Abstract
Summary
Pressure increases sarcoma cell adhesiveness via intracellular activation of Akt and FAK. Perioperative manipulation or forces in lymphatic or circulatory systems may potentiate local recurrence or distant metastasis.
Background
The effect of extracellular pressure on adhesion and adhesiogenic focal adhesion kinase (FAK) and Akt signaling in sarcomas was investigated.
Methods
Human sarcoma cells (HT-1080 fibrosarcoma, KHOS-240S osteosarcoma, A-673 rhabdomyosarcoma) were subjected to increased pressure followed by adhesion assay. Two cell lines were pretreated with the FAK inhibitor 1,2,4,5-benzenetetraamine tetrahydrochloride (Y15) or Akt IV inhibitor, followed by Western analysis for activated FAK and Akt. Parallel studies were conducted in cells from a resected human fibrous histiosarcoma.
Results
Pressure increased adhesion in all three sarcoma lines and primary histosarcoma cells by 7–18% (n=6, p<0.01 each). Pressure activated FAK and Akt (n=5, p<0.01). Inhibiting FAK or Akt inhibited FAK or Akt phosphorylation and the stimulation of adhesion by increased pressure (n=5 each, p<0.01 each).
Conclusion
Pressure increases sarcoma cell adhesiveness via Akt and FAK. Perioperative manipulation or forces in lymphatic or circulatory systems may potentiate local recurrence or distant metastasis.
Keywords: Akt, cell signaling, focal adhesion kinase, pressure, sarcoma
INTRODUCTION
During surgical procedures, tumor cells released into the circulation can adhere to distant sites and metastasize, while locally shed cells may adhere to surgical sites. Although some postoperative metastases reflect metastases too diminutive to have been detected preoperatively, iatrogenic tumor cell dissemination also causes recurrence. Adhesion of these shed tumor cells is crucial to metastasis. Previous studies 1–4,5 suggests that epithelial cancer cells can regulate their own adhesion to extracellular matrix proteins, endothelial cells, or surgical wounds via intracellular signals that ultimately regulate β1-integrin binding affinity.
The adhesiogenic signals are stimulated by forces such as extracellular pressure1,2 and nonlaminar shear5,6. Pressure may activate cancer cell signaling during passage though the lymphatics or circulation, surgical manipulation, laparoscopic insufflation,7 or postoperative increases in pressure due to third spacing. This force-activated inside-out signaling involves cytoskeleton-dependent mechanosensing8 that activates focal adhesion kinase (FAK) and Src2.
This phenomenon has previously been studied in epithelial cancers, including colon,1,2 breast,9 and head and neck cancers10. Epithelial and non-epithelial cancers originate from different tissues and express cell surface receptors differently,11 and thus might behave differently. We hypothesized that extracellular pressure also activates adhesiogenic signaling non-epithelial human cancers. We characterized the effects of pressure on three human sarcoma cell lines (HT-1080 fibrosarcoma, KHOS-240S osteosarcoma, A-673 rhabdomyosarcoma). We conducted parallel studies in primary sarcoma cells isolated directly from a surgically resected human fibrous histiosarcoma to confirm that our observations were not restricted to sarcoma cells adapted to culture conditions. We investigated pressure activation of FAK (a tyrosine kinase) and Akt (a serine-threonine kinase) and evaluated the effect of blocking these signals.
MATERIALS AND METHODS
Cells and reagents
We studied human HT-1080 fibrosarcoma, KHOS-240S osteosarcoma, A-673 rhabdomyosarcoma (ATCC, Manassas, VA), and primary human fibrous histiosarcoma cells isolated from a resected tumor by mincing and collagenase digestion as previously12. HT-1080, KHOS-240S, and histiosarcoma cells were maintained in Eagle’s Minimum Essential Medium (ATCC, Manassas, VA) with 10% fetal bovine serum (FBS) and A-673 cells in Dulbecco’s Modified Eagle’s Medium (Sigma, St. Louis, MO) with 10% FBS.
Pressure regulation
0–60 mmHg pressure above ambient was applied for 30 minutes using a prewarmed, airtight box with an inlet valve for gas and an outlet valve connected to a manometer as described.1–4 Control cells were incubated at 37°C under ambient pressure.
Adhesion assays
Cells were trypsinized at 80% confluence, resuspended in warm phosphate buffered saline (PBS), incubated with 1μL of Calcein-AM (Invitrogen, Carlsbad, CA) per 106 cells for 15 minutes at 37°C, centrifuged, and resuspended in culture medium. 500 μL of cells at 300,000 cells/mL were seeded into collagen-I-precoated 24-well plates. These were placed into the prewarmed pressure box, which was pressurized and replaced in a 37°C incubator. Control cells were handled similarly without increased pressure. After 30 minutes, the box was depressurized, non-adherent were cells washed away twice with warm PBS, and fluorescence/well was quantified using a SpectraMax microplate reader.
Inhibitors
HT-1080 and A-673 cells were pretreated with 20μM Y-15 (1,2,4,5-benzenetetraamine tetrahydrochloride, Sigma, St. Louis, MO) to inhibit FAK or 10μM Akt IV inhibitor (Calbiochem, Gibbstown, NJ) for 30 minutes at 37°C before assays. FAK and Akt activation was studied by Western blot for phosphorylated FAK(Y397) and Akt(S473).
Western blots
We assessed FAK and Akt phosphorylation under ambient or increased pressure for 30 minutes in HT-1080 cells and human fibrous histiosarcoma cells after lysing. suspended cells with protease inhibitors, measuring protein by bicinchoninic acid protein assay (Pierce, Rockford, IL), and resolving lysates by reducing 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis before transfer to nitrocellulose. Membranes were blocked (Odyssey blocking buffer, Li-Cor Biosciences, Lincoln, NE), incubated with antibody to activated Akt(S473) (Cell Signaling, Beverly, MA) or FAK(Y397) (BD Bioscience, San Diego, CA) overnight at 4°C, washed 3 times with 0.1% Tween 20 TBS, and incubated for 60 minutes with 10,000:1 secondary anti-rabbit or anti-mouse IgG (Li-Cor Biosciences). Membrane-bound secondary was detected with an Odyssey infrared imaging machine (Li-Cor Biosciences) within the linear range. FAK and Akt phosphorylation were expressed as the ratio of active phosphorylated FAK or Akt to total FAK or Akt for each blot, normalized to controls.
Statistical analysis
All data are represented as means ± SE. Differences between groups were analyzed by Student t-test or paired t-test as appropriate, seeking 95% confidence.
RESULTS
30 minutes of pressure 15 mmHg above ambient stimulated adhesion in all four cancer cell lines. Adhesion in HT-1080 increased 15±1% (n=6, P<0.001), in KHOS-240S 7±1% (n=6, P<0.01), in A-673 11±2% (n=6, P<0.01), and in primary human fibrous histiosarcoma cells 18±3% (n=6, P<0.001). 7.5– 60 mmHg increased pressures also promoted HT-1080 adhesion. 7.5 mmHg pressure stimulated HT-1080 adhesion by 12±2% (n=4, P<0.01) while 22.5, 30, 45, and 60 mmHg pressures each similarly increased adhesion by 16±3% (n=4, P<0.01 each).
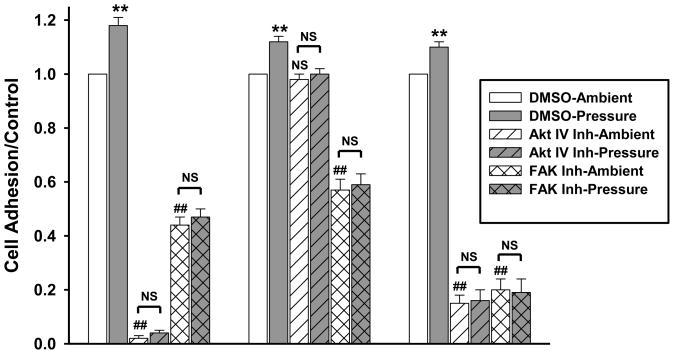
20μM Y15 or 10μM Akt IV inhibitor pretreatment prevented pressure-associated increased adhesion in fibrous histiosarcoma, HT-1080, and A-673 cells (Fig. 1), while a DMSO vehicle control did not affect the pressure response (n=5, p<0.01). KHOS-240S were not studied.
Fig. 1.
Fibrous histiosarcoma, HT-1080, and A-673cells were pre-treated with 10μM Akt IV inhibitor or 20μM Y15 (FAK Inh) before adhesion assay. Y15 or Akt IV inhibitor prevented pressure-associated increased adhesion in all three (n=5 each). FAK inhibitor reduced basal adhesion in all three cells and Akt IV inhibitor reduced basal adhesion in A-673 and fibrous histiosarcoma cells. **p<0.01 vs. DMSO ambient pressure; ##p<0.01 vs. DMSO ambient pressure; NS – not significant.
Akt IV inhibitor did not affect HT-1080 basal adhesion but blocked pressure-induced adhesion (n=5, p<0.01). Akt inhibition reduced basal A-673 adhesion 85±3% and histiosarcoma adhesion 98±1% (n=5, p<0.001 each), and blocked pressure-stimulated adhesion. Y15 decreased basal HT-1080 adhesion 43±4% (n=5, p<0.01), A-673 adhesion 80±4% (n=5, p<0.001), and fibrous histiosarcoma adhesion 56±3% (n=5, p<0.001), and blocked pressure-stimulated adhesion in all three.
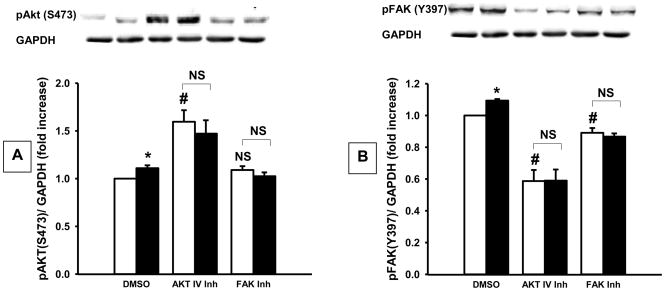
15 mmHg pressure for 30 minutes stimulated FAK and Akt phosphorylation in both HT-1080 (Fig. 2) and primary fibrous histiosarcoma (Fig. 3) cells. Phosphorylated FAK (Y397) increased by 9±1% (n=9, p<0.001) in HT-1080 cells while Akt (S473) phosphorylation increased 11±3% (n=9, p<0.01). Y15 decreased FAK phosphorylation by 11±2% (n=9, p<0.01) and prevented the pressure-stimulated increase in FAK phosphorylation. Akt IV inhibitor increased Akt phosphorylation 59±12% (n=9, p<0.001) and decreased FAK phosphorylation 41±7% (n=9, p<0.001), while preventing the pressure-induced increase in FAK or Akt phosphorylation.
Fig. 2.
Densitometric analysis of pressure (shaded bars) effects on (A) Akt and (B) FAK phosphorylation in HT-1080 cells vs. ambient pressure (open bars), with typical blots from one of nine similar studies. Increasing ambient pressure by 15 mm Hg (DMSO-closed bar) stimulated Akt and FAK phosphorylation vs. ambient pressure controls (DMSO-open bar). Akt IV inhibitor or Y15 (FAK inhibitor) prevented pressure-induced Akt and FAK phosphorylation vs. cells under ambient pressure. *p<0.05 vs. DMSO ambient pressure, #p<0.05 vs. DMSO ambient pressure, NS – not significant.
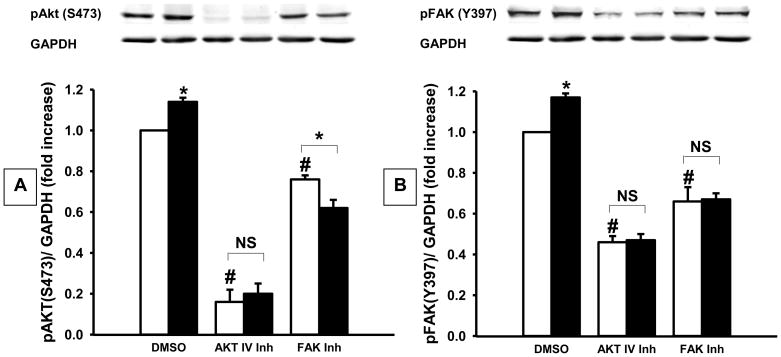
Fig. 3.
Densitometric analysis of the effects of pressure (shaded bars) on (A) Akt and (B) FAK phosphorylation in primary fibrous histiosarcoma cells vs. ambient pressure (open bars), with typical blots from one of four studies. Increasing ambient pressure by 15 mm Hg (DMSO-closed bar) stimulated Akt and FAK phosphorylation vs. ambient pressure controls (DMSO-open bar). Akt IV inhibitor or Y15 (FAK inhibitor) prevented pressure-induced Akt and FAK phosphorylation vs. cells under ambient pressure. *p<0.05 vs. DMSO ambient pressure, #p<0.05 vs. DMSO ambient pressure, NS – not significant.
Pressure stimulated FAK (Y397) and AKT phosphorylation 17±2% and 14±2% in primary histiosarcoma cells (n=4, p<0.001 each). Y15 decreased FAK and AKT phosphorylation by 33±7% and 24±2% (n=4, p<0.001 each), and prevented the pressure-stimulated increase in FAK and Akt phosphorylation. Akt IV inhibitor reduced Akt and FAK phosphorylation by 84±6% and 54±3% respectively (n=4, p<0.001 each) and blocked pressure-activation of FAK and Akt.
DISCUSSION
Extracellular forces influence intracellular signaling and adhesion in epithelial cancer cells. We investigated extracellular pressure on adhesion in non-epithelial cancer cells. Increasing ambient pressure to a relatively small degree (15 mmHg) increased adhesion in three sarcoma cell lines. The similar response by primary cells from a surgically resected human fibrous histiosarcoma suggests that this phenomenon is independent of cell line adaptations to culture.
The observed effect was not as large as previously seen in epithelial cells, as the magnitude of pressure-induced adhesion may differ by cell line. However, even small increases in tumor cell adhesion or FAK activation might be clinically significant for patients, particularly those with a high cancer cell burden. Metastasis is a stochastic process that begins with adhesion and continues as cells attempt to elude host responses. If more cells adhere, metastasis is more likely.
Modest changes in cell adhesion molecules are important in cancer prognosis. For instance, intercellular adhesion molecule (ICAM) expression is a strong independent prognostic factor for survival in non-small cell lung cancer.13 FAK phosphorylation is important in several tumor types14. For example, FAK (Y397) phosphorylation increases invasiveness of ovarian tumors.15 Other studies of acute myeloid leukemia have shown that FAK phosphorylation at Y397 correlates with enhanced cancer cell migration and drug resistance in vitro16.
Increased pressure stimulated FAK and Akt phosphorylation in the cells we studied. 15 mmHg increased pressure increased FAK Y397 phosphorylation by 28±7% and Akt S473 phosphorylation by 28±6% in Co26 human colon cancer cells.8 The sarcoma cells responded similarly but to a lesser extent. Whether the difference reflects differences in basal activity, pressure-responsiveness, or the kinetics of the response awaits further study.
Activation of the PI3-kinase (PI3K)/Akt signal pathway correlates with colon cancer cell invasion17 and is critical for integrin activation and cell adhesion.18 FAK is important in cell adhesion19 and survival. In human colon adenocarcinoma cells, FAK and Akt each translocate from the cytosol to the membrane in response to pressure, so FAK and Akt may interact.2
AKT IV inhibitor and Y15 reduced basal adhesion and disrupted pressure-induced adhesion in sarcoma cells. Y15 targets the FAK autophosphorylation site while Akt IV inhibitor is proposed to block Akt by targeting the ATP binding site of a kinase upstream of Akt but downstream of PI3K. These agents may not be suitable for patients, but represent proof of principle that FAK and Akt are useful targets to reduce force-activated sarcoma adhesion.
Interestingly, the Akt IV inhibitor increased HT-1080 Akt (S473) phosphorylation. This apparently paradoxical effect has been observed before.20,21 As others have suggested, the increase in Akt S473 phosphorylation by Akt IV inhibitor implies that this chemical may inhibit Akt differently than as proposed. In vitro kinase profiling shows that Akt-IV does not directly inhibit Akt kinase activity.20 The decrease in AKT phosphorylation by Akt IV in primary fibrous histiosarcoma cells suggests cell-specific variability in response of Akt phosphorylation to these novel compounds despite uniform prevention of downstream signaling attributable to Akt.
Cancer cells experience forces in various settings. Many tumors, including osteosarcomas, experience interstitial fluid pressure elevations up to 50 mmHg in vivo as they grow against a constraining stroma.22,23 Metastasizing tumor cells experience pressure and shear during passage through lymphatics or veins. Perioperatively, cancer cells are shed from tumors into the surgical site or the circulation during procedures, and the presence of free tumor cells on the outside of the surgical specimen is a poor prognostic sign for colon cancer.24 Surgeons apply force to tumors during dissection, irrigation, and laparoscopic insufflation to 15 mmHg pressure.
FAK inhibition decreases adhesion and causes human pancreatic cancer regression in vivo.25 This study suggests pressure also influences sarcoma adhesion and metastasis through Akt and FAK. The signals that modulate tumor cell adhesiveness may be targets to inhibit metastasis.
Footnotes
Presented at the 34th Annual Meeting of the Association of VA Surgeons, Indianapolis, IN, May 9-11, 2010, Supported in part by NIH RO1DK060771 (MDB).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basson MD, Yu CF, Herden-Kirchoff O, et al. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem. 2000;78:47–61. doi: 10.1002/(sici)1097-4644(20000701)78:1<47::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology. 2004;126:8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 3.van der Voort van Zyp J, Thamilselvan V, Walsh M, et al. Extracellular pressure stimulates colon cancer cell adhesion in vitro and to surgical wounds by Src (sarcoma protein) activation. Am J Surg. 2004;188:467–73. doi: 10.1016/j.amjsurg.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Craig DH, Gayer CP, Schaubert KL, et al. Increased extracellular pressure enhances cancer cell integrin-binding affinity through phosphorylation of β1-integrin at threonine 788/789. Am J Physiol Cell Physiol. 2009;296:193–204. doi: 10.1152/ajpcell.00355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawler K, Meade G, O’Sullivan G, Kenny D. Shear stress modulates the interaction of platelet-secreted matrix proteins with tumor cells through the integrin avb3. Am J Physiol. 2004;287:1320–7. doi: 10.1152/ajpcell.00159.2004. [DOI] [PubMed] [Google Scholar]
- 6.Thamilselvan V, Patel A, van der Voort van Zyp J, et al. Colon cancer cell adhesion in response to Src kinase activation and actin-cytoskeleton by non-laminar shear stress. J Cell Biochem. 2004;92:361–71. doi: 10.1002/jcb.20072. [DOI] [PubMed] [Google Scholar]
- 7.Wu JS, Brasfield EB, Guo LW, et al. Implantation of colon cancer at trocar sites is increased by low pressure pneumoperitoneum. Surgery. 1997;122:1–7. doi: 10.1016/s0039-6060(97)90256-7. [DOI] [PubMed] [Google Scholar]
- 8.Craig DH, Owen CR, Conway WC, et al. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest. 2008;118:3170–80. doi: 10.1172/JCI34279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downey C, Alwan K, Thamilselvan V, et al. Pressure stimulates breast cancer cell adhesion independently of cell cycle and apoptosis regulatory protein (CARP)-1 regulation of focal adhesion kinase. Am J Surg. 2006;192:631–5. doi: 10.1016/j.amjsurg.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Conway WC, Van der Voort van Zyp J, Thamilselvan V, et al. Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion. J Cell Biochem. 2006;98:1507–16. doi: 10.1002/jcb.20819. [DOI] [PubMed] [Google Scholar]
- 11.Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–14. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 12.Emenaker NJ, Basson MD. Short chain fatty acids inhibit human (SW1116) colon cancer cell invasion by reducing urokinase plasminogen activator activity and stimulating TIMP-1 and TIMP-2 activities, rather than via MMP modulation. J Surg Res. 1998;76:41–6. doi: 10.1006/jsre.1998.5279. [DOI] [PubMed] [Google Scholar]
- 13.Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non small cell lung cancer treated with chemotherapy with or without bevacizumab - an eastern cooperative oncology group study. Clin Cancer Res. 2008;14:1407–12. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 14.McLean GW, Carragher NO, Avizienyte E, et al. The role of focal adhesion kinase in cancer-a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 15.Grisaru-Granovsky S, Salah Z, Maoz M, et al. Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int J Cancer. 2004;113:372–8. doi: 10.1002/ijc.20607. [DOI] [PubMed] [Google Scholar]
- 16.Recher C, Ysebaert N, Beyne-Rauzy O, et al. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004;64:3191–7. doi: 10.1158/0008-5472.can-03-3005. [DOI] [PubMed] [Google Scholar]
- 17.Kermorgant S, Aparicio T, Dessirier, et al. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 2001;22:1035–42. doi: 10.1093/carcin/22.7.1035. [DOI] [PubMed] [Google Scholar]
- 18.Vanhaesebroeck B, Leevers SJ, Ahmadi K, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 19.Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007;21:1730–41. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- 20.Dunn EF, Fearns R, Connor JH. Akt inhibitor Akt-IV blocks virus replication through an Akt-independent mechanism. J Virol. 2009;83:11665–72. doi: 10.1128/JVI.01092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui QL, Zheng WH, Quirion R, et al. Inhibition of Src-like kinases reveals Akt-dependent and -independent pathways in insulin-like growth factor I-mediated oligodendrocyte progenitor survival. J Biol Chem. 2005;280:8918–28. doi: 10.1074/jbc.M414267200. [DOI] [PubMed] [Google Scholar]
- 22.Nathan SS, DiResta GR, Casas-Ganem JE, et al. Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res. 2006;11:2389–97. doi: 10.1158/1078-0432.CCR-04-2048. [DOI] [PubMed] [Google Scholar]
- 23.Diresta GR, Nathan SS, Manoso MW, et al. Cell proliferation of cultured human cancer cells are affected by the elevated tumor pressures that exist in vivo. Ann Biomed Eng. 2005;33:1270–80. doi: 10.1007/s10439-005-5732-9. [DOI] [PubMed] [Google Scholar]
- 24.Baskaranathan S, Philips J, McCredden P, et al. Free colorectal cancer cells on the peritoneal surface: correlation with pathologic variables and survival. Dis Colon Rectum. 2004;47:2076–9. doi: 10.1007/s10350-004-0723-8. [DOI] [PubMed] [Google Scholar]
- 25.Hochwald SN, Nyberg C, Zheng M, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009;8:2435–43. doi: 10.4161/cc.8.15.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]