Abstract
Background
Osteoporosis is a widespread but largely preventable disease. Improved adherence to screening and treatment recommendations is needed to reduce fracture and mortality rates. Additionally, clinicians face increasing demands to demonstrate proficient quality patient care aligning with evidence-based standards.
Methods
A three-stage, clinician-focused performance improvement (PI) continuing medical education (CME) initiative was developed to enhance clinician awareness and execution of evidence-based standards of osteoporosis care. Clinician performance was evaluated through a retrospective chart analysis of patients at risk or with a diagnosis of osteoporosis.
Results
Seventy-five participants reported their patient practices on a total of 1875 patients before and 1875 patients after completing a PI initiative. Significant gains were made in the use of Fracture Risk Assessment Tool (FRAX) (stage A, 26%, n=1769 vs. stage C, 51%, n=1762; p<0.001), assessment of fall risk (stage A, 46%, n=1276 vs. stage C, 89%, n=1190; p<0.001), calcium levels (stage A, 62%, n=1451 vs. stage C, 89%, n=1443; p<0.001), vitamin D levels (stage A, 79%, n=1438 vs. stage C, 93%, n=1439; p<0.001), and medication adherence (stage A, 88%, n=1136 vs. stage C, 96%, n=1106; p<0.001).
Conclusions
Gains in patient screening, treatment, and adherence were associated with an initiative promoting self-evaluation and goal setting. Clinicians must assess their performance to improve patient care and maintain certification. PI CME is a valid, useful educational tool for accomplishing these standards.
Introduction
Approximately 9 million Americans suffer from osteoporosis, and an additional 48 million have low bone mass; a majority of these patients are women.1 Approximately 50% of women older than 50 years will suffer an osteoporosis-related fracture in their lifetime, compared to fewer than 25% of men.2 Alarmingly, the number of cases of osteoporosis and related fractures is projected to rise—by 2025, an estimated 3 million fractures will occur due to osteoporosis.1,3 Although osteoporosis is a preventable disease, many patients do not receive appropriate testing to determine risk or establish a diagnosis.4,5 The general screening rate in primary care clinics is roughly 11%.6 Even among patients with previous fragility fractures, fewer than one-third are subsequently evaluated and treated for osteoporosis.
National organizations dedicated to the prevention of osteoporosis recognize the need for improved screening and treatment. For instance, the United States Preventive Services Task Force provides specific recommendations for the screening of postmenopausal women and those with an increased risk of fracture.5 Additionally, the recent 2010 National Osteoporosis Foundation guidelines include recommendations for African American, Asian, Latina, and other postmenopausal women, as well as men 50 years and older.4 Expanded guidelines also include revisions to the Fracture Risk Assessment Tool (FRAX) algorithm, a tool developed by the World Health Organization; the tool now accounts for U.S. fracture and mortality rates, thereby improving the tool's clinical utility.
The availability of updated evidence-based guidelines, however, is not enough to improve patient care. Measures, such as competency-based medical education, must be taken to ensure that changes are properly adopted and implemented. Indeed, the American Board of Internal Medicine (ABIM) and American Academy of Physician Assistants require self-evaluation of practice performance to maintain certification.7,8
At best, practicing clinicians are minimally able to self-identify current learning needs.9 Furthermore, traditional lectures require participants to be independently aware of deficits to apply knowledge gains.10 As a solution to address these educational needs, the American Medical Association (AMA) established the three-step performance improvement (PI) continuing medical education (CME) process.11 This model assists clinicians in assessing their individual retrospective practice of evidence-based performance measures, implementing goals to meet areas of assessed need, and reevaluating performance after a period of active change to catalyze long-term change.10
Given the need for improved osteoporosis screening and adoption of new guidelines, an ABIM-approved PI CME initiative was developed as a method for enabling clinicians to better meet standards of care. Primary care physicians, nurse practitioners, and physician assistants who treat patients with or at risk of osteoporosis participated in a series of self-evaluation, goal setting, and reevaluation to enhance patient care with the goals of improved screening, diagnosis, and treatment. The initiative was directed toward community-based practicing clinicians, who may not have the support of larger institutions where routine assessment of patient outcomes and clinician performance may be more common. The results of the PI initiative are reported here as a demonstration of the practice changes that may be achieved by clinicians actively engaging in practice-based learning.
Methods
An activity focused on the care of patients with or at risk of osteoporosis was developed based on the AMA PI CME model and in collaboration with expert faculty. Participant-recruitment methods included fax, e-mail, direct mail, and online advertising targeting the accredited provider's internal database of primary care clinicians, as well as purchased lists of AMA contacts within the target audience. Recruitment materials included a description of the AMA PI CME model, practical aspects of participating in the activity, and estimated time requirements.
Upon registration, participants were asked to complete a questionnaire assessing current practice patterns. In stage A, participants conducted retrospective patient chart reviews using a standardized data collection form to self-assess performance standards. Performance standards were based on nationally standardized performance measures in osteoporosis care established by The Joint Commission, The National Quality Forum, and the National Committee for Quality Assurance, as well as current evidence-based guidelines from other national organizations.4,5,12–16 Questions addressed the identification of fracture risk, development of appropriate treatment plans, and monitoring of clinical health indicators (Table 1). A report was provided to participants detailing their performance results and comparing their results with those of their peers enrolled in the PI CME activity and evidence-based standards. Participants were then asked to develop personalized, actionable goals to improve patient care and implement these goals over a period of 3 months (i.e., stage B). Participants then performed a second retrospective chart review (i.e., stage C); no instructions were given to provide data from unique patients. Results were compared with initial individual performance results, peer performance results, and evidence-based standards. A frequently asked questions page was linked to the activity Website providing a description of the AMA PI CME model, estimated time requirements for activity participation, and answers to other common inquiries.
Table 1.
Assessed Osteoporosis Performance Measures
| Category | Assessed performance measures and answer options |
|---|---|
| Risk assessment | • Future osteoporosis-related fracture risk calculated using the FRAX risk score |
| o Yes | |
| o No | |
| o N/A, has been on pharmacologic treatment for osteoporosis | |
| o Not necessary because patient has a low risk | |
| o I am unfamiliar with the FRAX risk score | |
| • Recorded BMD measurement by central DXA in chart | |
| o Result of most recent BMD testing by DXA | |
| o No result documented | |
| o Normal: T-score ≥−1.0 | |
| o Low bone mass: T-score between −1.0 and −2.5 | |
| o Osteoporosis: T-score ≤−2.5 | |
| Diagnosis and treatment | • Patient evaluated for secondary osteoporosis |
| • Does the chart contain documentation on the following: | |
| o Current estimated dietary calcium intake | |
| o Vitamin D [25(OH)D] level | |
| o Calcium intake | |
| o Vitamin D intake | |
| • Patient was counseled to start, increase, or maintain participation in a weight-bearing exercise program | |
| • If patient smokes, a cessation plan was discussed in the past 12 months | |
| • Patient counseled on excess alcohol consumption | |
| • Falls-risk screening performed in the past 12 months | |
| • For patients with osteoporosis, was patient prescribed antiresorptive therapy and/or anabolic therapy | |
| • For patient receiving antiresorptive therapy, which type of agent was prescribed | |
| o Bisphosphonate | |
| o Calcitonin | |
| o Estrogen | |
| o Parathyroid hormone | |
| o RANKL inhibitor | |
| • If patient suffered a hip or vertebral fracture, did at least one of the following actions occur within 6 months of the fracture: | |
| o Central DXA measurement performed | |
| o Pharmacologic treatment prescribed | |
| o N/A, patient has not had a hip or vertebral fracture | |
| o N/A, patient on pharmacologic treatment at time of fracture | |
| o None of the above | |
| • Patient's adherence with his/her osteoporosis-related medications been assessed within the last 12 months |
BMD, bone mineral density; DXA, dual x-ray absorptiometry; FRAX, Fracture Risk Assessment Tool; N/A, not applicable; RANKL, receptor activator of nuclear factor kappa-B ligand.
To support participant-improvement efforts, a complimentary CME-certified online publication was provided that included practice recommendations and several tools clinicians could implement in their practices to meet performance measures, including a calcium calculator, patient exercise tracking chart, and a medication adherence assessment form. The publication was made available in a downloadable format allowing participants to utilize the tools as an inexpensive means for achieving their performance-improvement goals. Within the publication, each performance measure was defined, its importance to optimizing patient outcomes was described, and, when appropriate, recommended methods to improve performance of these measures was discussed. There were no requirements surrounding the utilization of the CME-certified publication or implementation of the tools provided in the publication to complete stage B; however, participants were highly encouraged to implement the tools and other recommendations into their practice to help meet their performance goals. Expert faculty provided insight on the selection of performance standards and contributed to the development of the current practice pattern questionnaire and CME-certified online publication.
Patient charts were randomly selected by the participants. Inclusion criteria for the two chart reviews required that patients had been cared for by the participating clinician's practice for at least 1 year, had been seen by the participating clinician within the last 12 months, and had a diagnosis of osteoporosis or at least one documented risk factor. Risk factors were stratified by age and menopausal status (women 65 years or older, men 70 years or older, men and postmenopausal women 50 to 69 years of age taking medications or with conditions that increase osteoporosis risk, perimenopausal women with high-risk factors [e.g., low body weight, prior low-trauma fracture, high-risk medication]) and included fragility fracture after 50 years of age, use of medications associated with osteoporosis (e.g., glucocorticoids, antiseizure medications, aromatase inhibitors, androgen deprivation therapy/GnRH agonists, barbiturates, depomedroxyprogesterone acetate, lithium, thiazolidinediones, proton pump inhibitors, selective serotonin reuptake inhibitors), and presence of conditions associated with an increased risk of osteoporosis (e.g., smoking, rheumatoid arthritis, hyperthyroidism, hypogonadism, malabsorption, alcoholism, vitamin D deficiency).
Participants were incentivized to earn AMA PRA Category 1 Credits™. Upon completion of each stage, participants earned 5 credits; 5 additional credits were earned for completing all three stages for a total of 20 credits. Separately, credits could also be earned with the completion of the supporting online educational publication. Finally, the initiative was submitted to and accepted by the ABIM and approved for Maintenance of Certification (MOC) credit. Monetary compensation was not offered at any time.
Medical record selection, confidentiality, and exemption from consent
De-identified abstracted chart information was submitted by each participant from 25 patients in stage A and 25 patients in stage C for a total of 50 patients in accordance with ABIM MOC standards. No attempt was made to review charts from the same patients across stages, and no attempts were made to link the chart data by patient. Institutional review board approval was not sought because data were submitted and stored in a de-identified format.17
Statistical analysis
Noncompleter and completer practice patterns and stage A and stage C patient data were compared using Pearson's chi-square tests and independent t tests. Statistical analyses did not control for clustering of charts within participants. Results were considered statistically significant if the resulting p value was <0.05.
Results
Participant characteristics
A total of 366 participants registered and completed stage A of the initiative. Of those participants, 291 completed stage A (i.e., noncompleters), and 75 participants completed all three stages (i.e., completers). To assess the representativeness of completers in their practice characteristics and practice patterns, completers were compared with the larger group of noncompleters by assessing responses to the current practice patterns questionnaire administered prior to stage A. The majority of both completers and noncompleters were doctors (MDs), however more completers than noncompleters were MDs (Table 2). The most commonly identified specialty for completers was internal medicine, whereas family or general practice was most commonly identified by noncompleters. The distribution of practice type was similar between the two groups, with more than one-half of participants working in a solo or small group practice of fewer than five clinicians.
Table 2.
Participant Characteristics
| Completers n=75 | Noncompleters n=291 | p | |
|---|---|---|---|
| Degree | |||
| MD | 85% | 76% | <0.001* |
| DO | 5% | 4% | |
| PA | 9% | 14% | |
| NP | 0% | 5% | |
| Specialty | |||
| Endocrinology | 23% | 14% | <0.001* |
| Family/General Practice | 15% | 31% | |
| Internal Medicine | 44% | 22% | |
| OB/GYN | 1% | 4% | |
| Other | 17% | 28% | |
| Type of practice | |||
| Solo practice | 28% | 28% | 0.807* |
| Group practice<5 physicians | 24% | 22% | |
| Group practice>5 physicians | 21% | 17% | |
| Institutionally salaried (hospital/clinic) | 19% | 22% | |
| Institutionally salaried (HMO/managed care/insurance company) | 1% | 4% | |
| Other | 7% | 7% | |
| Years in practice | |||
| <1 | 0% | 4% | 0.257* |
| 1–10 | 36% | 29% | |
| 11–20 | 35% | 34% | |
| 21–30 | 20% | 19% | |
| >30 | 9% | 14% | |
| Average number of patients with osteoporosis seen each week | |||
| Mean | 12 | 11 | 0.326 |
| Median (range) | 10 (1–45) | 8 ( 0–100) | |
| Provision of direct, consultative, or administrative care for patients with osteoporosis | 98%† | 88%‡ | 0.032 |
| Approximate percentage of patients prescribed antiresorptive drug therapy if the patient has or is at risk of osteoporosis§ | |||
| <10 % | 4% | 20% | <0.001* |
| 10%–25% | 1% | 8% | |
| 26%–50% | 8% | 11% | |
| 51%–75% | 13% | 13% | |
| 76%–90% | 37% | 19% | |
| >90% | 36% | 29% | |
p value applies to the change within the group of data analyzed.
n=51
n=190
Defined as the patient having any of the following characteristics: a T-score of −2.5 or lower, history of prior fracture, low bone mass, and at least one factor associated with a high risk of fracture, or low bone mass and a FRAX 10-year major osteoporosis-related fracture risk of at least 20%.
HMO, health maintenance organization; MD, medical doctor; DO, doctor of osteopathy; NP, nurse practitioner; OB/GYN, obstetrics/gynecology; PA, physician assistant.
The two groups differed in only 2 of the 15 questions that evaluated perceived practice patterns. Completers were more likely to provide direct, consultative, or administrative care for patients with osteoporosis (noncompleters 88%, n=190 vs. completers 98%, n=51; p<0.032; Table 2). The other observed practice difference was completers reporting more frequent prescription of an antiresorptive drug to approximately 76% to 90% of their patients with or at risk of osteoporosis, defined as the patient having any of the following characteristics: a T-score of −2.5 or lower, history of prior fracture, low bone mass and at least one factor associated with a high risk of fracture, or low bone mass and a FRAX 10-year major osteoporosis-related fracture risk of at least 20% (noncompleters, n=291, 19% vs. completers, n=75, 37%; p<0.001). Similar reported practice patterns included the ability to implement process-improvement changes within their practice, presence of a system to identify at-risk patients who should be screened for osteoporosis, and percentage of patients with low bone mass or osteoporosis counseled about appropriate calcium and vitamin D intake, screened annually for fall-risk, counseled about smoking cessation if appropriate, recommended and/or tracked for exercise progress, and assessed for adherence (data not shown). In addition, both groups reported the similar presence of practice systems in place to assure counseling of appropriate calcium and vitamin D intake, annual screenings for fall-risk, discussion of smoking cessation if appropriate, counseling of exercise, and adherence assessment (data not shown).
Some reported practice patterns of completers prior to the initiative were notable. For instance, more than one-third did not have a system in place for identifying patients at risk of osteoporosis. In addition, nearly two-thirds did not have a system in place to assure annual fall-risk screenings in patients with osteoporosis or low bone mass. Finally, only one-third had a system in place to assess osteoporosis medication adherence (data not shown).
Plans for improvement by completer participants
Examples of submitted plans to attain performance improvement in stage C by completer participants included incorporating checklists in electronic medical records (EMR) or paper-based charts as a reminder to perform various processes, utilizing the FRAX tool via an EMR system, computer, or smartphone, using EMR filters to identify patients at risk of osteoporosis, creating and utilizing educational handouts, and devising questionnaires for new and returning patients to assess osteoporosis-related risk factors. In addition, participants reported utilizing staff members to assist with identifying patients with osteoporosis-related risk factors and providing education to patients. Barriers reported by participants that hindered effective implementation of their improvement plans were time constraints, costs of scans, tests, and treatments, lack of patient understanding of the risks associated with low bone density and treatment options available to treat osteoporosis, and patient adherence.
Patient characteristics, risk assessment, and diagnosis
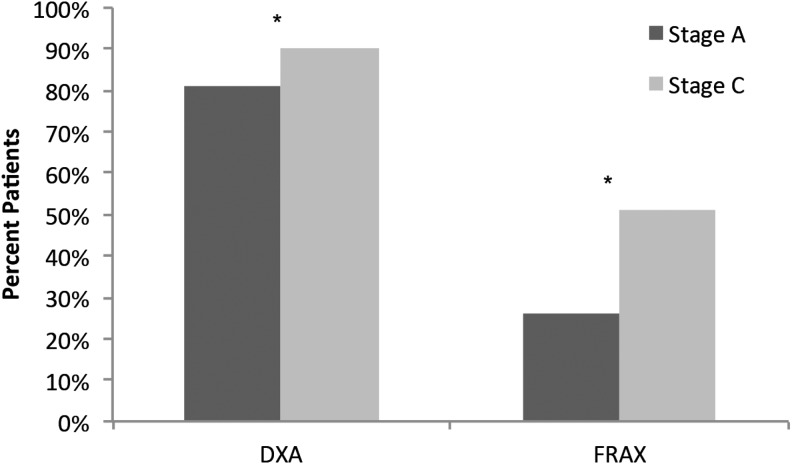
Analysis was restricted to the patient chart data submitted by 75 completer participants. These participants abstracted and submitted data from a total of 3750 patient charts—1875 in stage A and 1875 in stage C. Patients had a mean age of 69 years in both stages A and C, and the majority were female (Table 3). The percent of patients with no documented bone mineral density (BMD) results decreased (stage A, 17%, n=1875 vs. stage C, 10%, n=1875); whereas diagnosis of osteoporosis by central dual x-ray absorptiometry (DXA; defined as T-score ≤−2.5) increased from stage A (37%) to stage C (39%; p<0.001, for both). BMD was measured more often by DXA (p<0.001) after the initiative (Fig. 1). Furthermore, after the intervention, participants were more familiar with the FRAX risk score and used it nearly twice as often to calculate future osteoporosis-related fracture risk (stage A, 26%, n=1769 vs. stage C, 51%, n=1762; p<0.001; Fig. 1).
Table 3.
Patient Characteristics
| Stage A n=1875 | Stage C n=1875 | p | |
|---|---|---|---|
| Patient age | (n=1660) | (n=1689) | |
| Mean (SD) | 69 (11.7) | 69 (11.1) | 0.449* |
| Median (range) | 70 (7–99) | 69 (22–99) | |
| Sex | (n=1764) | (n=1756) | |
| Male | 15% | 16% | 0.367* |
| Female | 85% | 84% | |
| BMD Results by DXA | (n=1875) | (n=1875) | |
| Normal (T-score ≥−1.0)† | 13% | 17% | <0.001* |
| Low bone mass (T-score between −1.0 and −2.5) | 33% | 35% | |
| Osteoporosis (T-score ≤−2.5) | 37% | 39% | |
| No result documented | 17% | 10% |
p value applies to the change within the group of data analyzed.
Indicates the patient does not have osteoporosis or low bone mass, therefore additional questions regarding patient care were not completed.
FIG. 1.
Risk assessment for osteoporosis. Future osteoporosis-related risk calculated using bone mineral density (BMD) recorded by central dual x-ray absorptiometry (DXA) (stage A, n=1875, stage C, n=1875, p<0.001) and the Fracture Risk Assessment Tool (FRAX) risk score (stage A, n=1769, stage C, n=1762; p<0.001). *Denotes significant difference between stage A and stage C.
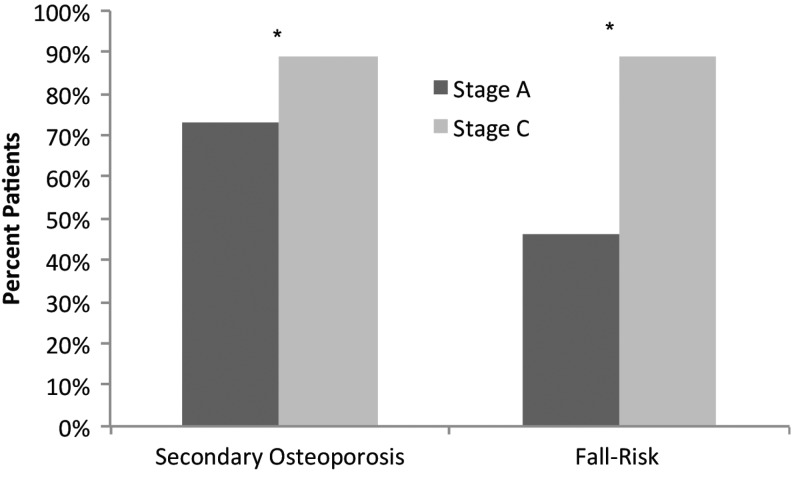
In stage C, 16% more patients were evaluated for secondary osteoporosis, and 43% more patients were assessed for risk of falls as compared with stage A (p<0.001 for both; Fig. 2). Participants were also more likely to document assessments of estimated dietary calcium intake and vitamin D (25-hydroxyvitamin D [25(OH)D]) levels after completing the initiative (Table 4). Furthermore, participants demonstrated significant improvements in all areas of assessed patient counseling (e.g., calcium intake, vitamin D intake, performing weight-bearing exercise, smoking cessation, and alcohol consumption) (Table 4).
FIG. 2.
Osteoporosis patient screening. Evaluation of secondary osteoporosis (stage A, n=1422, stage C, n=1426; p<0.001) and fall-risk within the past 12 months (stage A, n=1276, stage C, n=1190; p<0.001). *Denotes significant difference between stage A and stage C.
Table 4.
Documented Assessments of Calcium, Vitamin D, and Counseling
| Stage A n=1875 | Stage C n=1875 | p | |
|---|---|---|---|
| Documented estimated dietary calcium intake | (n=1451) | (n=1443) | |
| 62% | 89% | <0.001 | |
| Documented vitamin D [25(OH)D] levels | (n=1438) | (n=1439) | |
| 79% | 93% | <0.001 | |
| Participant provided patient counseling on: | |||
| Dietary calcium intake | (n=1457) | (n=1447) | |
| 85% | 96% | <0.001 | |
| Dietary vitamin D intake | (n=1450) | (n=1433) | |
| 84% | 96% | <0.001 | |
| Weight-bearing exercise | (n=1438) | (n=1424) | |
| 71% | 89% | <0.001 | |
| Smoking cessation | (n=351) | (n=424) | |
| 80% | 92% | <0.001 | |
| Alcohol consumption | (n=554) | (n=540) | |
| 53% | 84% | <0.001 | |
Treatment
A greater percentage of patients were prescribed pharmacologic therapies after the initiative (stage A, 79%, n=1237 vs. stage C, 83%, n=1188; p=0.038; Table 5). Specifically, a small but statistically significant increase in the use of denosumab (receptor activator of nuclear factor kappa-B ligand [RANKL] inhibitor) was observed (p=0.028), whereas a slight decrease in estrogen therapy was seen (p=0.041). For those patients with a history of hip or vertebral fracture, more patients received pharmacologic treatment (stage A, 11%, n=1231 vs. stage C, 14%, n=1301; p=0.026). Finally, patients were assessed for adherence to osteoporosis-related treatments more frequently (stage A, 88%, n=1136 vs. stage C, 96%, n=1106; p<0.001).
Table 5.
Treatment of Patients with Osteoporosis or Low Bone Mass
| Stage A n=1875 | Stage C n=1875 | p | |
|---|---|---|---|
| Patient prescribed antiresorptive therapy and/or anabolic therapy | (n=1237) | (n=1188) | |
| 79% | 82% | 0.038 | |
| Current therapies | (n=975) | (n=976) | |
| Bisphosphonate | 80% | 77% | 0.245 |
| Calcitonin | 3% | 3% | >0.999 |
| Estrogen | 3% | 1% | 0.041 |
| Parathyroid hormone | 6% | 4% | 0.086 |
| Estrogen agonist/antagonist | 5% | 7% | 0.101 |
| RANKL inhibitor | 5% | 8% | 0.028 |
| Other | 5% | 5% | 0.921 |
| For patients with history of hip or vertebral fracture, following actions occurred within 6 months of fracture (all that applied) | (n=1231) | (n=1301) | |
| Central DXA measurement | 20% | 20% | 0.632 |
| Pharmacologic treatment prescribed | 11% | 14% | 0.026 |
| None of the above | 69% | 67% | <0.001 |
| Adherence to osteoporosis-related medications assessed within the last 12 months | (n=1136) | (n=1106) | |
| 88% | 96% | <0.001 |
Discussion
Overall, improvements in clinical processes, including formal screening, provision of counseling, and therapeutic intervention, were associated with clinician participation in an osteoporosis PI CME initiative. Importantly, system-related deficiencies reported prior to the intervention by completer participants in identifying at-risk patients and screening patients with osteoporosis for fall risk improved significantly; after participation, nearly twice as many patients had documented FRAX risk score calculations, and 93% more patients underwent fall-risk screenings. Despite the significant increase in DXA assessment and use of FRAX, the percentage of women on therapy for osteoporosis was only minimally changed, possibly due to the high baseline rate of treatment in the cohort examined.
FRAX is a useful tool for calculating 10-year fracture probability in both women and men 50 years or older and is most helpful in determining fracture risk in patients with low BMD of the hip.4 Furthermore, scores help clinicians identify patients who require pharmacologic treatment, providing improved sensitivity and allowing for better identification of at-risk individuals than BMD testing alone.4,9,16,18 FRAX scores can also improve a patient's perception of future osteoporosis risk and provide motivation for changing bone health habits.19 Unfortunately, many clinicians are not appropriately applying the information in practice. Indeed, a recent study found that only one-third of FRAX-identified high-risk patients received treatment for osteoporosis.20 Results from this PI CME activity showed that the treatment of patients who received a diagnosis of osteoporosis through the use of BMD and FRAX testing improved; therefore, clinicians may benefit from participating in a PI CME activity.
Clinicians also significantly improved their documentation and recommendations of appropriate dietary consumption of calcium and vitamin D, both of which are critical to promoting and maintaining bone health; indeed, guidelines recommend advising patients to ensure adequate intake.4,9,16,21 A report by the Institute of Medicine (IOM) found that although most healthy individuals receive adequate intake, postmenopausal women and older individuals may be at risk of deficiencies.21 However, women taking calcium supplements may be at risk of taking too much of the nutrient. Clinicians are recommending high-dose supplements that match a women's total daily intake of 1200 mg.22 However, the average individual typically consumes 600 mg to 900 mg of calcium through diet; therefore, patients could exceed the safe upper limit of 2000 mg with the addition of a 1200-mg supplement.
Furthermore, conflict exists between the IOM and National Osteoporosis Foundation (NOF) recommendations for threshold 25-hydroxyvitamin D levels.22 The IOM recommends a more conservative 20 ng/mL, whereas the NOF recommends 30 ng/mL as a threshold minimum for adequate health. Although these NOF recommendations for adequate levels of vitamin D are higher than those of the IOM, they are well within the IOM's margin of safety.23
Despite a high level of baseline proficiency, after the intervention clinicians were significantly more likely to have patients undergo DXA testing. In addition to helping clinicians diagnose osteoporosis, DXA can be used to estimate disease severity, identify which patients require treatment, and monitor changes in bone mass over time.12 Further testing may be required to identify patients with secondary osteoporosis, which can be difficult to distinguish from primary osteoporosis.12,24 Notably, participants improved their evaluation of patients for secondary osteoporosis after the intervention as well.
Finally, participants increased their frequency of medication adherence assessments. Pharmacologic treatment of osteoporosis can be challenging due to lapses in patient compliance and persistence.25 A retrospective cohort study of more than 40,000 patients with osteoporosis found that approximately 45% did not routinely refill their prescriptions within the first year of treatment.26 This is unfortunate because evidence clearly demonstrates reductions in fracture rates with patients who are adherent to therapeutic interventions.27,28 Earlier identification of and action toward solving barriers to compliance may allow for improvement in the outcomes of these patients.
Unfortunately, a large number of participants who completed stage A failed to complete the initiative. Few differences were observed between completers and non-completers. Although 89% of noncompleters reported providing direct, consultative, or administrative care for patients with osteoporosis, nearly all completers also agreed with this statement. The most likely reason for the high rate of attrition was due to the time commitment required of PI CME activities.29 This is an inherent problem often observed with PI CME activities.29,30 Interestingly, a study examining the direct effect of PI participation on patient outcomes found minimal, if any, differences between patient outcomes among clinicians who completed various stages of PI.31 Participation in the process of PI itself contributed to greater differences in patient outcomes as compared with didactic CME activities. Therefore, it may be the initial act of self-assessment that provides the greatest value to PI CME, which suggests farther-reaching benefits than those observed here. In the future, participants may be more inclined to complete PI activities as the adoption of EMR and participation in ABIM MOC programs becomes more widespread.
Limitations include the retrospective and self-reported nature of the study, small sample sizes, and self-selection into the CME activity; additionally, changes observed may be due to better documentation practices. Chart selection and therefore the integrity of the data were reliant upon the participants. Bias may have been introduced through the selection of patient charts, yet this would have been self-defeating for participants because participants had an invested interest in self-improvement and compensation in the form of CME credit was not dependent upon performance outcomes. Several of the assessed measures were subject to interpretation, and participants were not required to submit information on exactly how measures were carried out. However, performance measures were defined in the CME-certified publication, and where relevant, recommendations were provided on ways in which to implement these measures. Unfortunately, measuring direct patient outcomes as a method of participant evaluation was not part of this study. However, other well-known national quality improvement (QI) initiatives in the areas of cardiology and surgical patient care have demonstrated that improvements in meeting performance measures are associated with improvements in patient outcomes.32,33 Both QI and PI initiatives are important methods for aiding clinicians in meeting performance measures. However, QI activities typically involve group assessments and may underestimate the contribution of individual clinicians while overestimating others. The value of PI lies within the evaluation of individual performance and enacting changes at the clinician level to improve patient care.
Conclusion
Clinicians require an accurate assessment of their performance of evidence-based standards to improve quality care of patients with or at risk of osteoporosis. This initiative was designed to assess and improve performance of community physicians who may not have access to large-scale healthcare systems and may be unaware of deficiencies in their provision of osteoporosis patient care. Numerous preventive measures can be taken to reduce facture risk in spite of the fact that many risk factors cannot be altered. By utilizing low-cost methods of improvement, clinicians can enhance their performance of osteoporosis screening and treatment measures, providing patients with a greater opportunity for prevention or slowed disease progression.
Acknowledgments
The authors thank Martha Inglis-Legall and Whitney Stevens for project management; Beth Wills for participant recruitment; Amy Sison and Mary Catherine Downes for outcomes management; Kenny Khoo for data management; Maggie Paul for assistance with data analyses; Sara Metzger for production assistance; and Rebecca Julian for editorial assistance.
Disclosure Statement
The initiative was supported by an unrestricted educational grant from Lilly USA, LLC. The funding source has no role in the execution, analysis, or development of the manuscript associated with this initiative. Dr. Greenspan discloses she has received consulting fees from Merck & Co., Inc., research support from Amgen, Eli Lilly and Company, Warner Chilcott, and Merck & Co., Inc. Dr. Bilezikian discloses he has received consulting fees from Amgen; Lilly USA, LLC; Johnson & Johnson; NPS Pharmaceuticals; and Merck & Co., Inc. and research support from Amgen, GlaxoSmithKline, and NPS Pharmaceuticals, Inc. Dr. Watts discloses that he has received consulting fees from Abbott Laboratories; Amgen; Endo Pharmaceuticals, Inc.; Medpace, Inc.; Johnson & Johnson; Lilly USA, LLC; Merck & Co., Inc.; Nitto Denko Corporation; Noven Pharmaceuticals, Inc.; Novo Nordisk; Pfizer, Inc.; and Quark Pharmaceuticals, Inc.; speaker fees from Amgen, Novartis Pharmaceuticals Corporation, and Warner Chilcott; research support from Merck & Co., Inc. and NPS Pharmaceuticals, Inc.; and is the co-founder, director, and stockholder of OsteoDynamics. For the remaining authors, no competing financial interests exist.
References
- 1.National Osteoporosis Foundation. What is osteoporosis? www.nof.org/articles/7. [Jun 3;2013 ]. www.nof.org/articles/7
- 2.National Osteoporosis Foundation. Debunking the myths. www.nof.org/articles/4. [Feb 20;2013 ]. www.nof.org/articles/4
- 3.National Osteoporosis Foundation. Washington, DC: National Osteoporosis Foundation; 2002. America's bone health: The state of osteoporosis and low bone mass in our nation. [Google Scholar]
- 4.National Osteoporosis Foundation. Washington, DC: National Osteoporosis Foundation; 2010. Clinician's guide to prevention and treatment of osteoporosis. [Google Scholar]
- 5.United States Preventive Services Task Force. Screening for osteoporosis: An update for the U.S. Preventive Task Force. Ann Intern Med. 2010;153:1–13. doi: 10.7326/0003-4819-153-2-201007200-00262. [DOI] [PubMed] [Google Scholar]
- 6.Lafata JE. Kolk D. Peterson EL, et al. Improving osteoporosis screening: Results from a randomized cluster trial. J Gen Intern Med. 2007;22:346–351. doi: 10.1007/s11606-006-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Board of Internal Medicine. Requirements for maintenance of certification. www.abim.org/moc/requirements-for-MOC.aspx. [Jan 29;2013 ]. www.abim.org/moc/requirements-for-MOC.aspx
- 8.National Commission on Certification of Physician Assistants. Certification maintenance. www.nccpa.net. [May 22;2013 ]. www.nccpa.net
- 9.Davis DA. Mazmanian PE. Fordis M. Van Harrison R. Thorpe KE. Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: A systematic review. JAMA. 2006;296:1094–1102. doi: 10.1001/jama.296.9.1094. [DOI] [PubMed] [Google Scholar]
- 10.American Medical Association Division of Continuing Physician Professional Development (CPPD) AMA PRA rules approved for performance improvement (PI) activities. 2004. www.ama-assn.org/resources/doc/cme/cppd15.pdf. [May 22;2013 ]. www.ama-assn.org/resources/doc/cme/cppd15.pdf
- 11.Kahn N. Bagley B. Tyler S. Performance improvement CME: Core of the new CME. American Medical Association CPPD report spring. 2007. www.ama-assn.org/resources/doc/cme/cppd22.pdf. [Jan 29;2013 ]. www.ama-assn.org/resources/doc/cme/cppd22.pdf
- 12.Watts NB. Bilezikian JP. Camacho PM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16:S1–S37. doi: 10.4158/ep.16.s3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Physicians. Current guidelines. www.acponline.org/clinical_information/guidelines/guidelines www.acponline.org/clinical_information/guidelines/guidelines
- 14.National Quality Forum. NQF endorsed standards. www.qualityforum.org/QPS www.qualityforum.org/QPS
- 15.National Committee for Quality Assurance. HEDIS and quality measurement. www.ncqa.org www.ncqa.org
- 16.North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17:25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services. Code of Federal Regulations. Title 45. Public welfare. Part 46. Protection of human subjects. 2009. www.hhs.gov/ohrp/policy/ohrpregulations.pdf. [Jan 25;2013 ]. www.hhs.gov/ohrp/policy/ohrpregulations.pdf [PubMed]
- 18.Leslie WD. Morin S. Lix LM, et al. Fracture risk assessment without bone density measurement in routine clinical practice. Osteoporos Int. 2012;23:75–85. doi: 10.1007/s00198-011-1747-2. [DOI] [PubMed] [Google Scholar]
- 19.Dunniway DL. Camune B. Baldwin K. Crane JK. FRAX® counseling for bone health behavior change in women 50 years of age and older. J Am Acad Nurse Pract. 2012;24:382–389. doi: 10.1111/j.1745-7599.2012.00700.x. [DOI] [PubMed] [Google Scholar]
- 20.Izuora KE. Alazraki N. Byrd-Sellers J. Tangpricha V. Nanes MS. Fracture assessment tool risk scores in bone density reports do not change physician prescribing behavior for osteoporosis. Am J Med Sci. 2011;342:5–8. doi: 10.1097/MAJ.0b013e31820aba02. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Nov 30, 2010. www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Report-Brief.aspx. [Feb 11;2013 ]. www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Report-Brief.aspx
- 22.Slomski A. IOM endorses vitamin D, calcium only for bone health, dispels deficiency claims. JAMA. 2011;305:453–454. doi: 10.1001/jama.2011.50. [DOI] [PubMed] [Google Scholar]
- 23.National Osteoporosis Foundation. Following is a statement by the National Osteoporosis Foundation on Vitamin D Recommendations. Nov 30, 2010. www.nof.org/news/219. [Feb 11;2013 ]. www.nof.org/news/219
- 24.Lewiecki EM. Prevention and treatment of postmenopausal osteoporosis. Obstet Gynecol Clin North Am. 2008;35:301–315. doi: 10.1016/j.ogc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Cramer JA. Gold DT. Silverman SL. Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 26.Solomon DH. Avorn J. Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414–2419. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 27.Siris ES. Harris ST. Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 U.S. claims databases. Mayo Clin Proc. 2006;81:1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 28.Caro JJ. Ishak KJ. Huybrechts KF. Raggio G. Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–1008. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP. Hoekstra JW. Larson DM, et al. A report of quality improvement in the care of patients with acute coronary syndromes. Crit Pathways in Cardiol. 2011;10:29–34. doi: 10.1097/HPC.0b013e318204eb8b. [DOI] [PubMed] [Google Scholar]
- 30.Goldhaber SZ. Ortel TL. Berry CA. Stowell SA. Gardner AJ. Improving physician performance of inpatient venous thromboembolism risk assessment and prophylaxis. Hospital Practice. 2013;41:123–131. doi: 10.3810/hp.2013.04.1061. [DOI] [PubMed] [Google Scholar]
- 31.Stowell SA. Baum HBA. Berry CA. Perri, et al. The impact of performance improvement strategies on the clinical care and outcomes of patients with type 2 diabetes. Clin Diabetes. 2013 doi: 10.2337/diaclin.32.1.18. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley SM. Huszti E. Warren SA. Merchant RM. Sayre MR. Nichol G. Duration of hospital participation in Get With the Guidelines–Resuscitation and survival of in-hospital cardiac arrest. Resuscitation. 2012;83:1349–1357. doi: 10.1016/j.resuscitation.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berenguer CM. Ochsner MG., Jr Lord SA. Senkowski CK. Improving surgical site infections: Using National Surgical Quality Improvement Program data to institute surgical care improvement project protocols in improving surgical outcomes. J Am Coll Surg. 2010;210:737–741. doi: 10.1016/j.jamcollsurg.2010.01.029. [DOI] [PubMed] [Google Scholar]