Abstract
Objective
To determine if dietary modifications with tomato products and/or a soy supplement affected circulating levels of IGF-1 and other markers of cell-signaling in postmenopausal women at risk for breast cancer.
Methods
Eligible and consented postmenopausal women at high risk for developing breast cancer were enrolled in a 26-week, two-arm (tomato and soy, 10 weeks each) longitudinal dietary intervention study in which each woman served as her own control. Changes in biochemical endpoints including Insuline-like Growth Factor (IGF)-1, IGF binding protein (BP)-3, estradiol, sex hormone binding globulin (SHBG), c-peptide, and insulin were measured for each intervention arm. Carotenoid and isoflavone levels were measured to assess adherence.
Results
Significant increases in carotenoid and isoflavone levels during the tomato and soy study arms, respectively, suggested that women were adherent to both arms of the intervention. The tomato-rich diet had little effect on cell-signaling biomarkers previously associated with breast cancer risk. However, results of the soy intervention showed that concentrations of IGF-1 and IGFBP-3 increased by 21.6 and 154.7 μmol/L, respectively (p=0.001 for both) and SHBG decreased by 5.4 μmol/L (p<0.001) after consumption of the soy protein supplement.
Conclusions
Increased soy protein intake may lead to small, but significant, increases in IGF-1 and IGFBP-3. Soy consumption also led to a significant decrease in SHBG, which has been hypothesized to promote, rather than prevent, cancer growth. Previous epidemiological studies, however, have confirmed soy’s protective effect on breast cancer. Additional investigation regarding the effect of soy on breast cancer risk and its mechanism of action appears warranted.
Keywords: breast cancer, tomato, soy, lycopene, isoflavone, insulin-like growth factor-1, inflammation, sex hormone
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer among women in the United States and worldwide (1, 2). Currently, women at high risk for developing breast cancer lack significant and practical primary preventive measures with which to protect themselves. Although tamoxifen and raloxifene have been consistently shown to reduce the risk of developing breast cancer (3), prophylaxis with these agents is accompanied by harsh side-effects, which may be responsible for the “exceptionally low” utilization of these agents for chemoprevention in otherwise healthy women (4). Dietary factors have been thought to account for roughly 30% of cancers in Western countries (5, 6), making diet second only to tobacco as a preventable cause of cancer. However, current research about breast cancer prevention and diet to date has been largely ecologic and unable to tease out specific dietary compounds that promote or prevent cancer. Recommendations for prevention are largely based on epidemiological or laboratory studies, and randomized intervention trials that investigate breast cancer prevention are rare. Presently, only increased alcohol consumption and postmenopausal obesity have shown consistent associations with breast cancer risk (7, 8). Research on other dietary factors including meat, dairy products, fruits and vegetables, fiber, fat, and phytoestrogens has been inconsistent (9-11). This translational gap in research has left large areas of uncertainty and controversy in both the scientific and lay communities regarding breast cancer risk and diet.
A common mechanism of action at the foundation of many dietary hypotheses is the effect of dietary modifications on insulin-like growth factor (IGF)-1 and IGF binding protein (IGFBP)-3 endocrine and tissue networks. IGF-1 is a mitogenic and antiapoptotic peptide hormone that plays an important role in cell regulation and tumorigenenisis. IGF-1 binds mainly to IGFBP-3 which both sequesters and regulates the effects of IGF-1. Increased levels of both IGF-1 and IGFBP-3 have been associated with breast cancer risk in animal and human studies (12-14). Recently, tomato and soy have separately been examined as potential chemopreventive agents for breast cancer that function through the IGF-1/IGFBP-3 signaling pathway.
Possible benefit from tomatoes is thought to be derived from lycopene, the carotenoid that provides the familiar red color to tomatoes. Lycopene has been shown in vitro to be a potent antioxidant (15), to reduce the risk of tumor progression (16, 17), and to have an antiproliferative effect on mammary tumors (18, 19). Human studies demonstrate that lycopene interacts with IGF-1 and IGFBP-3 (20), and can eliminate free oxygen radicals—thereby preventing DNA mutation, cell cycle alteration, and apoptotic disruption (21). However, human studies investigating the association between breast cancer risk and dietary intake of tomatoes, tomato products, and serum levels of lycopene, and between lycopene intake, IFG-1, IGFBP-3, and other biomarkers of cell growth and proliferation have been mixed (22-25).
Soy has also been studied extensively for its potential chemopreventive effects in breast cancer. Ecologic studies demonstrate that breast cancer risk for Western women is about six times higher than that of Asian women. Much of the variation in breast cancer incidence between Western and Asian women is explained by differences in established reproductive risk factors such as age at menarche, parity, age at births, and history of breastfeeding. However, it is hypothesized that diet may account for a notable portion of this difference (8, 26), and one of the many differences in the diets of Asian populations is that soy foods are consumed daily. Soy contains many substances hypothesized to inhibit breast cancer development, including phytoestrogens (isoflavones and lignans), protease inhibitors, saponins, and phytic acid (26, 27). Researchers have hypothesized that estrogen-like isoflavones in soy, similar to hormone-replacement therapy (28) and tamoxifen (29), lower IGF-1 levels. However, the results of animal and human studies investigating soy, IGF-1, IGFBP-3, and breast cancer risk have been mixed (26, 30-35).
Although some evidence exists for a protective effect of increased lycopene and soy consumption, independently, on breast cancer risk, further research is needed regarding the effects of lycopene and soy on the IGF-1 axis and other markers of cell signaling in relation to breast cancer progression. The primary objective of this study was to determine if dietary modifications with tomatoes and a soy supplement affected circulating IGF-1 levels in postmenopausal women at risk for developing breast cancer. Secondary aims were to determine the effects of these foods on other potential biomarkers of breast cancer risk including, IGFBP-3, estrodiol levels, sex hormone-binding globulin (SHBG), c-peptide, and insulin. We also assessed adherence to tomato- and soy-rich dietary modifications.
METHODS
Study Population
Postmenopausal women (i.e., no menstrual period for 12 months if over age 55 or no menstrual period for 12 months and a follicle-stimulating hormone (FSH) level > 30 if under age 55) at high risk for developing breast cancer were eligible to participate in the study. High risk was defined as having a body mass index (BMI) between 25 and 42kg/m2 (inclusive) and/or having a primary relative (i.e., mother, daughter, or sister) who had been diagnosed with breast cancer. To be eligible, women could not currently be taking hormone replacement therapy or a selective estrogen receptor modulator (SERM, e.g., tamoxifen or raloxifen). The study was approved by the Ohio State University Institutional Review Board.
Screening and Recruitment
Screening and recruitment occurred at medical and surgical oncology clinics, mammography centers, and the breast clinic at The Ohio State University Comprehensive Cancer Center. Physicians and their staff were asked to identify potential participants, and names of interested women were forwarded to the research staff for chart review. Prior to contacting women identified through chart review, permission was obtained from their physicians. Women were contacted via telephone by a trained research staff member who provided a general description (purpose and requirements) of the study and determined if the woman met study inclusion criteria. All potential subjects who were eligible and expressed an interest in participating were then scheduled to meet with a member of the research staff. At the initial visit, the research staff explained study requirements, answered potential participants’ questions, and obtained written informed consent and HIPAA authorization. Recruitment began February 2003 and was completed in September 2004.
Intervention
Consented and eligible women were enrolled in a 26-week, two-arm longitudinal dietary intervention study in which each woman served as her own control. The study consisted of three, two-week washout periods and two, 10-week dietary periods (Figure 1). During each two-week washout period, women were instructed to abstain from both tomato and soy products. For the 10-week tomato arm, women were instructed to consume approximately two or more tomato products daily to equal at least 25 milligrams (mg) of lycopene and not to consume any soy products. Women were asked to document the daily intake of tomato products on a worksheet that was provided to them. To aid in the consumption of tomato products, women were provided with tomato juice, tomato paste, and spaghetti sauce.
Figure 1.
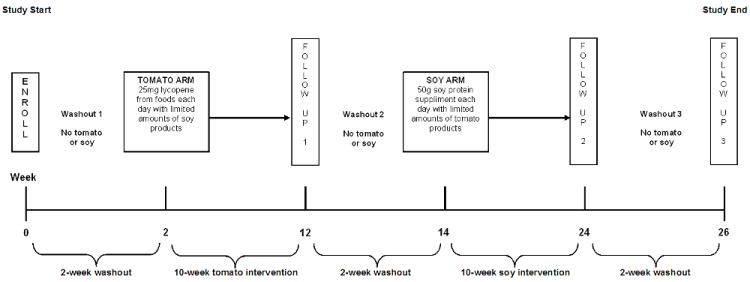
Study design schema
During the 10-week soy arm, each woman was given a powdered soy protein product (Dupont Technologies International, St. Louis, MO) that could be mixed with any liquid and provided 50 grams (g) of soy protein each day. Women were instructed to keep track of their daily consumption of soy by completing a provided soy calendar, and were told to limit the consumption of tomato products to 5mg of lycopene per day during the soy arm. Women were also given instructions and recipes to encourage consumption of the soy product. If a woman was taking a multivitamin, she was asked to replace it with a standard vitamin supplement provided by research staff because many brands of multivitamins contain certain elements that could have affected the results of the study. Women not already taking a vitamin supplement were not given a standard multivitamin.
Measurement
Dietary data were obtained via food frequency questionnaires at baseline, and three-day food records at baseline, during the tomato arm, and during the soy arm. Participant’s age, race, marital status, educational level, annual household income, and alcohol and tobacco consumption were determined by self-report at baseline. Height and weight were measured by the research staff at baseline. In addition, blood specimens and weight were obtained at the end of each washout period and at the end of each 10-week intervention period. Blood specimens were used to measure changes in biochemical endpoints including IGF-1, IGFBP-3, estradiol, SHBG, c-peptide, insulin, lycopene isomers, and carotenoids. Urine samples were also obtained to measure the effect of the dietary interventions on isoflavone levels, specifically the predominant soy isoflavones genistein and diadzein and their metabolic forms (dihydrogenistein and dihydrodiadzein, respectively). Adherence was measured using 1) completion of a daily tomato worksheet, 2) soy protein package counts, and 3) biochemical measures of adherence using blood carotenoid levels (lycopene and β-carotene) as markers of consumption of tomato products and urine isoflavone levels as markers of dietary soy consumption.
Statistical Analyses
Descriptive statistics were used to provide overall study population characteristics, categorize changes in biomarkers over the course of the study, and to analyze dietary compliance data. IGF-1 levels and secondary biomarker levels were measured before and after each 10-week treatment arm (tomato and soy, respectively). Treatment effects were estimated as the difference in pre- and post-treatment biomarker concentration levels after each washout period. To be analyzed as part of a treatment arm a woman had to complete the entire (10-week) arm of the study and have her follow-up assessments within 20 weeks of the beginning of the treatment arm.
Concentrations of biomarker endpoints were reported as means and standard deviations. The paired differences of several measures in both the tomato and soy arm were highly nonparametric, thus, intervention effects were estimated with exact Wilcoxon signed rank tests (nonparametric mean pairwise tests) that compared the biochemical concentration levels from the pre- and post-treatment periods, measured for both the tomato and soy arms. All analyses were conducted using SAS 9.2 (Cary, NC).
RESULTS
Overall, of 6,935 women initially screened for the study, 6,721 were ineligible to participate. Not being postmenopausal accounted for the large majority of ineligibles. Among eligible patients, 144 refused to participate. Seventy-four women were enrolled, however, four were deemed ineligible after enrollment and were not included in the analysis. Mean age of the 70 eligible women enrolled in the study was 57.2 years, and most women (58.6%) were between 50 and 60 years old. Additionally, most of the women were white (81.4%), married (72.9%), had some post-secondary education (85.5%), and had a combined household income of at least $50,000 annually (69.1%). The average baseline BMI of participating women was 30.0, which remained constant over the duration of the study (data not shown). All but nine of the 70 women had a BMI above normal, with 44.3% and 42.9% being overweight or obese, respectively. Most women (85.5%) were not current smokers and few women drank two or more alcoholic drinks per day (4.4%) (Table 1). Most women (45.7%) met the “high risk” eligibility criteria because they had both a family history of breast cancer and a BMI between 25-42 kg/m2. Women who met only the BMI or family history eligibility criteria comprised 42.9% and 11.4%, respectively, of the overall study population.
Table 1.
Demographic characteristics of participating women at baseline (n=70)
| Demographic Characteristic | n (%)* |
|---|---|
| Age (years) | |
| 40-49 | 8 (11.4) |
| 50-59 | 41 (58.6) |
| 60-69 | 18 (25.7) |
| 70-79 | 3 (4.3) |
| Race | |
| White | 57 (81.4) |
| Black | 13 (18.6) |
| Marital Status | |
| Married | 51 (72.9) |
| Unmarried | 19 (27.1) |
| Education† | |
| Graduate/professional degree | 12 (21.8) |
| Bachelor’s degree | 15 (27.3) |
| Associate’s degree | 4 (7.3) |
| Some post-secondary | 16 (29.1) |
| High school or less | 8 (14.6) |
| Annual Household Income† | |
| $0 - $24,999 | 6 (10.9) |
| $25,000 - $49,999 | 11 (20.0) |
| $50,000 - $74,999 | 13 (23.6) |
| $75,000 - $99,999 | 8 (14.6) |
| $100,000 - $149,999 | 8 (14.6) |
| $150,000 or higher | 9 (17.4) |
| Body Mass Index (BMI, kg/m2) | |
| Less than 18.5 (underweight) | 1 (1.4) |
| 18.5 - 24.9 (normal) | 8 (11.4) |
| 25.0 - 29.9 (overweight) | 31 (44.3) |
| 30.0 or higher (obese) | 30 (42.9) |
| Smoking Status‡ | |
| Never smoker | 33 (47.8) |
| Former smoker | 26 (37.7) |
| Current smoker | 10 (14.5) |
| Alcohol Consumption‡ (avg. drinks/day) | |
| None | 24 (34.8) |
| 1 or fewer | 42 (60.9) |
| 2 or more | 3 (4.4) |
15 patients were missing information about educational attainment and annual household income.
1 patient was missing information about tobacco and alcohol use.
Percentages may not add to 100.0% because of rounding.
Tomato Arm
One woman was not included in the tomato arm analyses because her follow-up assessment occurred 34 weeks after the baseline tomato assessment. Another five women did not complete the tomato arm follow-up assessment and four women discontinued the study prior to start of the tomato arm of the intervention, resulting in a total of 60 women for the tomato arm analyses. Means and standard deviations of the outcome measures for the tomato intervention at weeks 2 (post-washout) and 12 (post-tomato intervention) and for the paired differences (week 12 - week 2) are presented in Table 2. Mean pairwise differences showed no statistically significant changes in levels of IGF-1, IGFBP-3, estradiol, SHBG, c-peptide, or insulin at the end of the tomato intervention. Blood lycopene and β-carotene levels were used as biological markers of lycopene consumption. Analyses revealed that women were adherent to the tomato arm of the study, as evidenced by the concentrations of carotenoid isomer markers over time (Figure 2). Additionally, during the tomato arm, women were instructed to consume enough tomato products to equal at least 25mg of lycopene per day, and average self-reported lycopene consumption during the tomato arm of the study was 29.7mg/day.
Table 2.
Means, differences, and standard deviations of hormonal network biomarkers (μmol/L) for the tomato arm of the intervention (n=60)†
| Biomarker | Week 2 | Week 12 mean (sd) | Difference‡ | P§ |
|---|---|---|---|---|
| IGF-1 | 129.1 (39.7) | 132.0 (39.9) | 2.9 (21.1) | .19 |
| IGFBP-3 | 2,076.1 (441.5) | 2,055.2 (426.2) | -20.9 (167.7) | .56 |
| Estradiol | 14.6 (5.2) | 16.5 (9.7) | 1.9 (9.0) | .19 |
| SHBG | 50.2 (24.7) | 51.8 (25.0) | 1.7 (6.5) | .08 |
| c-peptide | 4.6 (2.7) | 4.6 (2.4) | .04 (2.0) | .55 |
| Insulin | 11.9 (11.6) | 10.6 (7.0) | 8.6 (.11) | .60 |
IGF-1 is insulin-like growth factor.
IGFBP-3 is insulin-like growth factor binding protein.
SHGB is sex hormone binding globulin.
One woman was excluded because of a late follow-up, and nine women were lost to follow-up.
Difference is calculated as Week 12 - Week 2.
P-values were calculated using exact Wilcoxon signed rank tests (nonparametric mean pairwise tests).
Figure 2.
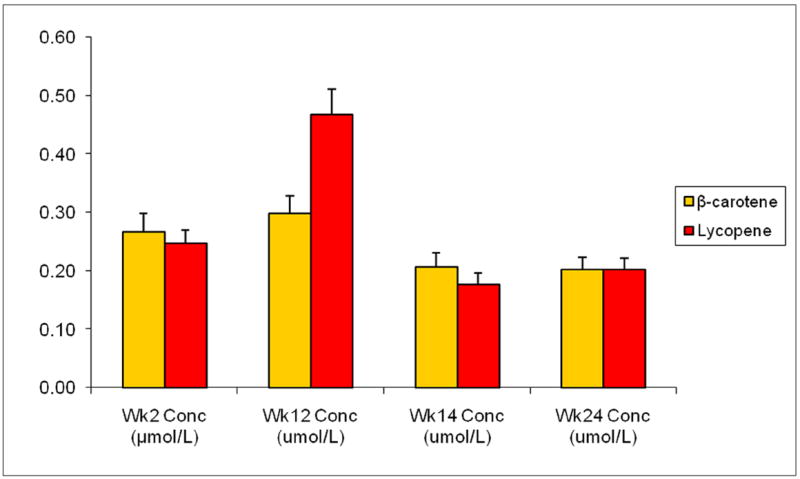
Blood serum concentrations (μmol/L) of β-carotene and lycopene by week as measures of tomato arm adherence (n=60)
Blood serum concentrations (μmol/L) of β-carotene and lycopene were measured at weeks 2, 12, 14, and 24. Weeks 2, 12, 14, and 24 represent the end of the first washout periods, the tomato intervention, the second washout period, and the soy intervention, respectively. Adherence is illustrated by the significant increase in serum carotenoids β-carotene and lycopene at week 12 compared to week 2 (exact Wilcoxon sign ranked test p<.01 for both).
Soy Arm
Four women were not included in the soy intervention analyses because their follow-up assessments occurred 20 or more weeks after the baseline soy assessment. Another two subjects did not have follow-up assessments, and nine women discontinued the study prior to the soy arm of the intervention, resulting in a total of 55 subjects for the soy analyses. Means and standard deviations of the outcomes measures for the soy intervention at weeks 14 (post-washout) and 24 (post-soy intervention) and for the paired differences (week 24 - week 14) are presented in Table 3. Mean pairwise differences showed that, after the soy intervention, there were statistically significant increases in levels of IGF-1 (difference=21.6 μmol/L ; p=.001) and IGFBP-3 (difference=154.7 μmol/L ; p=.001) and a statistically significant decrease in SHBG (difference=-5.4 μmol/L ; p< .001). Although women only consumed an average of 1.5 packets/day of soy protein (less than the goal of 2.0 packets/day), significant increases were seen in concentrations of several biomarkers of soy isoflavones including daidzein, dihydrodaidzein, genistein, and dihydrogenistein during the soy arm of the study (all p< .001) (Figure 3).
Table 3.
Means, differences, and standard deviations of hormonal network biomarkers (μmol/L) for the soy arm of the intervention (n=55)†
| Biomarker | Week 14 | Week 24 mean (sd) | Difference‡ | P§ |
|---|---|---|---|---|
| IGF-1 | 129.5 (42.1) | 151.0 (71.1) | 21.6 (59.1) | .001 |
| IGFBP-3 | 2,062.3 (462.8) | 2,217.0 (550.4) | 154.7 (343.8) | .001 |
| Estradiol | 14.2 (4.7) | 14.6 (6.5) | .37 (5.1) | .36 |
| SHBG | 53.1 (27.2) | 47.6 (26.0) | -5.43 (9.4) | <.001 |
| c-peptide | 4.5 (2.3) | 4.4 (2.3) | -.12 (1.2) | .50 |
| Insulin | 11.0 (9.2) | 10.4 (6.7) | -.62 (6.3) | .79 |
IGF-1 is insulin-like growth factor.
IGFBP-3 is insulin-like growth factor binding protein.
SHGB is sex hormone binding globulin.
Four women were excluded because of a late follow-up, and 11 women were lost to follow-up.
Difference is calculated as Week 24 - Week 14.
P-values were calculated using exact Wilcoxon signed rank tests (nonparametric mean pairwise tests).
Figure 3.
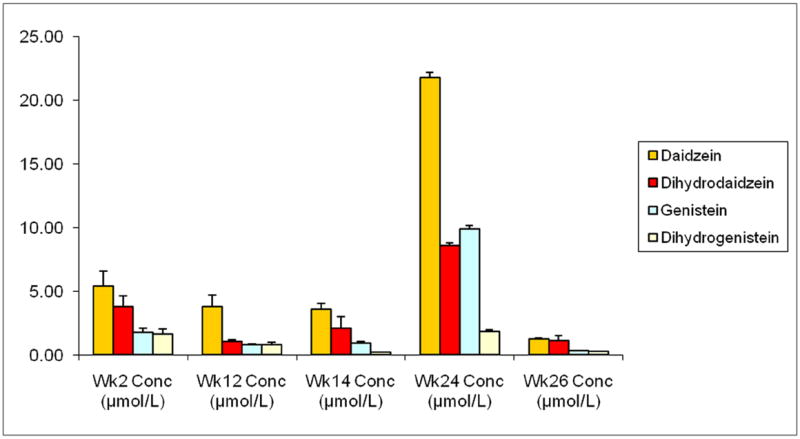
Urine concentrations (μmol/L) of isoflavone markers by week as measures of soy arm adherence (n=55)
Urine concentrations (μmol/L) of diadzein, dihydrodaidzein, genistein, and dihydrogenistein were measured at weeks 2, 12, 14, 24, and 26. Weeks 2, 12, 14, 24, and 26 represent the end of the first washout periods, the tomato intervention, the second washout period, the soy intervention, and the final washout period, respectively. Adherence is illustrated by the significant increase in all measured urinary isoflavones at week 24 compared to week 14 (exact Wilcoxon sign ranked test p<.001 for all).
DISCUSSION
To reduce the human and economic burden of breast cancer, effective methods of primary prevention must be identified. Currently, little is known about how a woman can practically lower her risk for the disease. In assessing the potential chemopreventive effects of separate tomato- and soy-rich diets among postmenopausal women at high risk for breast cancer, we chose to avoid a “reductionist” approach, which focuses only on a pure substance (such as lycopene or soy isoflavones diadzein and genistein), as soy and tomato products may have a number of phytochemicals with the potential to reduce cancer risk. Thus, we tested individual biochemical effects of the introduction of 10-week, tomato- and soy-rich diets under the assumption that the benefits of a food product may be much greater than the benefits achieved with a single biochemical component, and that whole food products offer a safe and potentially more effective intervention.
The six biochemical endpoints we chose to evaluate have all been previously hypothesized to be potential biomarkers for breast cancer risk. Experimental evidence suggests that insulin and insulin-like growth factors may play a role in breast pathology through their mitogenic and anti-apoptotic effects on normal, benign, and malignant breast epithelial cells (36). IGF binding proteins, on the other hand, are postulated to reduce growth stimulus by binding circulating IGFs and through a direct anti-proliferative effect on cells. In addition, studies have reported an increased risk of breast cancer with increasing concentrations of c-peptide in both pre- and postmenopausal women (36). Circulating sex steroid hormones such as testosterone and estradiol are associated with an increased risk of breast cancer in postmenopausal women as well, and predominantly bind to sex hormone binding globulin (SHBG) which limits the amount of ‘free’ circulating androgens and estrogens (37). Thus, assessing the effect of tomato- and soy-rich dietary interventions on potential breast cancer biomarkers may provide insight about reducing breast cancer risk in a safe and practical manner.
The average daily intake of lycopene in the United States has been reported to be 2-5mg/day (38), and women in our study consumed nearly 30mg/day, on average, during the tomato arm of the intervention. Results of this study, however, suggest that a tomato-rich diet had little effect on cell signaling biomarkers previously associated with breast cancer risk. There was a marginally significant increase in SHBG (p=0.08) after the tomato intervention, which could lead to a decrease in the biological availability of sex hormones, however, the effect size was small. Previous studies investigating the effects of tomatoes and its primary active ingredient, lycopene, on breast cancer risk have had mixed results, but largely have shown no effect (22-25).
Results of the soy intervention showed that blood-level concentrations of IGF-1 and IGFBP-3 significantly increased after consumption of the soy protein supplement (p=0.001 for both). If soy does protect against the development of breast cancer as shown in previous epidemiological studies (34, 35, 39), and the mechanism of protection is via the IGF-1/IGFBP-3 pathway, the opposite result would have been expected. This study, however, is not the first to report an increase in IGF-1 and IGFBP-3 following soy supplementation. A study of healthy women reported increases in IGF-1 and IGFBP-3 after a one-week phytoestrogen supplementation (40) as did a longitudinal study examining the effect of dietary protein and soy isoflavones among prostate cancer patients (41). Furthermore a study of healthy men consuming a soy protein drink (42), and a study of postmenopausal women also receiving soy protein reported increases in IGF-1 levels (43). A small increase in IGF-1 levels was reported in a 2-year intervention of premenopausal women as well (44). Thus, there now seems to be relatively consistent evidence that soy (either in protein supplement or whole food form) does not lower serum levels of IGF-1 or IGFBP-3. Rather, as hypothesized by Maskarinec et al. (44) and the authors of this study, an increased protein intake inherent in consuming additional soy products and in soy protein supplementation may lead to small, but significant, increases in IGF-1 and IGFBP-3.
Results of the soy intervention also showed a significant decrease in SHBG, which could lead to an increase in the biological availability of sex hormones. Other authors have raised such concerns about isoflavones in soy, specifically, that their estrogenic effects may promote, rather than prevent, cancer and estrogen-dependent mammary tumor growth (45, 46). However, previous studies have shown that soy isoflavones increase the synthesis of SHBG (opposite to our results) (45, 47), and a recent, large, and population-based study of soy confirmed its protective effect on breast cancer (32). Additional investigation to elucidate this effect appears warranted.
Overall, although there is strong in vitro evidence (17, 18, 48-52) and moderate in vivo evidence (19, 53) that lycopene protects against the development of breast cancer by affecting levels of biomarkers known to influence cell cycling and tumorigenesis, human studies have largely found no association (10, 54-56). Our results support previous null human findings regarding lycopene and breast cancer risk. The reasons for the lack of association between lycopene and a reduction in levels of biomarkers of oxidative stress and cell signaling associated with cancer progression in human studies could be many and include: 1) high levels of carotenoids (including lycopene) in the serum may actually be markers for other compound(s) responsible for protection against breast cancer, 2) carotenoids could be affected by other factors (e.g., smoking), and 3) different methods and exposure levels have been used for in vitro, in vivo, and human studies.
Regarding soy, several human studies have shown no effect of soy on IGF-1 and IGFBP-3 levels (57, 58). Others, including ours, have shown small, statistically significant increases in IGF-1, IGFBP-3, or both (42, 44). Thus, the majority of published literature does not support the notion that soy’s protective effect on breast cancer functions through the IGF-1/IGFBP-3 axis. It is more likely, that soy’s breast cancer chemoprevention effects (if they truly exist) stem from phytoestrogens (namely isoflavones) that act as SERMs (59). Like other SERMs, however, phytoestrogens have both estrogenic and antiestrogenic properties, and more studies are needed to assess their safety. Although many studies have shown a protective effect of soy consumption on breast cancer risk (33) and better post-diagnostic breast cancer survival among women who consume soy (32, 60), questions still remain about phytoestrogen’s ability to stimulate breast tissue and promote estrogen-dependent tumor growth.
This study is not without limitations. Sample size was small, and not all enrolled participants completed each arm (tomato and soy) of the study. Adherence to both the tomato and soy diets for individuals who completed the study, however, was high as confirmed by both dietary calendars and biomarker confirmation, and, compared to other tomato and soy dietary intervention studies, our sample size was actually rather large (55, 56, 61-65).
Another limitation of this study is that our results are generalizable only to postmenopausal women at high risk for breast cancer. Previous research describing the relationship between IGF-1 and IGFBP-3 on breast cancer has suggested effect modification by age (i.e., menopausal status). Research about the age-dependent effects of IGF-1 and IGFBP-3 on breast cancer risk, however, is largely contradictory. Early research indicated that increasing circulating concentrations of IGF-1 and IGFBP-3 were related to increased risk of breast cancer in premenopausal women only (12-14, 55, 56). More recent research, however, has suggested that there is no effect of IGF-1 and IGFBP-3 on breast cancer risk in premenopausal women (66-68), and, instead, that higher levels of IGF-1 and IGFBP-3 are associated with increased risk of breast cancer in women older than age 50 (66, 67). Thus, future studies examining the relationship between IGF-1 and IGFBP-3, diet, and breast cancer risk should be especially mindful of age-specific effects and examine the outcomes of dietary modification in both pre- and postmenopausal women separately.
Other limitations of this study include the fact that only dietary information regarding tomato and soy intake were recorded. Thus, we know little about whether the additional tomato products and the soy supplement were consumed in addition to each participant’s normal diet, or if the participants’ diets were altered to compensate for the addition of tomato and soy supplementation (i.e., tomato and soy products were substituted for other foods or meals). Thus, future studies should focus on parsing out whether the biological effects of dietary modification with tomato and soy products are the result of supplementation or substitution.
In spite of limitations, this study is one of few prospective studies to analyze the effects of tomato and soy dietary modifications on several biomarkers associated with breast cancer risk in a U.S. population. Additionally, the crossover design of this study with appropriate washout periods provides highly valid results that cannot be achieved with case-control studies performed previously. Finally, adherence to each dietary arm was high and loss-to-follow-up was relatively low.
The true effect of soy phytoestrogens on breast cancer risk is still unknown. There is a great deal of in vitro evidence that supports a protective role of soy isoflavones on the development of breast cancer (59). A suggested mechanism is that phytoestrogens are weaker estrogens than ovarian estrogens, and these weaker estrogens competitively inhibit the proliferative action of natural estrogens (69). A number of these studies, however, demonstrated that soy at low concentrations may act as an estrogen agonist and stimulate proliferation while at higher concentrations it acts antagonistically to estrogen, inhibiting cell growth (i.e., biphasic effects) (10, 54-56).
Further, it is possible that only long-term exposure to soy phytoestrogens is protective, as there is evidence that soy may only be beneficial if consumed in utero or before puberty (70, 71). Postmenopausal women, however, do not produce ovarian estrogen, and a sudden addition of soy phytoestrogens may cause increased breast cell proliferation (46), and is a significant cause for concern. We noted an increase in SHBG among postmenopausal women at high risk for breast cancer that supports this cautionary tone. Thus, future studies should continue to examine soy as a potential chemopreventive agent for breast cancer based upon promising in vitro, in vivo, and epidemiological evidence, but also be mindful of a potential differentiation of effects based on 1) at what age and for how long soy is introduced into the diet, 2) menopausal status, 3) current and past use of estrogen replacement therapy, tamoxifen, or other SERMs, 4) the hormone receptor status of the breast tumor, and 5) whether soy is introduced as a dietary supplement to a standard western diet, or as a dietary substitution for other (potentially more fatty) sources of protein typical of the American diet. Regardless, soy’s mechanism of action in breast tissue may likely hold the key to the etiology of breast disease itself, and should continue to be investigated.
Acknowledgments
The project described was supported by grants from the Breast Cancer Research Foundation and Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Breast Cancer Research Foundation, the National Center for Research Resources, or the National Institutes of Health.
References
- 1.American Cancer Society. Cancer Facts & Figures 2009. Atlanta, GA: American Cancer Society, Inc.; 2009. [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–98. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 19:443–6. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 6.World Health Organization. Cancer: diet and physical activity’s impact. Geneva, Switzerland: Noncommunicable Diseases and Mental Health Cluster (NMH), World Health Organization; 2003. [Google Scholar]
- 7.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ, Allen NE, Spencer EA, Travis RC. Nutrition and breast cancer. Breast. 2003;12:412–6. doi: 10.1016/s0960-9776(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 10.Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285:769–76. doi: 10.1001/jama.285.6.769. [DOI] [PubMed] [Google Scholar]
- 11.Wu AH, Pike MC, Stram DO. Meta-analysis: dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst. 1999;91:529–34. doi: 10.1093/jnci/91.6.529. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 13.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein 3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 14.Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, et al. Serum insulin-like growth factor-I and breast cancer. Int J Cancer. 2000;88:828–32. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Bohm F, Tinkler JH, Truscott TG. Carotenoids protect against cell membrane damage by the nitrogen dioxide radical. Nat Med. 1995;1:98–9. doi: 10.1038/nm0295-98. [DOI] [PubMed] [Google Scholar]
- 16.Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, et al. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis. 1997;18:89–92. doi: 10.1093/carcin/18.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Zhang LX, Cooney RV, Bertram JS. Carotenoids up-regulate connexin43 gene expression independent of their provitamin A or antioxidant properties. Cancer research. 1992;52:5707–12. [PubMed] [Google Scholar]
- 18.Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutrition and cancer. 1995;24:257–66. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- 19.Sharoni Y, Giron E, Rise M, Levy J. Effects of lycopene-enriched tomato oleoresin on 7,12-dimethyl-benz[a]anthracene-induced rat mammary tumors. Cancer detection and prevention. 1997;21:118–23. [PubMed] [Google Scholar]
- 20.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer epidemiology biomarkers and prevention : a publication of the American Association for Cancer Research cosponsored by the American Society of Preventive Oncology. 2001;10:861–8. [PubMed] [Google Scholar]
- 21.Nakachi K, Matusuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors. 2000;13:49–54. doi: 10.1002/biof.5520130109. [DOI] [PubMed] [Google Scholar]
- 22.Freudenheim JL, Marshall JR, Vena JE, Laughlin R, Brasure JR, Swanson MK, et al. Premenopausal breast cancer risk and intake of vegetables, fruits, and related nutrients. J Natl Cancer Inst. 1996;88:340–8. doi: 10.1093/jnci/88.6.340. [DOI] [PubMed] [Google Scholar]
- 23.Potischman N, McCulloch CE, Byers T, Nemoto T, Stubbe N, Milch R, et al. Breast cancer and dietary and plasma concentrations of carotenoids and vitamin A. The American journal of clinical nutrition. 1990;52:909–15. doi: 10.1093/ajcn/52.5.909. [DOI] [PubMed] [Google Scholar]
- 24.Wane D, Lengacher CA. Integrative review of lycopene and breast cancer. Oncol Nursing Forum. 2006;33:127–34. doi: 10.1188/06.ONF.127-137. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Tang G, Russell RM, Mayzel KA, Stampfer MJ, Willett WC, et al. Measurement of retinoids and carotenoids in breast adipose tissue and a comparison of concentrations in breast cancer cases and control subjects. Am J Clin Nutr. 1997;66:626–32. doi: 10.1093/ajcn/66.3.626. [DOI] [PubMed] [Google Scholar]
- 26.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. Review. J Natl Cancer Inst. 2006;98:1275–84. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 27.Fournier DB, Erdman JW, Jr, Gordon GB. Soy, its components, and cancer prevention: a review of the in vitro, animal, and human data. Cancer Epidemiol Biomarkers Prev. 1998;7:1055–65. [PubMed] [Google Scholar]
- 28.Chang S, Wu X, Yu H, Spitz MR. Plasma concentrations of insulin-like growth factors among healthy adult men and postmenopausal women: associations with body composition, lifestyle, and reproductive factors. Cancer Epidemiol Biomarkers Prev. 2002;11:758–66. [PubMed] [Google Scholar]
- 29.Decensi A, Bonanni B, Guerrieri-Gonzaga A, Gandini S, Robertson C, Johansson H, et al. Biologic activity of tamoxifen at low doses in healthy women. J Natl Cancer Inst. 1998;90:1461–7. doi: 10.1093/jnci/90.19.1461. [DOI] [PubMed] [Google Scholar]
- 30.Gann PH, Kazer R, Chatterion R, Gapstur S, Thedford D, Helenowski I, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: Effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- 31.Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, et al. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. American journal of epidemiology. 2001;154:434–41. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 32.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–71. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 34.Wu AH, Wan P, Hankin J, Tseng C-C, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–6. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. Journal of the National Cancer Institute. 2003;95:906–13. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 36.Schairer C, Hill D, Sturgeon SR, Fears T, Pollak M, Mies C, et al. Serum concentrations of IGF-I, IGFBP-3 and c-peptide and risk of hyperplasia and cancer of the breast in postmenopausal women. Int J Cancer. 2004;108:773–9. doi: 10.1002/ijc.11624. [DOI] [PubMed] [Google Scholar]
- 37.Thompson DJ, Healey CS, Baynes C, Kalmyrzaev B, Ahmed S, Dowsett M, et al. Identification of common variants in the SHBG gene affecting sex hormone-binding globulin levels and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:3490–8. doi: 10.1158/1055-9965.EPI-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care. 2002;5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 39.Dai Q, Shu XO, Jin F, Potter JD, Kushi LH, Teas J, et al. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. British journal of cancer. 2001;85:372–8. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodside JV, Campbell MJ, Denholm EE, Newton L, Honour JW, Morton MS, et al. Short-term phytoestrogen supplementation alters insulin-like growth factor profile but not lipid or antioxidant status. J Nutr Biochem. 2006;17:211–5. doi: 10.1016/j.jnutbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Dewell A, Weidner G, Sumner MD, Barnard RJ, Marlin RO, Daubenmier JJ, et al. Relationship of dietary protein and soy isoflavones to serum IGF-1 and IGF binding proteins in the Prostate Cancer Lifestyle Trial. Nutr Cancer. 2007;58:35–42. doi: 10.1080/01635580701308034. [DOI] [PubMed] [Google Scholar]
- 42.Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutrition. 2002;132:2605–608. doi: 10.1093/jn/132.9.2605. [DOI] [PubMed] [Google Scholar]
- 43.Arjmandi BH, Khalil DA, Smith BJ, Lucas EA, Juma S, Payton ME, et al. Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J Clin Endocrinol Metab. 2003;88:1048–54. doi: 10.1210/jc.2002-020849. [DOI] [PubMed] [Google Scholar]
- 44.Maskarinec G, Takata Y, Murphy SP, Franke AA, Kaaks R. Insulin-like growth factor-1 and binding protein-3 in a 2-year soya intervention among premenopausal women. British journal of nutrition. 2005;94:362–67. doi: 10.1079/bjn20051525. [DOI] [PubMed] [Google Scholar]
- 45.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67:398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Velentzis LS, Woodside JV, Cantwell MM, Leathem AJ, Keshtgar MR. Do phytoestrogens reduce the risk of breast cancer and breast cancer recurrence? What clinicians need to know. Eur J Cancer. 2008;44:1799–806. doi: 10.1016/j.ejca.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Wang Y, Mo B. The effects of carotenoids on the proliferation of human breast cancer cell and gene expression of bcl-2. Zhonghua yu fang yi xue za zhi. 2002;36:254–7. [Chinese journal of preventive medicine]. [abstract] [PubMed] [Google Scholar]
- 49.Prakash P, Russell RM, Krinsky NI. In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. The Journal of nutrition. 2001;131:1574–80. doi: 10.1093/jn/131.5.1574. [DOI] [PubMed] [Google Scholar]
- 50.Amir H, Karas M, Giat J, Danilenko M, Levy R, Yermiahu T, et al. Lycopene and 1,25-dihydroxyvitamin D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutrition and cancer. 1999;33:105–12. doi: 10.1080/01635589909514756. [DOI] [PubMed] [Google Scholar]
- 51.Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–36. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 52.Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12:2109–14. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]
- 53.Nagasawa H, Mitamura T, Sakamoto S, Yamamoto K. Effects of lycopene on spontaneous mammary tumour development in SHN virgin mice. Anticancer research. 1995;15:1173–8. [PubMed] [Google Scholar]
- 54.Gandini S, Merzenich H, Robertson C, Boyle P. Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. European journal of cancer. 2000;36:636–46. doi: 10.1016/s0959-8049(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 55.McEligot AJ, Rock CL, Flatt SW, Newman V, Faerber S, Pierce JP. Plasma carotenoids are biomarkers of long-term high vegetable intake in women with breast cancer. The Journal of nutrition. 1999;129:2258–63. doi: 10.1093/jn/129.12.2258. [DOI] [PubMed] [Google Scholar]
- 56.Pool-Zobel BL, Bub A, Muller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–50. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- 57.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Dietary soy and fats in relation to serum insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 levels in premenopausal Japanese women. Nutr Cancer. 2003;45:185–9. doi: 10.1207/S15327914NC4502_07. [DOI] [PubMed] [Google Scholar]
- 58.Vrieling A, Voskuil DW, Bueno de Mesquita HB, Kaaks R, van Noord PA, Keinan-Boker L, et al. Dietary determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1, -2 and -3 in women in the Netherlands. Cancer Causes Control. 2004;15:787–96. doi: 10.1023/B:CACO.0000043429.51915.c6. [DOI] [PubMed] [Google Scholar]
- 59.Duffy C, Cyr M. Phytoestrogens: potential benefits and implications for breast cancer survivors. J Womens Health (Larchmt) 2003;12:617–31. doi: 10.1089/154099903322404276. [DOI] [PubMed] [Google Scholar]
- 60.Guha N, Kwan ML, Quesenberry CP, Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395–405. doi: 10.1007/s10549-009-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu LJ, Anderson KE, Grady JJ, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer research. 2000;60:4112–21. [PubMed] [Google Scholar]
- 62.Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16alpha-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer research. 2000;60:1299–305. [PubMed] [Google Scholar]
- 63.McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, Potten CS, et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. The American journal of clinical nutrition. 1998;68:1431S–5S. doi: 10.1093/ajcn/68.6.1431S. [DOI] [PubMed] [Google Scholar]
- 64.Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. Journal of the National Cancer Institute. 1998;90:1830–5. doi: 10.1093/jnci/90.23.1830. [DOI] [PubMed] [Google Scholar]
- 65.Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, et al. Effects of soy foods on ovarian function in premenopausal women. British journal of cancer. 2000;82:1879–86. doi: 10.1054/bjoc.1999.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:763–8. doi: 10.1158/1055-9965.EPI-06-0960. [DOI] [PubMed] [Google Scholar]
- 67.Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C, Olsen A, et al. IGF-I, IGFBP-3 and breast cancer risk in women: The European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2006;13:593–605. doi: 10.1677/erc.1.01150. [DOI] [PubMed] [Google Scholar]
- 68.Schernhammer ES, Holly JM, Hunter DJ, Pollak MN, Hankinson SE. Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in The Nurses Health Study II. Endocr Relat Cancer. 2006;13:583–92. doi: 10.1677/erc.1.01149. [DOI] [PubMed] [Google Scholar]
- 69.Jakes RW, Duffy SW, Ng F-C, Gao F, Ng E-H, Seow A, et al. Mammographic parenchymal patterns and self-reported soy intake in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev. 2002;11:608–13. [PubMed] [Google Scholar]
- 70.Adami HO, Signorello LB, Trichopoulos D. Towards an understanding of breast cancer etiology. Semin Cancer Biol. 1998;8:255–62. doi: 10.1006/scbi.1998.0077. [DOI] [PubMed] [Google Scholar]
- 71.Hilakivi-Clarke L, Cabanes A, Olivo S, Kerr L, Bouker KB, Clarke R. Do estrogens always increase breast cancer risk? J Steroid Biochem Mol Biol. 2002;80:163–74. doi: 10.1016/s0960-0760(01)00184-4. [DOI] [PubMed] [Google Scholar]


