to ALA (Fig. 4). The relative intensity of the m/z 239 fragment ion after normalization to the sum of intensities of the abundant fragment ions (i.e., m/z 183, m/z 169 and m/z 239) was plotted with the isomeric composition of the mixtures (i.e., the percentages of GLA in the mixtures containing different molar ratios of GLA and ALA). This plot exhibited a linear relationship with a correlation coefficient of 0.999 (Fig. 4 inset, solid line). Interestingly, this linear relationship was nearly identical to the linearity established from the tandem mass spectra of the two authentic 18:3 FA isomers (Fig. 4 inset, dashed red line). This result demonstrated that this linear relationship encompasses the entire range of the isomeric compositions of the mixtures.
Analysis of Fatty Acid Double Bond Positional Isomers in Human Serum
To demonstrate the application of this approach to the identification and quantification of FA double bond positional isomers in biological samples, we analyzed the isomers of non-esterified FAs and esterified FAs in TGs present in human serum. As described above, the PIS of m/z 183 of AMPP-derivatized FAs can globally profile a diversity of FAs with high sensitivity and specificity. The PIS of m/z 183 of human serum samples identified the complete serum FA profiles with extremely low background noise and high sensitivity (Fig. 5 top panels). This AMPP-derivatized FA profiling approach displayed some very low abundance FA species in human serum (Fig. 5 top panels, insets) that are typically non-detectable by direct analysis in the negative-ion mode (Fig. S6). Additionally, AMPP derivatization of FAs resulted in the remarkable preservation of unsaturated FAs from degradation at −20°C (Fig. S7). Furthermore, these FA profiles can be directly utilized for quantitation of FAs in biological samples with appropriate internal standards for many months after AMPP derivatization (Table S1).
The developed method was used to determine double bond positional isomers of non-esterified FAs and esterified FAs in TGs in normal human serum. Two positional isomers of 18:3 FA in low abundance (e.g., less than 1.5% of total non-esterified FAs; See Table S1) were identified in human serum, i.e., GLA and ALA. The coexistence of the two isomers were demonstrated by the difference in the relative intensity of the m/z 239 fragment ion and the coexisting fragment ions at m/z 347 and m/z 349 (Figs. 5A_c and 5B_c) in comparison to the mass spectra of authentic ALA (Fig. 4a) and GLA (Fig. 4f). Notably, the detection of GLA in serum samples has previously been frequently missed48–50 due to its low abundance or unanticipated presence during SRM-based targeted analysis.
The compositions of the 18:3 FA isomeric mixtures in human serum were calculated based on experimentally determined linearity of the normalized m/z 239 fragment ion intensities vs. the compositions of isomeric mixtures (Fig. 4 inset). The results demonstrated that non-esterified 18:3 FA was comprised of 12% of GLA and esterified 18:3 FA in TGs was comprised of 45% of GLA. This three-fold difference in isomeric composition demonstrates the specificity of FA double bond positional isomers in discrete lipid classes in human serum. In addition, ω-3 polyunsaturated FAs (e.g., ALA and its downstream product DHA) and ω-6 polyunsaturated FAs (e.g., GLA and its downstream product arachidonic acid) compete for the same enzyme systems for their de novo synthesis, stereospecific oxidation to produce their respective signaling metabolites, and esterification into cellular membrane lipids.7,51–54 Recent work has emphasized the anti-inflammatory effects of ω-3 FAs7,55–57 while ω-6 FAs promote the progression of multiple inflammatory processes.51,53 The ability to precisely determine the ratio of GLA vs. ALA in biological systems provides important insights into the effects of dietary manipulation on the production of downstream ω-3/ω-6 products that likely can serve as novel biomarkers and therapeutic targets for lipid-related metabolic diseases.51,53,56
To verify the accuracy of the quantitation of FA double bond positional isomers by the developed method, two isomeric mixtures were prepared from the authentic 18:3 FA isomers at the designated ratios (i.e., 12% or 45% of GLA in the mixtures). The tandem mass spectra of the two prepared mixtures were identical to those from human serum (Figs. 5A_d vs. 5A_c, and Figs. 5B_d vs. 5B_c). In addition, spectra of the two mixtures were generated in silico by linearly combining the relative intensities of tandem mass spectra of the authentic 18:3 FA isomers (Figs. 4a and 4f) at the designated ratios. The simulated spectra precisely matched with the mass spectra of human serum (Figs. 5A_e vs. 5A_c, and Figs. 5B_e vs. 5B_c). Collectively, these results by multiple independent approaches validated the quantitative analysis of FA isomeric mixtures using the developed strategy.
In contrast, the other 18 carbon-containing unsaturated FAs were comprised of a single isomer (i.e., Δ9 18:1 FA and Δ9,12 18:2 FA) in both non-esterified FAs and those esterified in TGs in human serum. This result was substantiated by the identical tandem mass spectra of non-esterified (Fig. 5A_a), esterified (Fig. 5B_a) and authentic Δ9 18:1 FA (Fig. 2b). Similarly, identical mass spectra were observed for 18:2 FA (i.e., Figs. 5A_b and 5B_b, and Fig. 2c).
We specifically point out that the approach of simulating spectra of isomeric mixtures from individual tandem mass spectra of authentic FA isomers (e.g., Fig. 5A_e and Fig. 5B_e) can be extended to encompass the quantitation of FAs having three or more isomers. For example, the composition of a mixture comprising three 18:1 FA isomers (i.e., Δ6, Δ9 and Δ11 18:1 FAs) was identified from the simulated in silico spectrum that best fits the experimental tandem mass spectrum of the mixture (Table S2 and Fig. S8).
CONCLUSION
A direct infusion-based tandem mass spectrometric approach was developed to identify and quantify the double bond positional isomers of unsaturated FAs after charge-switch derivatization with AMPP. The identification of FA positional isomers was achieved through tandem mass spectrometric analysis of AMPP-derivatized FAs that provides: (1) a diagnostic fragment ion that defines the location of the proximal double bond; and (2) a series of 40 Th fragment ions that substantiate the assignment of the multiple double bond locations in polyunsaturated FAs. The quantitation of FA isomeric pairs that coexist in biological systems was accomplished by utilizing the linear relationship between the normalized intensity of a characteristic fragment ion (e.g., AMPP-derivatized prop-2-enoic acid at m/z 239) and the composition of corresponding isomeric mixtures. The quantitation of FAs containing three or more positional isomers was established through comparisons of in silico spectral simulations to the experimental tandem mass spectra of mixtures of FA isomers. This strategy identified substantial differences in the double bond positional isomers of 18:3 FA (i.e., ratio of GLA to ALA) in non-esterified FAs vs. esterified FAs in TGs in normal human serum. Through the detailed analysis of FA double bond positional isomers, new insights into alterations of FA metabolism in health and disease can be obtained.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants P01 HL57278 and RO1 HL118639 (RWG). RWG has financial relationships with LipoSpectrum LLC and Platomics, Inc.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Additional information including fragmentation routes and mass spectra as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fernandis AZ, Wenk MR. Curr Opin Lipidol. 2007;18:121–128. doi: 10.1097/MOL.0b013e328082e4d5. [DOI] [PubMed] [Google Scholar]
- 2.Gross RW, Han X. Chem Biol. 2011;18:284–291. doi: 10.1016/j.chembiol.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Meer G, Voelker DR, Feigenson GW. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett WFD, Tieleman DP. Biochim Biophys Acta - Biomembranes. 2013;1828:1765–1776. doi: 10.1016/j.bbamem.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Hunte C, Richers S. Curr Opin Struct Biol. 2008;18:406–411. doi: 10.1016/j.sbi.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Seara H, Rog T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M, Reigada R. Biophys J. 2008;95:3295–3305. doi: 10.1529/biophysj.108.138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascio G, Schiera G, Di Liegro I. Curr Diabetes Rev. 2012;8:2–17. doi: 10.2174/157339912798829241. [DOI] [PubMed] [Google Scholar]
- 8.Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, Zhao Z, Sims HF, Cerqua R, Cade WT, Han X, Gross RW. J Lipid Res. 2013;54:1312–1225. doi: 10.1194/jlr.M034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiebish MA, Yang K, Sims HF, Jenkins CM, Liu X, Mancuso DJ, Zhao Z, Guan S, Abendschein DR, Han X, Gross RW. J Biol Chem. 2012;287:25086–25097. doi: 10.1074/jbc.M112.340521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyfried TN, Shelton LM. Nutr Metab. 2010;7:1743–7075. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundstrom SL, Balgoma D, Wheelock AM, Haeggstrom JZ, Dahlen SE, Wheelock CE. Curr Pharm Biotechnol. 2011;12:1026–1052. doi: 10.2174/138920111795909087. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Kelly OJ, Ilich JZ. Lipids. 2013;48:787–802. doi: 10.1007/s11745-013-3803-5. [DOI] [PubMed] [Google Scholar]
- 13.Mancuso DJ, Kotzbauer P, Wozniak DF, Sims HF, Jenkins CM, Guan S, Han X, Yang K, Sun G, Malik I, Conyers S, Green KG, Schmidt RE, Gross RW. J Biol Chem. 2009;284:35632–35644. doi: 10.1074/jbc.M109.055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MC, Dunn SR, Altvater J, Dove SG, Nette GW. Anal Chem. 2012;84:5976–5983. doi: 10.1021/ac3006523. [DOI] [PubMed] [Google Scholar]
- 15.Pham HT, Trevitt AJ, Mitchell TW, Blanksby SJ. Rapid Commun Mass Spectrom. 2013;27:805–815. doi: 10.1002/rcm.6503. [DOI] [PubMed] [Google Scholar]
- 16.Kamphorst JJ, Fan J, Lu W, White E, Rabinowitz JD. Anal Chem. 2011;83:9114–9122. doi: 10.1021/ac202220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang WC, Adamec J, Regnier FE. Anal Chem. 2007;79:5150–5157. doi: 10.1021/ac070311t. [DOI] [PubMed] [Google Scholar]
- 18.Sommer U, Herscovitz H, Welty FK, Costello CE. J Lipid Res. 2006;47:804–814. doi: 10.1194/jlr.M500506-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Kerwin JL, Wiens AM, Ericsson LH. J Mass Spectrom. 1996;31:184–192. doi: 10.1002/(SICI)1096-9888(199602)31:2<184::AID-JMS283>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Jensen NJ, Tomer KB, Gross ML. Anal Chem. 1985;57:2018–2021. [Google Scholar]
- 21.Deterding LJ, Gross ML. Org Mass Spectrom. 1988;23:169–177. [Google Scholar]
- 22.Tomer KB, Crow FW, Gross ML. J Am Chem Soc. 1983;105:5487–5488. [Google Scholar]
- 23.Hsu FF, Turk J. J Am Soc Mass Spectrom. 1999;10:600–612. doi: 10.1016/S1044-0305(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 24.Hsu FF, Turk J. J Am Soc Mass Spectrom. 2008;19:1673–1680. doi: 10.1016/j.jasms.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu FF, Turk J. J Am Soc Mass Spectrom. 2010;21:657–669. doi: 10.1016/j.jasms.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell TW, Pham H, Thomas MC, Blanksby SJ. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2722–2735. doi: 10.1016/j.jchromb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Brown SH, Mitchell TW, Blanksby SJ. Biochim Biophys Acta. 2011;11:807–817. doi: 10.1016/j.bbalip.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Sun C, Black BA, Zhao YY, Ganzle MG, Curtis JM. Anal Chem. 2013;85:7345–7352. doi: 10.1021/ac401242z. [DOI] [PubMed] [Google Scholar]
- 29.Sun C, Zhao YY, Curtis JM. Anal Chim Acta. 2013;762:68–75. doi: 10.1016/j.aca.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Thomas MC, Mitchell TW, Blanksby SJ. Methods Mol Biol. 2009;579:413–441. doi: 10.1007/978-1-60761-322-0_21. [DOI] [PubMed] [Google Scholar]
- 31.Pham HT, Maccarone AT, Campbell JL, Mitchell TW, Blanksby SJ. J Am Soc Mass Spectrom. 2013;24:286–296. doi: 10.1007/s13361-012-0521-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JI, Tao WA, Cooks RG. Anal Chem. 2011;83:4738–4744. doi: 10.1021/ac1030946. [DOI] [PubMed] [Google Scholar]
- 33.Pham HT, Ly T, Trevitt AJ, Mitchell TW, Blanksby SJ. Anal Chem. 2012;84:7525–7532. doi: 10.1021/ac301652a. [DOI] [PubMed] [Google Scholar]
- 34.Yoo HJ, Hakansson K. Anal Chem. 2010;82:6940–6946. doi: 10.1021/ac101217x. [DOI] [PubMed] [Google Scholar]
- 35.Castro-Perez J, Roddy TP, Nibbering NM, Shah V, McLaren DG, Previs S, Attygalle AB, Herath K, Chen Z, Wang SP, Mitnaul L, Hubbard BK, Vreeken RJ, Johns DG, Hankemeier T. J Am Soc Mass Spectrom. 2011;22:1552–1567. doi: 10.1007/s13361-011-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang K, Zhao Z, Gross RW, Han X. Anal Chem. 2011;83:4243–4250. doi: 10.1021/ac2006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mochizuki Y, Inagaki S, Suzuki M, Min JZ, Inoue K, Todoroki K, Toyo’oka T. J Sep Sci. 2013;36:1883–1889. doi: 10.1002/jssc.201300083. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Franke AA. Anal Chem. 2011;83:3192–3198. doi: 10.1021/ac103093w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leng J, Wang H, Zhang L, Zhang J, Wang H, Guo Y. Anal Chim Acta. 2013;758:114–121. doi: 10.1016/j.aca.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Vrkoslav V, Cvacka J. J Chromatogr A. 2012;12:244–250. doi: 10.1016/j.chroma.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 41.Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M, Turecek F, Gelb MH. Anal Chem. 2010;82:6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X, Yang K, Gross RW. Mass Spectrom Rev. 2012;31:134–178. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang K, Cheng H, Gross RW, Han X. Anal Chem. 2009;81:4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunstone FD. Fatty Acid and Lipid Chemistry. Springer US; 1996. pp. 1–34. [Google Scholar]
- 45.Ecker J, Liebisch G, Scherer M, Schmitz G. J Lipid Res. 2010;51:2686–2694. doi: 10.1194/jlr.M007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claeys M, Nizigiyimana L, Van den Heuvel H, Derrick PJ. Rapid Commun Mass Spectrom. 1996;10:770–774. [Google Scholar]
- 47.Stahlman M, Pham HT, Adiels M, Mitchell TW, Blanksby SJ, Fagerberg B, Ekroos K, Boren J. Diabetologia. 2012;55:1156–1166. doi: 10.1007/s00125-011-2444-6. [DOI] [PubMed] [Google Scholar]
- 48.Lindberg M, Midthjell K, Bjerve KS. Br J Nutr. 2013;109:1123–1134. doi: 10.1017/S0007114512002759. [DOI] [PubMed] [Google Scholar]
- 49.Grapov D, Adams SH, Pedersen TL, Garvey WT, Newman JW. PLoS One. 2012;7:e48852. doi: 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou L, Lian K, Yao M, Shi Y, Lu X, Fang L, He T, Jiang L. Cardiovasc Diabetol. 2012;11:126–134. doi: 10.1186/1475-2840-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeppesen C, Schiller K, Schulze MB. Curr Diab Rep. 2013;13:279–288. doi: 10.1007/s11892-012-0362-8. [DOI] [PubMed] [Google Scholar]
- 52.Cook HWMCR. In: Biochemistry of Lipids, Lipoproteins and Membranes. Vance DEV, JE, editors. 2002. p. 181. [Google Scholar]
- 53.Lottenberg AM, da Afonso MS, Lavrador MS, Machado RM, Nakandakare ER. J Nutr Biochem. 2012;23:1027–1040. doi: 10.1016/j.jnutbio.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Karlstrom BE, Jarvi AE, Byberg L, Berglund LG, Vessby BO. Am J Clin Nutr. 2011;94:26–33. doi: 10.3945/ajcn.110.006221. [DOI] [PubMed] [Google Scholar]
- 55.Kazemian P, Kazemi-Bajestani SM, Alherbish A, Steed J, Oudit GY. Cardiovasc Drugs Ther. 2012;26:311–320. doi: 10.1007/s10557-012-6397-x. [DOI] [PubMed] [Google Scholar]
- 56.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. Adv Nutr. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weylandt KH, Nadolny A, Kahlke L, Kohnke T, Schmocker C, Wang J, Lauwers GY, Glickman JN, Kang JX. Biochim Biophys Acta. 2008;11:634–641. doi: 10.1016/j.bbadis.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.