Abstract
TBI induces a neuroinflammatory response frequently associated with increased intracranial pressure (ICP). We investigated the effects of alcohol and increased extracellular pressure on murine BV-2 microglial proliferation and cytokine responses to lipopolysaccharide (LPS) stimulation. BV-2 cells were cultured under 0 or 30mmHg increased extracellular pressure without or with ethanol (100mM) or LPS (10ng/ml) for 24 hours. We assessed cell proliferation via MTS assay and secretion of the pro-inflammatory cytokines TNF-α, IL-6, and MCP-1 by ELISA. Increased pressure and LPS stimulation each promoted proliferation. Ethanol pretreatment blocked these effects. Basal TNF-α and IL-6 secretion were at the limits of delectability. Basal MCP-1 production was stimulated by pressure, which was blocked by ethanol. Even this low LPS dose stimulated microglial secretion of TNF-α, IL-6 and MCP-1. Pressure inhibited LPS-stimulated production of these proinflammatory cytokines, while ethanol pretreatment blocked LPS-stimulated cytokine production. The combination of pressure and ethanol further reduced TNF-α, IL-6 and MCP-1 secretion by LPS-stimulated microglial cells. Alcohol’s anti-inflammatory effects may contribute to the reduced TBI mortality that some have described in acutely intoxicated patients, while pressure downregulation of inflammatory cytokine release could create a negative feedback that ameliorates inflammation in TBI.
Keywords: Proinflammatory cytokines, microglial cells, MCP-1, IL-6, mechanotransduction
INTRODUCTION
Up to two million Americans experience traumatic brain injury (TBI) annually and 230,000 are hospitalized (17). 35-81% of TBI patients are intoxicated and 42% were heavy drinkers before injury (20). TBI involves both focal and diffuse brain injuries, inducing a neuroinflammatory response frequently associated with increased intracranial pressure (ICP) that leads to brain ischemia, herniation, and death (11).
ICP’s above 20 mm Hg (27 cm H2O) are high. We studied 30 mm Hg, resembling in vivo ICP after TBI. We previously described effects of similar pressures on cell proliferation and signaling in microglial cells (24), epithelial cells (8, 22), macrophages (21), monocytes (21) and fibroblasts (4). Pressures of 15 mmHg and 30 mmHg above ambient have similar effects on microglial cells (24). Lee reported that 100mM ethanol activated BV-2 cells but 10 mM ethanol did not (13). We studied 100mM ethanol here.
Microglial cells, the resident macrophages of the brain, sense CNS infection or injury and play a pivotal role in the CNS host response (14). Brain injury or infection activate microglial cells to produce pro-inflammatory mediators including tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and monocyte chemotactic protein 1 (MCP-1, CCL2). These cytokines initiate and reinforce inflammation in the CNS (12). By these actions, activated microglial cells may aggravate neuronal damage (6) and may be targets for immunosuppression after acute spinal cord or brain injury.
Alcohol generally adversely influences the nervous system, but its specific effect on brain inflammation is incompletely understood. We sought to determine how ethanol influences inflammatory microglial activation under increased pressure consistent with the increased ICP of TBI, using low dose LPS as a mild inflammatory stimulus.
MATERIALS and METHODS
Materials
Ethanol (200 proof) was from Fisher (Pittsburgh, PA), lipopolysaccharide (LPS from Salmonella enteric, L7770) from Sigma (St. Louis, MO), DMEM/F-12 with 2 mM glutamine, 0.05% trypsin-EDTA, 0.4% trypan blue stain and penicillin/streptomycin from Gibco (Gaithersburg, MD), and MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt, from Promega (Madison, WI). Enzyme-linked immunosorbent assay (ELISA) kits for Interleukin-6 (IL-6), TNF-α and MCP-1 were from BD Biosciences (San Diego, CA). Immortalized BV-2 mouse microglial cells developed by Dr. Blasi (5) were donated by Dr. Van Eldik at the University of Kentucky. The cells were maintained in DMEM/F-12 supplemented with 10% FBS, 100 IU/ml penicillin and 100 mg/ml streptomycin at 37°C with 5% CO2.
Pressure model
Pressure was increased 30 mmHg above ambient for some cell culture conditions with consistent temperature, pH and pO2 as previously (27).
Proliferation
Proliferation was assessed by colorimetric MTS assay as described by the manufacturer (Promega Corporation, Madison, WI).
TNF-α, MCP-1 and IL-6
1×106 cells/well were incubated overnight at 37°C in complete medium, rinsed twice with basal medium and pretreated with basal medium alone, 100mM ethanol, 10ng/ml LPS or ethanol and LPS for 30 min. After pretreatment, the ethanol was aspirated and the cells redosed with ethanol and LPS. Cells were then incubated for 24 hours at ambient or 30mmHg pressure, and medium TNF-α, MCP-1, and IL-6 were assayed by sandwich ELISA (BD Biosciences Pharmingen).
Statistical Analysis
All experiments were done independently at least three times with similar results. Data sets were analyzed using paired or unpaired t-tests using Bonferroni corrections as appropriate. Statistical significance was considered p<0.05.
RESULTS
Ethanol and LPS regulate pressure-stimulated microglial proliferation
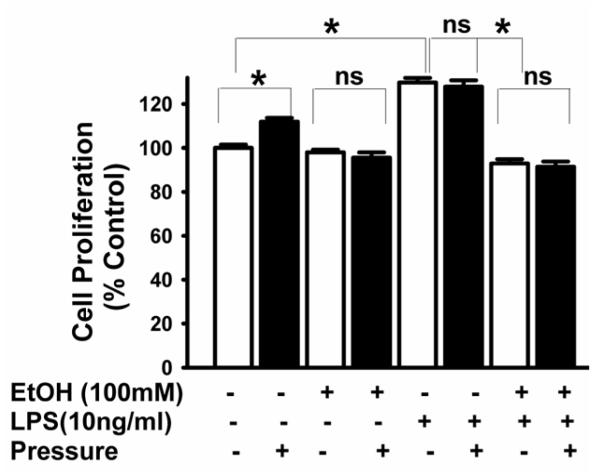
Increased pressure stimulated microglial proliferation without affecting viability (n=3, p<0.05, Fig 1). Pretreatment with ethanol did not alter basal proliferation at ambient pressure (n=3) but blocked pressure-induced proliferation. LPS increased basal proliferation (n=3, p<0.05, Fig 1) and increased pressure did not further enhance proliferation in these cells. Ethanol pretreatment blocked LPS-induced proliferation at ambient pressure as well as pressure-stimulated proliferation (n=3, p<0.05). BV-2 cells treated with ethanol, LPS or both with or without increased pressure did not demonstrate any differences in trypan blue exclusion compared to untreated control cells (data not shown).
Fig 1. Effect of extracellular pressure, LPS, ethanol and the combination of LPS plus ethanol on microglial proliferation.
Pressure and LPS significantly stimulated proliferation compared to control cells at ambient pressure (◻P < .05, n = 3) and ethanol blocked both pressure-induced and LPS-induced proliferation without affecting basal proliferation (P>0.05 considered non-significant (ns).
Pressure and ethanol modulate LPS-stimulated inflammatory responses
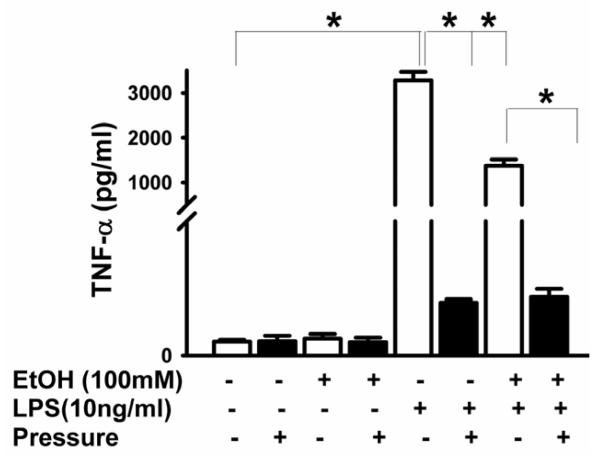
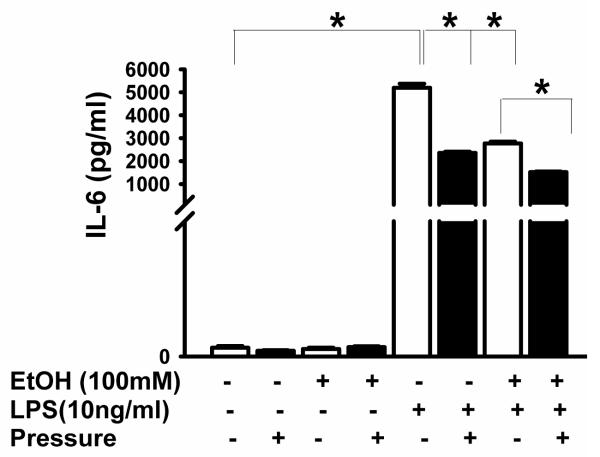
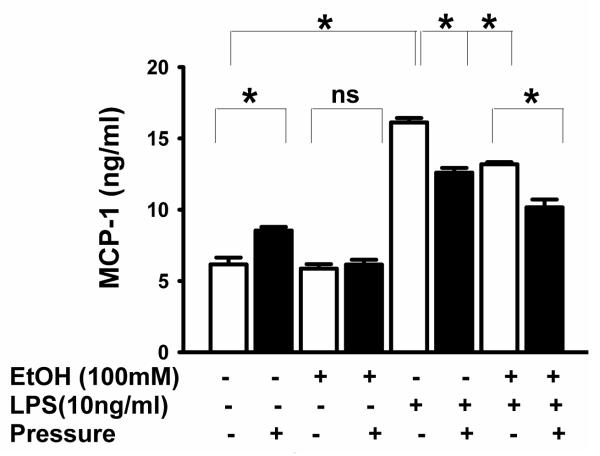
BV-2 TNF-α and IL-6 were undetectable without LPS (Figs 2 and 3) but MCP-1 was detectable even without LPS (Fig 4). LPS enhanced TNF-α secretion (n=3, p<0.05, Fig 2), IL-6 (p<0.05, Fig 3), and MCP-1 (p<0.05, Fig 4). Increased pressure completely blocked LPS-stimulated TNF-α secretion (n=3, p<0.05, Fig 2). Furthermore, ethanol pretreatment inhibited LPS-induced TNF-α production at ambient pressure (n=3, p<0.05, Fig 2). Interestingly, increased pressure completely inhibited TNF-α production in cells treated with either LPS alone or LPS and ethanol (Fig 2). Either pressure or ethanol (p<0.05, Fig 3) blocked LPS-stimulated IL-6 secretion. Pressure further inhibited IL-6 secretion after combined LPS-ethanol treatment vs. LPS-ethanol treated cells at ambient pressure (p<0.05, Fig 3). Like LPS, pressure alone stimulated MCP-1 secretion (p<0.05, Fig 4), and ethanol blocked both pressure-induced and LPS-induced (p<0.05, Fig 4) MCP-1 secretion. However, pressure inhibited LPS-stimulated MCP-1 production either alone or when LPS was combined with ethanol (p<0.05, Fig 4).
Fig 2. Effect of extracellular pressure, LPS, ethanol and the combination of LPS plus ethanol on TNF-α production.
LPS significantly increased TNF-α secretion in comparison of ambient control (~P < .05, n = 3). Pressure and ethanol blocked LPS-induced TNF-α production (◻P < .05, n = 3).
Fig 3. Effect of extracellular pressure, LPS, ethanol and the combination of LPS plus ethanol on IL-6 production.
LPS significantly increased IL-6 secretion in comparison of ambient control (◻P < .05, n = 3). Pressure and ethanol blocked LPS-induced IL-6 production (◻P < .05, n = 3).
Fig 4. Effect of extracellular pressure, LPS, ethanol and the combination of LPS plus ethanol on MCP-1 production.
Pressure and LPS significantly increased MCP-1 secretion compared to ambient pressure controls (◻P < .05, n 3). Ethanol blocked release of MCP-1 in response to pressure and LPS (◻P < .05, n = 3), (P>0.05 considered non-significant (ns).
DISCUSSION
In general, more intoxicated polytrauma victims display increased need for intensive care, more complications, and higher mortality than sober patients (19). In contrast, TBI victims with high blood alcohol levels do not necessarily fare worse than sober TBI patients (2, 3, 15). Indeed, a high admission blood alcohol level (≥230 mg/dL) is even associated with lower mortality in some TBI subsets(2). The mechanisms of the effect of alcohol on the host response to CNS trauma remain obscure, and the interplay among alcohol-mediated modulation of immune cell activation, inflammatory reaction, and ICP alteration in the disease progression following TBI is poorly understood.
Microglial cells are the major CNS inflammatory cell (12). Like other members of the monocyte-macrophage family, these resident CNS macrophages are activated by injurious and infectious stimuli to produce inflammatory mediators that initiate and reinforce the inflammatory response (12, 16).
Increased ICP is common in CNS inflammation. We found that increased extracellular pressure stimulates microglial proliferation and MCP-1 secretion even without an inflammatory stimulus. However, increased pressure inhibits LPS-induced production of the pro-inflammatory cytokines TNF-α, IL-6, and MCP-1 by microglial cells, and does not further stimulate proliferation in the setting of inflammatory stimulation. While the differences related to alcohol were not very large, they are highly statistically significant. Even small changes in inflammatory cytokine levels may be amplified by the cytokine cascade to have profound biological effects.
Taken together, these observations suggest that while pressure may stimulate microglial proliferation, it also creates a strong negative feedback that downregulates inflammatory cytokines. This may be protective during the response to TBI. An appropriate inflammatory reaction clears devitalized tissue and initiate tissue repair and regeneration. However, overwhelming inflammation that further increases ICP would be harmful. How pressure regulates microglial cytokine production awaits elucidation. Others have shown that increased ICP exerts anti-inflammatory effects by inducing IL-10 in the peripheral tissues through sympathetic nervous system activation (23).
Further investigation of the signals by which pressure regulates microglial activation will improve our understanding of this novel mechanism of protection in the CNS inflammatory response. Similar to our results, both extracellular pressure Shiratsuch, 2005 #33} and ethanol (1) inhibit LPS-stimulated TNF-α secretion in human monocytes.
We studied constant high pressure. Fluctuating pressure might have different effects since turning another force (cyclic strain) on and off may have a greater effect than simply maintaining cyclic strain (9). This will be a subject for future study.
Conversely, 100mM alcohol blocked the mitogenic effects of pressure or LPS. Alcohol also facilitated pressure-induced inhibition of microglial TNF-α, IL-6 and MCP-1 production in response to LPS. Thus alcohol may restrain the local inflammatory response to TBI, perhaps contributing to the apparent amelioration of mortality observed clinically. The effects of combining ethanol and LPS on microglial cytokine secretion have not previously been studied. Bovadjieva reported that ethanol without LPS stimulates rat microglial TNF-α secretion (5), but we could not detect significant TNF-α secretion changes in the absence of LPS. Murine BV-2 microglial cells may secrete less TNF-α at baseline than rat cells. In contrast to our results, Qin reported that ethanol potentiated LPS-induced TNF-α and MCP-1 content in whole brain tissue (18), but Qin studied chronic ethanol and LPS exposure over 10 days in mice intact organisms so this may reflect differences between acute direct effects of ethanol and LPS on microglial cells and indirect neurohumoral effects of longstanding chronic exposure in vivo.
The mechanism by which LPS, alcohol and pressure act on BV-2 cells wait further study. Cao reported that NF-κB-regulated NOTCH-1 signaling mediates LPS-stimulated BV-2 cell activation (7). However, Cao studied a high LPS dose (1μg/ml) with effects different from the 10ng/ml LSP studied here. Alcohol is a small molecule that affects many intracellular signals and its effects remain to be elucidated. Indeed, alcohol may act by changing the conformation of many different proteins. Ingber (10) postulated that physical forces like pressure change the conformation of the cytoskeleton and the signal molecules associated with it. We have previously identified numerous intracellular kinases modulated by pressure in epithelial cells (8)(22), macrophages (21) monocytes (21) and fibroblasts (4). Thus it would not be unreasonable to hypothesize that alcohol-pressure interactions might be mediated by structural conformational changes in one or more intracellular signal molecules. This awaits further study. This study delineates potential anti-inflammatory mechanisms by which increased ICP and ethanol intoxication might ameliorate TBI-induced inflammation. Certainly, ICP increases and ethanol have other deleterious effects, while the effects of these stimuli on the release of anti-inflammatory cytokines await study. However, further studies directed at understanding how alcohol and extracellular pressure damp microglial pro-inflammatory cytokine secretion may help to elucidate the pathobiology of TBI.
Acknowledgments
Supported in part by a VA Merit Grant and NIH R01 DK067257 (both MDB), and NIH R01 AA019676 (PZ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arbabi S, Garcia I, Bauer GJ, et al. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–7445. [PubMed] [Google Scholar]
- 2.Berry C, Ley EJ, Margulies DR, et al. Correlating the blood alcohol concentration with outcome after traumatic brain injury: too much is not a bad thing. Am Surg. 2011;77:1416–1419. [PubMed] [Google Scholar]
- 3.Berry C, Salim A, Alban R, et al. Serum ethanol levels in patients with moderate to severe traumatic brain injury influence outcomes: a surprising finding. Am Surg. 2010;76:1067–1070. [PubMed] [Google Scholar]
- 4.Bhalla S, Shiratsuchi H, Craig DH, et al. beta(1)-integrin mediates pressure-stimulated phagocytosis. Am J Surg. 2009;198:611–616. doi: 10.1016/j.amjsurg.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyadjieva NI, Sarkar DK. Role of microglia in ethanol’s apoptotic action on hypothalamic neuronal cells in primary cultures. Alcohol Clin Exp Res. 2010;34:1835–1842. doi: 10.1111/j.1530-0277.2010.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrnes KR, Stoica BA, Fricke S, et al. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain. 2007;130:2977–2992. doi: 10.1093/brain/awm179. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q, Kaur C, Wu CY, et al. Nuclear factor-kappa beta regulates Notch signaling in production of proinflammatory cytokines and nitric oxide in murine BV-2 microglial cells. Neuroscience. 2011;192:140–154. doi: 10.1016/j.neuroscience.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 8.Downey C, Craig DH, Basson MD. Isoform-specific modulation of pressure-stimulated cancer cell proliferation and adhesion by α-actinin. Am J Surg. 2011;202:520–523. doi: 10.1016/j.amjsurg.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc Res. 1991;42:245–254. doi: 10.1016/0026-2862(91)90059-k. [DOI] [PubMed] [Google Scholar]
- 10.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 11.Juul N, Morris GF, Marshall SB, et al. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. J Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- 12.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Jeong J, Son E, et al. Ethanol selectively modulates inflammatory activation signaling of brain microglia. J Neuroimmunol. 2004;156:88–95. doi: 10.1016/j.jneuroim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 15.Lustenberger T, Inaba K, Barmparas G, et al. Ethanol intoxication is associated with a lower incidence of admission coagulopathy in severe traumatic brain injury patients. J Neurotrauma. 2011;28:1699–1706. doi: 10.1089/neu.2011.1866. [DOI] [PubMed] [Google Scholar]
- 16.McGeer PL, Kawamata T, Walker DG, et al. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 17.Opreanu RC, Kuhn D, Basson MD. Influence of alcohol on mortality in traumatic brain injury. J Am Coll Surg. 2010;210:997–1007. doi: 10.1016/j.jamcollsurg.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin L, He J, Hanes RN, et al. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehm J, Gmel G, Sempos CT, et al. Alcohol-related morbidity and mortality. Alcohol Res Health. 2003;27:39–51. [PMC free article] [PubMed] [Google Scholar]
- 20.Salim A, Ley EJ, Cryer HG, et al. Positive serum ethanol level and mortality in moderate to severe traumatic brain injury. Arch Surg. 2009;144:865–871. doi: 10.1001/archsurg.2009.158. [DOI] [PubMed] [Google Scholar]
- 21.Shiratsuch H, Basson MD. Differential regulation of monocyte/macrophage cytokine production by pressure. Am J Surg. 2005;190:757–762. doi: 10.1016/j.amjsurg.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MF, Woo RK, Gomez R, et al. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif. 2004;37:427–441. doi: 10.1111/j.1365-2184.2004.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woiciechowsky C, Volk HD. Increased intracranial pressure induces a rapid systemic interleukin-10 release through activation of the sympathetic nervous system. Acta Neurochir Suppl. 2005;95:373–376. doi: 10.1007/3-211-32318-x_76. [DOI] [PubMed] [Google Scholar]
- 24.Yu G, Dymond M, Yuan L, et al. Propofol’s effects on phagocytosis, proliferation, nitrate production, and cytokine secretion in pressure-stimulated microglial cells. Surgery. 2011;150:887–896. doi: 10.1016/j.surg.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]