Abstract
Dendritic cell (DC)-based vaccine strategies have gained increasing popularity in recent years. Methods for ex vivo generation of immunocompetent mature DCs still require optimization. DCs have been shown to phenotypically mature under elevated pressure. We compared the effects of pressure on DC maturation with LPS- and cytokine-stimulation. Human monocyte-derived immature or LPS- and cytokine-matured DCs were exposed to ambient or 40mmHg increased pressure for 12-hrs., then assessed for expression of CD80, CD86, CD40, MHC-I/II, and inflammatory cytokine production. DCs were also evaluated for capacity to stimulate T-cell proliferation by co-culture with allogeneic lymphocytes. Pressure significantly increased cytokine production and expression of all surface molecules on immature DC other than MHC-I and CD40. Pressure/LPS-treated DCs displayed further upregulation of MHC-I, CD40, and IL-12p70. Cytokine-matured DCs appeared less responsive to pressure. T-cell proliferation correlated with MHC expression. Results suggest mechanical stimulation of DCs may provide a useful adjuvant to TLR-agonist maturation strategies.
Keywords: dendritic cell, maturation, vaccine, pressure, mechanotransduction
INTRODUCTION
Dendritic cells (DCs) are often considered the sentinels of the human immune system. They are the most potent of antigen presenting cells, and are critical for stimulating effective antigen-specific T-cell responses necessary for protection from foreign pathogens [1]. As such, the use of patient-derived DCs for both antitumor and antiviral immunization strategies has gained increasing popularity in recent years. The majority of these approaches generate autologous DCs in culture from a patient’s peripheral blood monocytes, the DCs are loaded with target antigen ex vivo and then infused back into the patient with the hope of inducing a robust antigen-specific CD4+ and/or CD8+ T-cell response.
While the clinical success of most adoptive immune therapy protocols has been modest, the generation of optimally-activated or fully immunocompetent DCs ex vivo is typically regarded as one of the main factors limiting DC vaccine potential [2]. Activated or mature DCs (mDCs) are almost exclusively used for these studies due to their greater expression of costimulatory molecules, increased production of cytokines, and overall greater immunogenicity compared with immature DCs (iDCs) [3]. The classic mature DC phenotype is more specifically noted by an increase in expression of major histocompatibility complex (MHC) molecules, CD40, CD80 and CD86 costimulatory molecules, increased IL-12 cytokine secretion, and the acquired ability to home to lymph nodes through upregulation of CC chemokine receptors [1]. DC maturation is characteristically stimulated in response to inflammatory stimuli originating from invading pathogens or dying cells [4]. A variety of biological agents and microbial compounds have been employed successfully for generating mDCs ex vivo. Two of the most commonly used methods include toll-like receptor (TLR) stimulation using the bacterial derivative, lipopolysaccharide (LPS), and the cytokine cocktail: IL-1β, IL-6, and TNF-α, with or without PGE2 [5,6]. Yet, the degree of phenotypic dichotomy existing between mDCs matured by alternate methods still supports a need for greater optimization of these protocols.
Contrary to conventional cell signaling paradigms, it is becoming increasingly evident that less tangible mechanical forces such as pressure and shear can influence diverse aspects of cell biology [7]. We recently demonstrated that overnight exposure of human monocyte-derived DCs to 40 mmHg increased extracellular pressure significantly impacts expression of cell surface maturation markers [8]. DCs may be exposed to a wide array of physiologic pressures ranging from -1 mmHg below atmospheric pressure to +3 mmHg in the skin [9], elevations as high as 50 mmHg in tumor microenvironments and sites of inflammation [10], lymphatic pressures up to 60 mmHg [11], and even greater pressure fluctuations during vascular transit. We hypothesized that pressure-induced phenotypic changes may reflect an adaptive response mechanism enhancing DC immunogenicity under increased lymphatic pressures or at sites of inflammation. Furthermore, since DCs can be cultured under elevated pressures ex vivo during antigen loading and activation, and alternate vaccination sites (e.g. subcutaneous, intradermal, or intravenous) each have distinct mechanical environments, a better understanding of this phenomenon might allow for its exploitation in the design of DC vaccination strategies.
We therefore sought to compare the maturation effects of 12-hour exposure to 40 mmHg increased pressure against cell treatments with either lipopolysaccharide or a cytokine cocktail of IL-1β, IL-6, and TNF-α, as well as whether these effects were additive and could be used in combination. Dendritic cells were generated by granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 treatment of monocytes isolated from healthy human donors. We assessed DC maturation by evaluating the expression of surface markers CD80, CD86, CD40, MHC class I and MHC class II. IL-12p70, IL-6, IFN-γ, and TNF-α cytokine production were assessed in parallel. Finally, to further address whether resulting phenotypic differences between DC maturation methods had a functional impact on T-cell stimulatory capabilities, we evaluated CD4+ and CD8+ T-cell proliferation following stimulation with experimentally-treated allogeneic DCs.
MATERIALS AND METHODS
Cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy human donors using Lymphocyte Separation Medium (Mediatech, Herndon, VA). DCs were derived from plastic-adherent monocytes after 7 days in RPMI 1640 medium with 10% autologous serum, supplemented with 1000 U/mL GM-CSF (Amgen/Immunex, Thousand Oaks, CA) and 500 U/mL IL-4 (Peprotech, Rocky Hill, NJ). On day 6, DCs were treated overnight with 100 ng/mL of LPS (E. coli serotype 026:B6, Sigma), a cytokine cocktail of 10 ng/ml IL-1β, 1000 U/ml IL-6 and 10 ng/ml TNF-α (Peprotech), or left untreated in order to generate age-matched mature and immature DC populations. Lymphocytes were isolated from parallel populations of non-adherent mononuclear cells.
Pressure application
Pressure was controlled using an airtight Lucite box with an inlet valve for gas application and an outlet valve connected to a manometer [12]. The box was prewarmed to 37°C to prevent internal temperature and pressure fluctuations. Temperature was maintained at 37±2°C and pressure at 40±1.5 mmHg throughout the duration of the 12-hour experiment. Control cell populations were maintained at ambient pressure in the same incubator.
DC staining and flow cytometric analysis
Following exposure to experimental conditions, dendritic cells were washed twice with phosphate buffered saline (PBS) and fixed with 1% paraformaldehyde. After fixation, cells were washed twice with staining buffer containing 0.2% BSA and 0.02% sodium azide in PBS and incubated with anti-CD80 (PE-L307.4), anti-CD86 (PE-IT2.2), anti-HLA-DR (FITC-G46-6), anti-HLA-class I (FITC-BB7.2) and anti-CD40 antibodies (BD Pharmingen, San Diego, CA) for 30 minutes at 4°C. DCs were then washed and analyzed with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and WinMDI software. Flow cytometric analysis was performed strictly on cells within a standard DC gate, based on size and granularity.
Cytokine enzyme-linked immune absorbent assay (ELISA)
Following exposure to ambient or increased pressure conditions, supernatant was collected from DCs seeded at 2.5 × 105 cells/mL in RPMI-1640 medium supplemented with 10% autologous serum. Concentrations of immunoreactive IL-12p70, IL-6, IFN-γ, and TNF-α in supernatants were determined by ELISA kits according to the manufacturer’s recommendations (BD Biosciences).
T cell stimulation by allogeneic DCs
Isolated lymphocytes were labeled with 5 µM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Inc., Eugene, OR) at 37°C for 10-minutes, washed, and stimulated at a ratio of 1:5 with experimentally-treated allogeneic DCs. Mixed lymphocytes and DCs were co-cultured for 3 days before being isolated, stained with either anti-CD4 or anti-CD8 allophycocyanin (APC)-conjugated antibodies (BD Biosciences) and assessed for proliferation by flow cytometric analysis of double-positive stained cells. Modfit Proliferation Wizard (Verity Software House Inc., Topsham, MN) and CellQuest software (BD Biosciences) were used for determination of cell proliferation index.
Statistical analysis
Statistical analysis was done using SigmaStat software (SPSS, Inc., Chicago, IL). Student’s paired or unpaired t tests were employed as appropriate. A 95% confidence interval was set a priori as the desired level of statistical significance.
RESULTS
Exposure to increased extracellular pressure stimulates expression of DC surface maturation markers
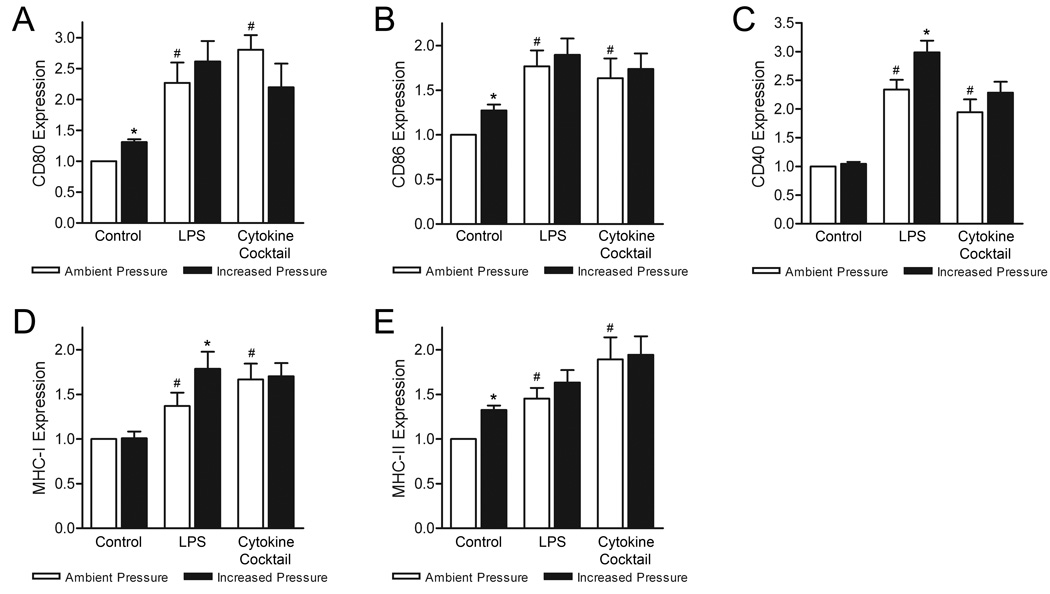
Previous studies suggest increased extracellular pressure may act as a maturation stimulus for DCs [8]. To expand upon these initial findings, we assessed DC surface expression of CD80, CD86, CD40, MHC class I and MHC class II molecules on immature, LPS-matured, and cytokine-matured DCs following 12-hour exposure to either ambient or 40 mmHg increased pressure conditions. Under ambient conditions, both LPS and IL-1β/IL-6/TNF-α cytokine treatment significantly increased expression of all surface markers compared with untreated DCs (Fig. 1), in agreement with known DC maturation trends [1,13]. Consistent with our previous findings, iDC exposure to elevated pressure increased expression of CD80, CD86, and MHC-II by 31±5% (n=6; p<0.01), 28±7% (n=6; p<0.02), and 33±5% (n=6; p<0.01), respectively. DC surface expression of CD40 and MHC-I molecules were unaffected. Interestingly, exposure of LPS-matured DCs to elevated pressure stimulated a further increase in both CD40 and MHC-I expression by 65±21% (n=6; p<0.01) and 39±15% (n=6; p<0.02). This effect was not observed with cytokine-matured DCs. Pressure-stimulated changes in CD80, CD86, and MHC-II expression on LPS- and cytokine-matured DCs were only modest and deemed insignificant. In contrast to our initial report [8], where these changes were significant on LPS-matured DCs, the more subtle effects observed here likely reflect the greater number of donors used for these studies as well as inherent differences in responsiveness between donors.
Figure 1. Effect of increased pressure, LPS, and cytokine stimuli on DC surface maturation markers.
DC surface expression of (A) CD80, (B) CD86, (C) CD40, (D) MHC class I, and (E) MHC class II molecules were compared between iDCs, and LPS- and cytokine-matured DCs following culture under ambient (open bars) or 40 mmHg increased pressure (closed bars) conditions. Expression data is representative of the mean fluorescence intensity (MFI) of each marker normalized to respective iDC ambient pressure controls and graphically expressed as mean ± SEM (n=6). *P<0.05 compared with respective ambient pressure control; #P<0.05 compared with respective iDC ambient pressure control.
DC inflammatory cytokine production is enhanced under elevated pressure
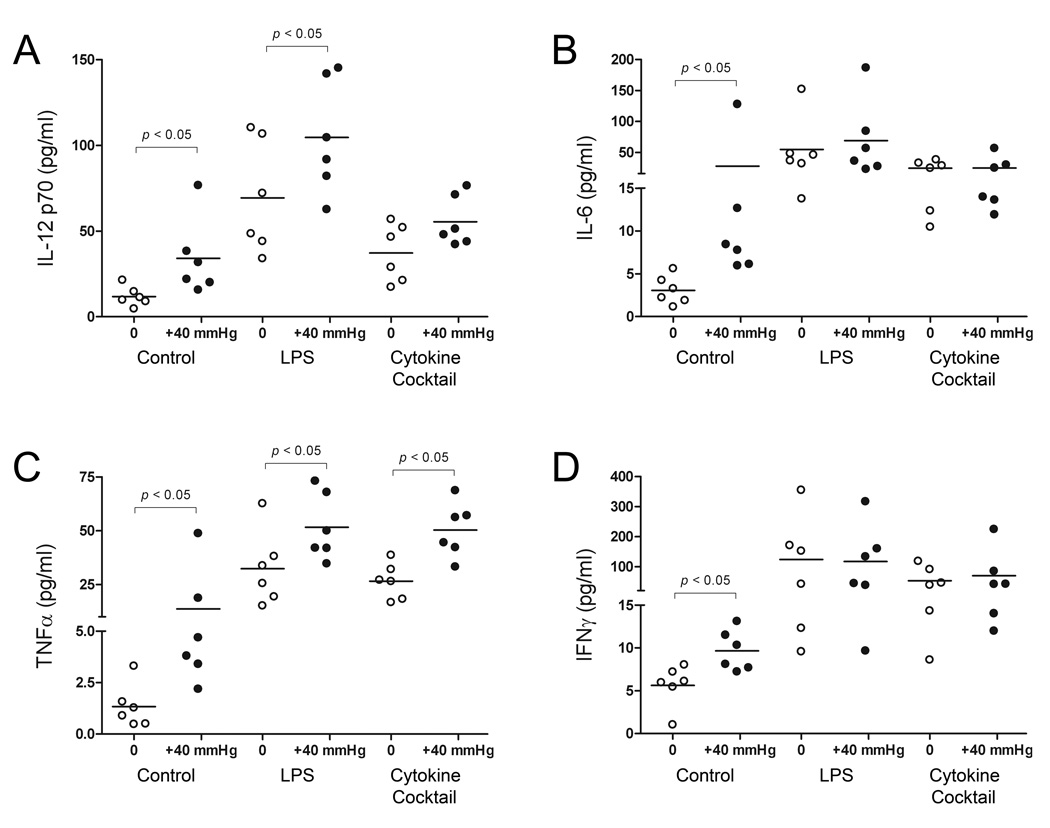
Mature DC cytokine profiles exhibit a high degree of plasticity depending on the nature of their maturation stimulus [14]. We therefore sought to assess the effects of extracellular pressure on DC production of IL-12p70, IL-6, IFN-γ and TNF-α, and compare the cytokine profiles of LPS- and cytokine-matured DCs in cell supernatants by ELISA (Fig. 2). Age-matched iDCs maintained under ambient conditions expressed low levels of all four cytokines. 12-hour exposure to elevated pressure stimulated a 186±77% (n=6; p<0.05) increase in IL-12p70 release compared with iDC controls. LPS-matured DCs displayed a 487±113% (n=6; p<0.01) increase in IL-12p70 release compared to iDC under ambient conditions, and increased production by an additional 199±104% (n=6; p<0.01) under elevated pressure. Cytokine-matured DCs displayed a 214±58% (n=6; p<0.02) increase in IL-12p70 release compared with iDCs, and display a further 49±16% (n=6; differences not significant) increase under pressure. Elevated pressure stimulated a 817±651% (n=6; p<0.05) increase in iDC IL-6 production compared with iDC ambient pressure controls. LPS-matured DCs produced 1692±656% (n=6; p<0.01) greater IL-6 compared to iDC controls, but displayed a highly variable 367±823% (n=6; differences not significant) additional increase under elevated pressure. Cytokine maturation increased DC IL-6 release by 705±151% (n=6; p<0.01), but this population of mDCs again failed to display any further pressure-mediated cytokine production. Pressure-treated iDCs displayed a 914±556% (n=6; p<0.05) increase in TNF-α release. Both LPS- and cytokine-matured DCs displayed a significant 2318±522% (n=6; p<0.001) and 1881±253% (n=6; p<0.001) increase in TNF-α production compared to iDC under ambient conditions. Interestingly, both LPS- and cytokine-matured DCs also increased production of TNF-α under elevated pressure by an additional 1333±472% (n=6; p<0.04) and 1673±388% (n=6; p<0.01), respectively. Pressure similarly stimulated a 72±17% (n=6; p<0.02) increase in IFN-γ production in iDCs. Under ambient conditions, both LPS- and cytokine-matured DCs significantly increased expression of IFN-γ by 2092±964% (n=6; p<0.05) and 837±314% (n=6; p<0.04), respectively, yet neither displayed any further response under pressure.
Figure 2. Effect of increased pressure, LPS, and cytokine maturation stimuli on DC cytokine production.
Supernatants from iDCs, and LPS- and cytokine-matured DCs were harvested following exposure to either ambient (open bars) or 40 mmHg increased pressure (closed bars) conditions. Levels of (A) IL-12p70, (B) IL-6, (C) TNF-α, and (D) IFN-γ in cell supernatants were measured by ELISA. Data points are representative of individual donors and values are expressed in pg/mL. Horizontal bars represent mean values under each condition (n=6).
Pressure-stimulated DC upregulation of MHC molecules enhances T-cell stimulatory capacity
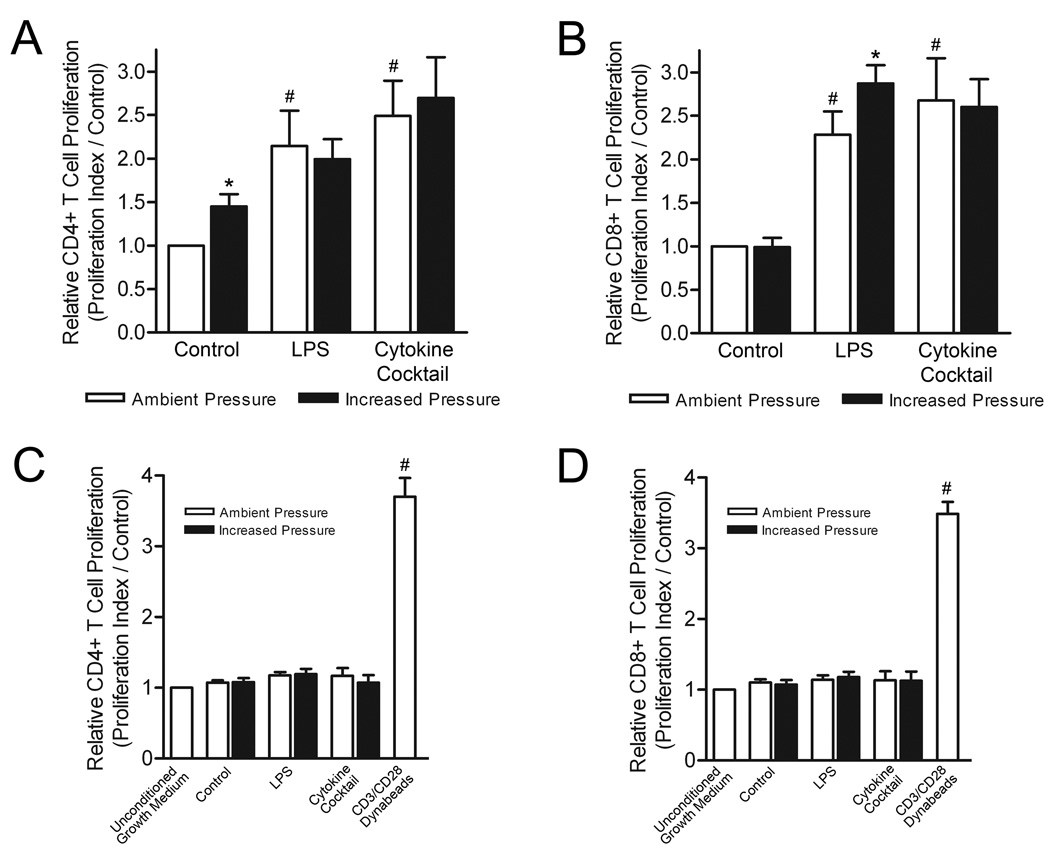
Differences in DC phenotypic maturation and cytokine secretion are of functional relevance primarily in their regard to T-cell stimulation. Thus we finally assessed whether the observed pressure-mediated changes in DC surface marker expression and cytokine production would have a functional impact on CD4+ and CD8+ T-cell proliferation. Human lymphocytes were isolated from non-adherent mononuclear cell populations and labeled with CFSE. Following exposure of immature and alternatively matured DCs to either ambient or increased pressure conditions, DCs were co-cultured with allogeneic lymphocytes at a ratio of 5:1, DCs to lymphocytes. After 3 days, lymphocyte populations were harvested and stained with either anti-CD4 or anti-CD8 APC-conjugated antibodies. CD4+ and CD8+ T-cell proliferation index values were determined by flow cytometric analysis of APC+CFSE+ cell populations (Fig. 3A & B). CD4+ T-cells stimulated with pressure-matured DCs exhibited a 45±17% (n=6; p<0.05) greater proliferation index than those co-cultured with iDCs maintained under ambient conditions. LPS-and cytokine-matured DCs both stimulated significantly greater CD4+ T-cell proliferation than iDC controls, while neither mDC population displayed enhanced stimulatory capacity when pretreated with pressure. In contrast to CD4+ T-cell proliferation results, pressure-matured DCs did not display any enhanced stimulatory capacity towards CD8+ T-cell populations. To the contrary, LPS-matured DCs enhanced CD8+ T-cell proliferation by 128±30% (n=6; p<0.01) compared with iDCs under ambient conditions, and stimulated a further 59±21% (n=6; p<0.05) increase in CD8+ T-cell proliferation index following prolonged exposure to elevated pressure. Cytokine-matured DCs displayed a similar CD8+ T-cell stimulatory capacity as LPS-matured DCs under ambient conditions, but failed to display a functional pressure-mediated stimulatory response.
Figure 3. Effect of increased pressure, LPS, and cytokine maturation stimuli on DC capacity to stimulate T-cell proliferation.
CFSE-labeled T-cells were co-cultured for three days with either iDCs, LPS- or cytokine-matured DCs exposed to ambient (open bars) or 40 mmHg increased pressure (closed bars) conditions. T-cell proliferation was determined by flow cytometric analysis of CFSE label intensity in co-cultured (A) CD4+ and (B) CD8+ T-cells. The stimulatory effect of DC-conditioned medium on (C) CD4+ and (D) CD8+ T-cell proliferation was similarly assessed. Proliferation data is representative of T-cell proliferation index values normalized to respective iDC ambient pressure controls and graphically expressed as mean ± SEM (n=6). *P<0.05 compared with respective ambient pressure control; #P<0.05 compared with respective iDC ambient pressure or unconditioned medium control.
To discern whether the enhanced stimulatory capacity of pressure-stimulated DCs reflects increased expression of cell surface molecules, increased cytokine production, or a combination of both, we additionally assessed CD4+ and CD8+ T-cell proliferation following 3 day culture in the cytokine-rich conditioned medium from parallel populations of experimentally treated DCs (Fig. 3C & D). Both CD4+ and CD8+ T-cell proliferation were severely impaired in the absence of physical engagement with DCs. No proliferative differences were observed between T-cell subsets regardless of the conditioned medium source (n=6). Moreover, only subtle, insignificant differences in proliferative index were detected between T-cell populations cultured in conditioned versus non-conditioned control medium.
DISCUSSION
Although we have previously reported that increased extracellular pressure imparts a novel DC maturation stimulus, in the current study we have expanded upon these findings by comparing the effects of pressure, LPS- and cytokine-stimulation on DC co-stimulatory and MHC molecule expression, inflammatory cytokine secretion, and T-cell stimulatory capacity. Our results suggest environmental pressure may influence both the phenotypic and functional maturity of DCs through upregulation of cell surface molecules and increased cytokine secretion, resulting in enhanced T-cell stimulatory capacity. While pressure-mediated DC maturation effects were less robust than those achieved through LPS or cytokine stimulation, the synergistic impact of LPS treatment in conjunction with increased pressure supports the potential use of mechanical stimulation as an adjuvant to some conventional DC maturation protocols.
The ability of elevated pressure to trigger an increase in costimulatory and MHC molecule surface expression is consistent with DC responses to other inflammatory signals and supports the idea that environmental pressure can act as a DC maturation stimulus. Yet, the inability of pressure treatment alone to stimulate iDC CD40 expression, necessary for induction of effective T-cell responses, suggests the pressure stimulus is ill-suited for independent use as a method of DC maturation [15]. Pressure was however capable of markedly enhancing CD40 and MHC-I expression following TLR stimulation by LPS, consistent with prior observations [8]. The mechanism underlying observed differences in the response to pressure between iDCs and LPS-matured DCs requires some speculation. Full activation or “licensing” of mDCs requires CD40 cross-linking with CD40L on T cells in the draining lymph nodes [2]. It is therefore plausible that the inability of pressure to stimulate CD40 expression on iDCs is an adaptive response preventing autoimmunity by abating DC activation under elevated lymphatic pressures in the absence of foreign antigen. Alternatively, following TLR stimulation by antigen, components of the pressure-sensitive signaling pathway in DCs may cross-talk with downstream TLR signaling molecules and function synergistically.
While the pressure-stimulated signaling cascades impacting DC maturation are currently unknown, three mitogen-activated protein kinases (MAPKs), p38 MAPK, JNK, and ERK, are reported to differentially regulate DC phenotypic maturation in response TLR and cytokine stimulation [16]. These reports suggest DC phenotypic maturation and cytokine secretion are positively regulated by p38 MAPK and JNK phosphorylation, and negatively regulated by ERK. Interestingly, a previous study from our lab found that elevated pressure stimulates macrophage phagocytic function in a p38 MAPK-dependent manner [17]. Moreover, this same study found that macrophage ERK phosphorylation was significantly reduced under elevated pressure conditions. Thus, it is also possible that the differences in sensitivity to pressure stimuli of CD40 and MHC-I molecules between immature and LPS-matured DCs are exemplitive of dual LPS-mediated p38 MAPK activation and pressure-mediated ERK inhibition. Yet, why cytokine-matured DCs appear less sensitive to elevated pressures is unclear.
Generation of an effective T cell response requires activation of naïve T-cells by MHC-peptide complexes on mDCs in the context of appropriate costimulatory molecules. Pathogen-specific responses are further defined by additional soluble signals provided by mDCs that influence the appropriate polarization of Th1/Th2 cell responses [18]. Th1-skewed immune responses are characterized by the preferential induction of antigen-specific IFN-γ-secreting CD4+ T cells capable of supporting long-lasting CD8+ T cell responses, essential for effective tumor and viral immunity [19]. In contrast, Th2 responses primarily mediate host protection against parasitic infection, but are non-protective against tumor and viral pathogens [20]. High production of IL-12, TNF-α, and IFN-γ by DCs typically promote Th1 responses whereas Th2 responses result from low IL-12 and TNF-α, and high IL-6 [21]. Consistent with our results, DC stimulation with LPS is known to generate type-1 mDCs expressing high levels of IL-12 compared with cytokine-matured DCs [22]. Exposure of iDCs to elevated pressure stimulated a significant increase in secretion of all four cytokines assessed. IL-6 production by pressure-treated iDCs was of similar magnitude to cytokine-matured DCs whereas pressure-treated iDC IL-12p70 and IFN-γ production was relatively meager in comparison to type-I LPS-matured DCs. The relatively low IL-12p70 production and high IL-6 production by pressure-treated iDCs suggests exposure to elevated pressure in the absence of other maturation stimuli may generate type-2 mDCs promoting Th2 cell responses. Alternatively, exposure of LPS-matured DCs to elevated pressure stimulated a further increase in IL-12p70 production suggesting pressure may act in conjunction with some maturation stimuli to promote further Th1 polarization.
Our assessment of T-cell proliferation following co-culture with alternatively treated DCs suggests pressure-mediated changes in DC phenotype can directly impact T-cell stimulatory capacity. However, the observed increase in CD4+ T-cell proliferation following T-cell co-culture with pressure-treated iDCs, and enhanced CD8+ T-cell proliferation following co-culture with pressure-stimulated LPS-matured DCs more specifically suggests increased MHC expression is the predominant mediator of this effect. DC cytokine production was insufficient to stimulate T-cell proliferation in the absence of cell-cell interaction. Likewise, it follows that the differences in cytokine profiles of conditioned medium from alternatively stimulated DCs were also insufficient to measurably impact T-cell proliferation. Further investigation is required to determine whether opposing DC cytokine profiles could still be sufficient to polarize specific Th1 or Th2 cell responses in the absence of any proliferative effect.
In summary, these findings suggest prolonged exposure of iDCs to increased extracellular pressure stimulates an aberrantly mature phenotype capable of stimulating CD4+ T-cells, but likely prone to polarization of Th2 immune responses. However, pressure-activated signaling also appears to act synergistically with TLR stimulation, further potentiating a type-I mDC phenotype. Thus, pressure stimuli may provide divergent signals dependent on the state of DC activation. Although superior to pressure stimulation alone, the use of cytokines such as IL-1β, IL-6, and TNF-α for DC maturation still falls short in producing potent Th1-polarizing DCs compared with TLR agonists, such as LPS, due to low induction of IL-12 [22]. Interestingly, while in vitro results would suggest otherwise, cytokine-based maturation strategies have still been used preferentially by most DC vaccine clinical trials to date. Consequently, the best approach to generating immunocompetent DCs remains unclear. Our current findings raise the possibility that mechanical pressure may be used as an adjuvant to enhance the efficacy of other conventional DC maturation methods. Elucidation of the intracellular signals modulating DC responses to pressure may provide a better understanding of whether mechanical stimuli operate via unique pathways or overlap with other known inflammatory signals, and thus whether these signals may be successfully manipulated to enhance DC immunogenicity.
ACKNOWLEDGMENTS
Supported in part by NIH RO1 DK60771 (MDB) and Department of Veterans Affairs Merit Review (MDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avigan D. Dendritic cells: development, function and potential use for cancer immunotherapy. Blood Rev. 1999;13:51–64. doi: 10.1016/s0268-960x(99)90023-1. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 6.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 7.Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst. 2004;1:53–68. [PubMed] [Google Scholar]
- 8.Craig DH, Schaubert KL, Shiratsuchi H, Kan-Mitchell J, Basson MD. Increased pressure stimulates aberrant dendritic cell maturation. Cell Mol Biol Lett. 2008;13:260–270. doi: 10.2478/s11658-007-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res. 1991;51:6691–6694. [PubMed] [Google Scholar]
- 10.Nathan SS, DiResta GR, Casas-Ganem JE, Hoang BH, Sowers R, Yang R, Huvos AG, Gorlick R, Healey JH. Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res. 2005;11:2389–2397. doi: 10.1158/1078-0432.CCR-04-2048. [DOI] [PubMed] [Google Scholar]
- 11.Modi S, Stanton AW, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol. 2007;583:271–285. doi: 10.1113/jphysiol.2007.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basson MD, Yu CF, Herden-Kirchoff O, Ellermeier M, Sanders MA, Merrell RC, Sumpio BE. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem. 2000;78:47–61. doi: 10.1002/(sici)1097-4644(20000701)78:1<47::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, Lander ES, Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 15.Tong AW, Stone MJ. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 2003;10:1–13. doi: 10.1038/sj.cgt.7700527. [DOI] [PubMed] [Google Scholar]
- 16.Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Shiratsuchi H, Basson MD. Activation of p38 MAPKalpha by extracellular pressure mediates the stimulation of macrophage phagocytosis by pressure. Am J Physiol Cell Physiol. 2005;288:C1083–C1093. doi: 10.1152/ajpcell.00543.2004. [DOI] [PubMed] [Google Scholar]
- 18.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 19.Hokey DA, Larregina AT, Erdos G, Watkins SC, Falo LD., Jr Tumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunity. Cancer Res. 2005;65:10059–10067. doi: 10.1158/0008-5472.CAN-05-1692. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 22.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]