Abstract
Humans are often exposed to music beginning at birth (or even before birth), yet the study of the development of musical abilities during infancy has only recently gained momentum. The goals of the present study were to determine whether young infants (ages four to seven months) spontaneously moved rhythmically in the presence of music, and whether the presence of visual information in addition to music would increase or decrease infants’ movement. While nearly all infants moved in the presence of music, very few infants demonstrated rhythmic movement. Results revealed that, when visual information was present, and particularly when infants appeared to show focused attention toward the visual information, infants moved less than when only auditory information was present. The latter result is in agreement with most studies of sensory dominance in adults in which visual stimuli are dominant over auditory stimuli.
Keywords: auditory dominance, infant, music, rhythmic movement, synchronization, visual dominance
Why do people engage in musical behavior? Some of the oldest physical artifacts found in human and protohuman excavation sites are musical instruments such as bone flutes (Levitin, 2006). Today, musical behavior is a universal phenomenon shared by all cultures (Brown, Merker, & Wallin, 2000); however, there is variability in how different cultures engage in musical behavior. For instance, in Western society, there is a distinct classification between those who are considered music performers and music listeners (Levitin, 2006). Distinctions such as this are likely both a cause and a result of the systematization of music education in Western society. Expertise in a domain such as music is said to require 10 years of intense and deliberate preparation by an individual (Ericsson, Krampe, & Tesch-Romer, 1993). It does not, however, take a musical expert to enjoy or listen to music. Music appears in many areas of everyday life, such as in shopping centers and restaurants, as the background of a homework session, and from the car radio. Music also appears in important events and ceremonies such as weddings, presidential inaugurations, and holiday celebrations. And yet music can be enjoyed and produced by even the youngest of people: children. Although songs geared especially toward children are a common feature in educational and play contexts, children in Western society are often exposed to a variety of musical genres and in different contexts (e.g., as a performer, as a listener, as a dancer). Even during infancy, nature seems to have a way of introducing rhythmic melodies, both through non-social sources (e.g., television and stereo) and social sources (e.g., singing in caregiver–infant interactions). The subject of this study, and a growing body of research, is how musical behavior develops during infancy: when, and under what conditions, do infants’ behaviors begin to resemble the sophisticated musical behaviors of adults?
One of the main theories regarding the development of musical behavior is that caregivers express emotional information to infants through infant-directed song (Trainor, 1996). For example, caregivers may sing a playful melody to express happiness and liveliness or sing a lul-laby to soothe an infant and communicate that it is time to go to sleep. Another developmental theory is that mothers produce music in order to regulate an infant’s emotional state (Trainor, 1996). Specifically, music provides a way for infants to learn about social interaction and self-regulation before developing linguistic competence. Additionally, maternal singing has been found to modulate arousal in infants, leading to slight increases in arousal in infants with low baseline arousal levels, and slight decreases in arousal in infants with higher baseline arousal levels (Shenfield, Trehub, & Nakata, 2003).
Regardless of its purpose, maternal singing appears to be culturally universal, both in its occurrence and in its characteristics. Just as infant-directed speech commonly incorporates certain melodic features, infant-directed singing shares specific features across different cultures, such as repetition and a narrow pitch range (Trehub & Trainor, 1998). Infants prefer songs sung in this manner to songs sung in a non-maternal style, and it is unlikely that this preference is due solely to musical exposure during gestation or early infancy, because hearing infants born to deaf parents demonstrate similar preferences for music sung in a maternal style (Masataka, 1999; Trainor, 1996). These findings suggest that music plays an important social role, particularly in the mother–infant relationship.
The developmental perspective also proposes that, in addition to the auditory features of music, music also possesses many visual features. Caregivers often incorporate gestural features into speech directed at infants and young children (Shatz, 1982). Just as maternal speech is full of expressive vocal features that make it music-like, maternal singing often involves expressive movements that make it dance-like (Longhi, 2009). These musical and dance-like qualities of maternal communication are also found in deaf mother-infant dyads. For example, deaf caregivers adopt a style or register of sign language that has notable parallels to infant-directed speech, and the signing of deaf mothers to their infants involves rhythmic and repetitive movements (Trehub & Nakata, 2001–2002).
As infants develop, they are gradually introduced to a culture of music that provides both auditory and visual components through nursery rhymes, pre-school-aged songs, and media geared towards children (e.g., Sesame Street, The Wiggles, Barney, among others), and infants are encouraged to, and rewarded for, expressing themselves through music in many ways. Synchronized, rhythmic movement to music, such as clapping, tapping, dancing, singing and ensemble performance, appears to be a universal human behavior as it has been observed across all known cultures (Hannon & Trehub, 2005; Nettl, 2000). It should be noted that an ability to move rhythmically to music does not imply the ability to move in synchrony with music. Rhythmic movements involve repeated, similar movements, which may or may not occur in the presence of external stimuli such as music. Synchronous rhythmic movements occur when motor movements are timed to occur in synchrony with external stimuli such as music. While numerous studies have established the ability of young children to perceive rhythm and move rhythmically in the presence of music (Denac, 2008; Drake, Jones, & Baruch, 2000; Kirschner & Tomasello, 2009; McAuley, Jones, Holub, Johnston, & Miller, 2006; Provasi & Bobin-Begue, 2003), only recently have investigators begun to focus on infants’ ability to move rhythmically in the presence of music, and the effect of training on perception of different rhythms in auditory stimuli (Gerry, Faux, & Trainor, 2009; Longhi, 2009; Phillips-Silver & Trainor, 2005; Zentner & Eerola, 2010). Various studies have found that infants can detect rhythm; for instance, in their study of 11–12-month-old infants, Hannon and Trehub (2005) found that infants can readily differentiate musical rhythms (see Thorpe & Trehub, 1989; Trehub, 1987; and Trehub, 1990 for further examples). In fact, a study using event-related potentials (ERP) has shown that the ability to detect a change in rhythm is present in neonates (Winkler, Haden, Ladinig, Sziller, & Honing, 2009). Thus, well before infants appear capable of moving rhythmically, they are capable of perceiving external auditory rhythms.
In addition to infants’ abilities to detect rhythm, two studies have demonstrated auditory-motor associations in infants (Gerry et al., 2009; Phillips-Silver & Trainor, 2005). In these studies, infants who were bounced to a certain rhythm later preferred the rhythm they were bounced to over another rhythm. While infants appear capable of perceiving rhythms, only three studies have demonstrated infants’ ability to produce rhythmic movements in the presence of music (Longhi, 2009; Mazokopaki & Kugiumutzakis, 2009; Zentner & Eerola, 2010). Rhythmic movement to music is plausible because young infants demonstrate a number of stereotyped rhythmic movements, defined by Thelen as a minimum of three subsequent movements of the same type, such as leg kicking, arm waving, and torso swaying (Thelen, 1979). There is a significant increase in stereotyped movements between .9–2.8 and 3.2–5.0 months of age, with a peak in stereotyped movements between 5.5 and 9.7 months of age. Similarly, Kravitz and Boehm (1971) found a median age of onset of 2.7 months for rhythmic foot kicking, and 6.1 months for rhythmic body rocking.
While Thelen (1979) interprets stereotyped movements like those described earlier as developmental precursors to complex, voluntary movements, and under little voluntary control, she nevertheless notes that these movements may be elicited by particular environmental stimuli. We therefore hypothesized that young infants (ages 4–7 months) might demonstrate rhythmic, stereotyped movements in the presence of music.
Because various rhythmic movements have been found to have different ages of onset (Kravitz & Boehm, 1971), and because rhythmic movements peak between 5.5 and 9.7 months of age (Thelen, 1979), infants’ ages may be an important factor for rhythmic movement. While Gerry et al. (2009) and Phillips-Silver and Trainor (2005) tested seven-month-old infants, and Zentner and Eerola (2010) tested infants from five to 24 months of age, Longhi (2009) tested infants as young as three months old. In her study, Longhi (2009) demonstrated that even three-month-old infants could move in synchrony to songs sung during mother-infant interactions. Unlike Longhi’s (2009) study, we examined infants’ movements outside the context of mother-infant interactions; instead of songs sung by the mother, infants listened to non-lyrical music played from a speaker. We expected that infants’ movements in the presence of music might not be as refined as those of adults, as adults have the advantages of greater development of motor abilities, coordination and practice. Accordingly, Zentner and Eerola (2010) found that, while infants as young as five months moved rhythmically in the presence of music, infants and toddlers (up to 24 months old) did not move in synchrony with the music. Yet, just as infants babble before they speak (Goswami, 2008), perhaps spontaneous movements (both arrhythmic and rhythmic) in the presence of music are a developmental precursor of the sophisticated synchronous movements adults are capable of producing. Perhaps infants’ movements in the presence of music would be reflected as simple quick movements of the head, torso, hands, feet, or any combination of the four.
Because music often incorporates both visual and auditory features, the existence of sensory dominance comes into question. Sensory dominance refers to a hierarchy in sensory perception. For example, if hearing is dominant over vision in infancy, if an infant is presented with a toy that produces sounds and lights, the infant’s sense of hearing may provide the infant with more salient information than their sense of sight. Sensory dominance seems especially likely in early infancy, since the development of the auditory system precedes the development of the visual system both structurally and functionally (Lewkowicz, 1988). While in the womb, infants can learn by association through the use of their auditory system and it is not until after birth that infants can begin to utilize their visual system effectively and fully as their eyesight continues to develop (Goswami, 2008).
Studies with adults have repeatedly found that visual stimuli are dominant over auditory stimuli (Colavita, 1974; Colavita & Weisberg, 1979; Hecht & Reiner, 2009; Koppen, Levitan, & Spence, 2009; Lukas, Philipp, & Koch, 2010; Sinnett, Spence, & Soto-Faraco, 2007; Van Damme, Crombez, & Spence, 2009). For instance, Colavita (1974) instructed adult participants to press one key to indicate that a tone was played, and a different key to indicate that a light was flashed. When participants were presented with bimodal trials (on which the tone and light were presented simultaneously), nearly all participants indicated that the light was flashed. There are some exceptions to visual dominance, however, when rhythmic movement is involved. Studies with adults have demonstrated that, when adults are required to move rhythmically (i.e., tap a finger) in synchrony with a stimulus, they are more accurate when presented with auditory stimuli than when presented with visual stimuli (Patel, Iversen, Chen, & Repp, 2005; Repp & Penel, 2002, 2004). When participants are presented with both auditory and visual stimuli, responses are more similar to responses to auditory stimuli (alone) than responses to visual stimuli (alone; Repp & Penel, 2002). Similarly, when adults must synchronize their finger tapping to either an auditory or visual stimulus, and then an asynchronous distractor stimulus of the other modality is used, auditory distractors appear to be much more salient than visual distractors (Repp & Penel, 2004). Taken together, these results suggest that, when moving rhythmically, adults demonstrate auditory dominance, a finding at odds with the majority of the sensory dominance literature.
While several studies with infants have demonstrated infants’ ability to match synchronous auditory and visual information (Lewkowicz, 1986, 1992; Spelke, 1979; Spelke, Born, & Chu, 1983), the issue of sensory dominance in infancy is less clear. Lewkowicz (1988) found that infants demonstrated auditory dominance when they viewed a flashing checkerboard and heard a pulsing sound. While infants detected changes in the visual stimulus after habituation to just the visual stimulus, infants did not detect changes in the visual stimulus when bimodal (audiovisual) stimuli were used. In a subsequent study, however, Lewkowicz (1994) found that, when infants habituated to circles moving upwards and downwards on a screen, accompanied by the sound of a bouncing ball, infants were most sensitive to changes in the visual stimulus (rather than the auditory stimulus). Instead of using a habituation-dishabituation paradigm, the present study was designed to assess sensory dominance by measuring infants’ movement to auditory stimuli both when visual information was, and was not, present.
In addition to the question of whether auditory or visual stimuli are dominant in infants, the source of sensory dominance is also unclear. Posner, Nissen, and Klein (1976) propose that sensory dominance is the product of non-sensory processes, such as attention. Their explanation for visual dominance is that, because the visual system is less adept than the auditory system at alerting people to important cues, people essentially compensate for this weakness in vision by attending more to visual stimuli. Conversely, Colavita (1974) proposes that visual dominance is the product of sensory processes, namely the direct connections between the superior colliculus and motor centers of the ventral tegmentum, medulla, and spinal cord. Regardless of whether sensory dominance is primarily a product of sensory or non-sensory processes, several studies suggest that attention can modulate sensory dominance (Colavita, 1974; Colavita & Weisberg, 1979; Posner et al., 1976; Sinnet et al., 2007). In each of these studies, however, while attentional manipulations could reduce visual dominance, no manipulations were successful at eliminating visual dominance in adults.
In the present study, we examined whether infants aged four to seven months would move spontaneously in the presence of music. We attempted to minimize the influence of mothers on their infants’ movement, and thus examined infants’ movement in a relatively asocial context (as opposed to within a typical mother-infant interaction). We also examined whether infants demonstrated sensory dominance while listening to music in the presence of visual information. We predicted that infants would be able to detect an auditory periodic beat and convey this information through the rhythmic movement of their head, torso, hands, feet or a combination of any of the four. However, if a visual stimulus is introduced that moves in synchrony with the auditory stimulus, and if infants demonstrate visual dominance, we predicted a reduction in the infant’s movement, whether in synchrony with the periodic beat or not. Because attentional manipulations can reduce visual dominance in adults, we expected that infants’ focused attention to the video monitor might modulate sensory dominance. Thus, if visual stimuli are dominant over auditory stimuli in infancy, we predicted not only a reduction in movement when visual stimuli were present, but a further reduction in movement when infants directed focused attention to the visual stimuli. Conversely, if infants demonstrate auditory dominance, we predicted that infants would show similar levels of movement regardless of the presence of visual information, and regardless of their attention toward the video monitor.
Method
Participants
Participants were 51 healthy, full-term infants (24 female, 27 male). Two additional participants were excluded from the study due to technical difficulties. Mother-infant pairs were recruited from around the university area and local community using methods such as radio announcements, newspaper advertisements, and posters in doctors’ offices. The infants’ ages ranged from 3.68 to 6.9 months (M = 5.00 months, SD = .88). The mothers’ ages ranged from 18 to 39 years (M = 27.95 years, SD = 5.51). The ethnic composition of the sample was 25% non-Hispanic Caucasian, 55% Hispanic, and 20% other, or more than one, ethnicity. Participants in this study were recruited as part of another study, which required two experimental sessions. Infants typically completed the present study during the second experimental session. Mothers received US$20 for their participation in each session.
Materials
Participants sat approximately two feet from a large Daewoo Plasma Display Panel (94 × 53 cm). Infants were seated on their mother’s lap facing the monitor. The monitor displayed either a chapter from a Baby Einstein™ Mozart DVD, which is geared towards infants (the visual information condition), or black and white static (the no visual information condition). Auditory stimuli were broadcast through a small speaker placed in front of the monitor but not in the way of the visual stimuli. A video camera placed directly above the monitor recorded infants’ movements for later coding and analysis.
Procedure
Infants were placed on their mother’s lap facing the monitor. Mothers were instructed to only assist their infant in sitting by holding their infant by his or her waist.1 To help reduce the mothers’ influence over their infant’s movement, headphones were placed over the mothers’ ears, which played white noise at a level that was tolerable, and yet loud enough to mask the music the infants heard. Because mothers held their infant in their lap, we did not obstruct mothers’ vision. Mothers were asked to not influence their infant’s movement in any way during the experiment.
The experiment consisted of two conditions in which every infant participated: the visual information condition and the no visual information condition. In the visual information condition, the visual stimuli on the monitor moved in approximately synchronous periodic beat with the music. These visual stimuli consisted of brightly colored toys (mostly self-propelled) and puppets moving to the music, which were intended to capture the infants’ visual attention. In the no visual information condition, black and white static could be seen on the monitor; however, this visual stimulus did not move in synchronous periodic beat with the music. The infants listened to two different songs: Mozart’s Piano Sonata No. 11 in A, 3rd movement, K331 (the Turkish March) and Mozart’s Piano Sonata No. 16 in C, 2nd movement, K545 (Andante) that came from the Baby Einstein™ Mozart DVD collection, one of which accompanied each visual condition. For example, if a participant heard the Turkish March and saw the visual stimuli from the DVD first, that participant would next hear Andante but black and white static would accompany the song. The pairing of song and visual information was counterbalanced among participants. These songs were chosen over other possible songs primarily because the visual stimuli that accompanied them on the Baby Einstein™ DVD were deemed to be comparatively interesting. The tempo of the Turkish March was approximately 135 beats per minute, and the tempo of Andante was approximately 119 beats per minute. Infants also listened to a third song, Eine Kleine Nachtmusik, which was always paired with black and white static. Because the third condition did not vary the visual stimulus, this third condition was not included for analysis. The order of song presentation was counterbalanced among participants; however, because of the original inclusion of a third song, this led to the Turkish March occurring before Andante in roughly two-thirds of the sessions. Due to differing lengths of the song excerpts, the Turkish March lasted three minutes and Andante lasted five minutes, however, only a one-minute segment of each song was coded for infants’ movement.2 The entire experiment took less than 10 minutes.
Infants were video recorded in both visual conditions. Two coders independently viewed a one-minute video segment for every participant in both visual conditions. The one-minute segment started at the same measure for each of the two songs, beginning approximately five seconds after the song began.3 Coders watched one-second segments of the video in real time, and rated the infant’s movement for that second, as well as whether the infant directed attention toward the video monitor during that second. The coders did not listen to the videos while rating infants’ movements and they were unaware of which song was being played and which visual condition the infant was participating in. After the independent ratings were made, the videos were reviewed by both an experimenter and the second coder, and disagreements in coding were discussed and resolved. Inter-rater reliability was assessed through kappas computed for each video. Kappas of above .85 were used. If kappas fell below .85, videos were recoded. The coding scheme for the videos is described in the following section.
Coding system for analyzing movement and attention
One of the following levels (0–3) was assigned for movement for each second of one-minute segment for each visual condition:
Level 0: not rhythmic movement. Includes involuntary movement such as sneezes, coughs, yawns, etc. The mother may be repositioning the baby so that one cannot tell if the baby is moving on its own. The baby could also be distracted by playing with his/her own shoes and clothes or is moving his/her hand or other object to place in mouth. Any movement considered as indicating fussing behavior was coded as level 0.
Level 1: slight movement, not rhythmic. There is not very much movement of the limbs: infant is moving just the eyes, a single finger or toe, or slightly moving the mouth.
Level 2: clear movement, not rhythmic. There is easily visible movement through the digits, hands, head, a foot, a leg, an arm or any combination of these.
Level 3: rhythmic movement (two or more instances of the same movement, in succession). Large amounts of repeated movement are detected which includes swaying, rocking, movement of more than one limb at the same time, head banging, clapping, bouncing (initiated by the infant only), legs pushing in squatting movements, or combinations of any of the above.
For attention, each one-minute video segment was also coded regarding whether the infant exhibited visual orienting toward the monitor. Each second was labeled as either ‘not paying attention’ or ‘paying attention’.
Results
Preliminary analyses
For all analyses, we excluded observations coded as ‘0’ for movement (which included involuntary movements such as sneezing and movements caused by the mother), and included only movements coded as voluntary (movement levels 1–3). Because fussing behaviors were coded as ‘0’, movement due to fussing was also excluded from analysis. A total of 2,652 observations (out of 6,120), or 43%, were coded as ‘0’ and therefore excluded from analyses. For each infant, we used the remaining coded movement levels to calculate the average movement level in each 60-second video.4 Thus each infant had two measures of average movement, one for each visual information condition. An exception occurred when an infant had only movement level ‘0’ in a given condition; in that case, he or she had no average movement for that condition, and was excluded from those analyses. The percentage of observations of each movement level (levels 1–3) in each condition is reported in Table 1, as well as the number of participants who were included in each analysis.
Table 1.
The percentage of observations in each condition coded as movement code 1, 2, or 3, the average movement level in each condition, and the number of participants included in each condition. Observations coded as 0 (involuntary movements, fussing behaviors, etc.) are excluded
| Movement code 1 (slight movement) |
Movement code 2 (clear movement) |
Movement code 3 (rhythmic movement) |
Average movement (SD) |
N | |
|---|---|---|---|---|---|
| Gender | 49 | ||||
| Female | 26% | 74% | 1% | 1.81 (.26) | |
| Male | 22% | 74% | 4% | 1.82 (.30) | |
| Previous exposure to DVD | 49 | ||||
| No exposure | 22% | 76% | 2% | 1.83 (.26) | |
| Exposure | 2 7% | 68% | 5% | 1.78 (.32) | |
| Song | 49 | ||||
| Andante | 18% | 79% | 3% | 1.90 (.26) | |
| Turkish March | 29% | 69% | 2% | 1.80 (.33) | |
| Song order | 49 | ||||
| First | 2 7% | 72% | 1% | 1.78 (.31) | |
| Second | 19% | 76% | 5% | 1.92 (.27) | |
| Visual information | 46 | ||||
| No visual information | 17% | 79% | 4% | 1.91 (.28) | |
| Visual inforzmation | 28% | 70% | 2% | 1.79 (.31) | |
| Attention | 46 | ||||
| No attention | 6% | 89% | 5% | 2.00 (.19) | |
| Attention | 27% | 71% | 2% | 1.79 (.30) | |
| Visual information (VI), Attention (A) |
36 | ||||
| No VI, no A | 6% | 86% | 9% | 2.04 (.28) | |
| VI, no A | 5% | 93% | 2% | 1.98 (.17) | |
| No VI, A | 20% | 77% | 3% | 1.87 (.32) | |
| VI, A | 29% | 69% | 2% | 1.79 (.34) |
We first examined whether infants demonstrated rhythmic movement in the presence of auditory stimuli. Only five participants demonstrated rhythmic movement for periods longer than one second (three participants demonstrated more than 15 seconds of rhythmic movement within the 60-second time period). While the remaining 46 participants did not demonstrate rhythmic movement for more than one second, all but two participants demonstrated clear movement (movement level 2). Because these two participants did not demonstrate any voluntary movement during the study, they were excluded from analyses, leaving a sample size of 49 infants.
Because the infants in our sample ranged from four to seven months old, and infants’ rhythmic movement increases during this time (Thelen, 1979), one might expect an effect of age on infants’ movement. For the entire sample, a Pearson correlation revealed no effect of age, in months, on infants’ average level of movement (r = .15, p = .31). For the five infants who demonstrated rhythmic movement, we analyzed rhythmic movement (movement level 3) separately from the other movement levels, and found no effect of age on infants’ rhythmic movement (r = .23, p = .12). Because we found no effect of age on movement, we did not examine age in further analyses.
We next examined whether infants’ movement varied as a function of gender, since generally greater activity levels have been found in male infants than in female infants (Campbell & Eaton, 1999). However, when we examined the average coded movement (movement levels 1-3) for each participant, there was no difference in the average level of movement between the genders (Males, M = 1.82, SD = .30; Females, M = 1.81, SD = .26; F(1,47) = .001, p = .97).
Because the Baby Einstein™ video series is a commercial product marketed to mothers with infants, a number of infants (15 infants; 31%) had previously been exposed to the stimuli used in the study. There was, however, no effect of previous exposure on infants’ average level of movement (no exposure, M = 1.83, SD = .26; exposure, M = 1.78, SD = .32; F(1, 47) = .27, p = .61).
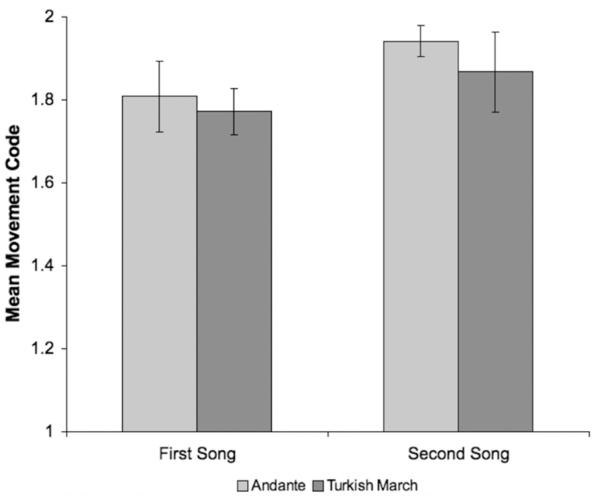
We next examined whether there was an effect of the song infants heard or the order in which they heard each song. Three infants were excluded from these analyses due to a lack of voluntary movement in at least one condition, leaving a sample size of 46 infants.5 Paired t-tests revealed significantly more movement to Andante than to the Turkish March (Andante, M = 1.90, SD = .26, Turkish March, M = 1.80, SD = .33; t(45) = 2.10, p < .05), as well as significantly more movement to the song that was played second than to the song that was played first (first song, M = 1.78, SD = .31; second song, M = 1.92, SD = .27; t(45) = 3.10, p < .01). A 2 (song: Andante or Turkish March)× 2 (song order: first or second) analysis of variance (ANOVA) revealed no significant interaction between song and order on infants’ movement, F(1,91) = .12, p = .74 (Figure 1). Because there was no interaction between song and order, we combined all conditions for further analysis.
Figure 1.
Mean level of movement for each song based on the order in which the song was played. Bars represent standard errors
Visual information
Our second hypothesis was that, due to competition between auditory and visual information, infants would show a reduction in movement in the presence of auditory stimuli if visual stimuli were also present. Three infants were excluded from these analyses due to a lack of voluntary movement in at least one condition, leaving a sample size of 46 infants. Results revealed that infants demonstrated significantly more movement when no visual information was present (no visual information, M = 1.91, SD = .28; visual information, M = 1.79, SD = .31; t(45) = 2.74, p (two-tailed) < .01). These results support our hypothesis, suggesting that the presence of visual information interferes with movements produced in the presence of auditory stimuli.
Attention
Because of the role attention plays in visual perception (Treisman & Gelade, 1980) and sensory dominance (Colavita & Weisberg, 1979; Posner et al., 1976; Sinnett et al., 2007), we examined whether infants’ attention to the monitor influenced their movement. Results revealed that, like the presence of visual information, directed attention also interferes with infants’ movement; infants moved more when they did not direct attention toward the monitor (no attention, M = 2.00, SD = .19; attention, M = 1.79, SD = .30; t(45) = 5.84, p (two-tailed) < .001). This result is consistent with those obtained by Bacher and Robertson (2001), who found that visual attention and movement were inversely related in three-month-old infants.
Visual information and attention
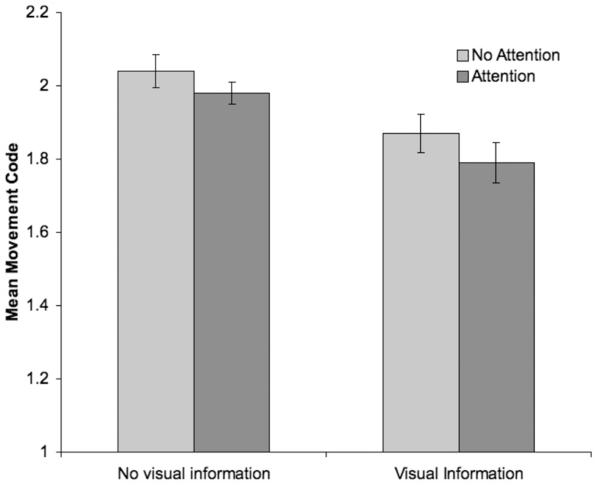
While our results thus far indicate a main effect of visual information and attention to the monitor, they do not indicate whether attention to the different visual stimuli (the DVD visual stimuli or the black and white static) influenced movement. Thus we separately examined the role of attention to the monitor when visual information was, and was not, present. We excluded participants for whom at least one condition lacked voluntary movement or in which there were no observations (e.g., when visual information was present, some participants directed their attention to the monitor the entire time; thus they had no data for the visual information/no attention condition, and were excluded from analyses), leaving a sample size of 36 infants. Both when visual information was, and was not, present, t-tests revealed significantly less movement when infants directed attention toward the monitor (no visual information, no attention, M = 2.04, SD = .28; no visual information, attention, M = 1.87, SD = .32; t(35) = 3.83, p (two-tailed) < .001; visual information, no attention, M = 1.98, SD = .17, visual information, attention, M = 1.79, SD = .34, t(35) = 4.19, p (two-tailed) < .001).6 Overall, our results demonstrated that the most movement occurred when no visual information was present, and that the least movement occurred when the DVD visual stimuli were present and infants were paying attention to the monitor (Figure 2). Again, these results support our hypothesis that infants’ movement to auditory stimuli is reduced in the presence of visual stimuli.
Figure 2.
Mean level of movement based on attention to the monitor and the presence of visual information (DVD stimuli or black and white static). Movement was coded on a 0-3 scale, with analyses excluding movement that was coded 0. Bars represent standard errors
Discussion
While nearly all infants demonstrated noticeable movement in the presence of music, only a few infants moved rhythmically. This result is at odds with the findings of Longhi (2009), Mazokopaki and Kugiumutzakis (2009), and Zentner and Eerola (2010); however, several differences in experimental design may explain this discrepancy. First, the infants in the present study (ages 4–7 months) were somewhat younger than those tested by Zentner and Eerola (2010) (ages 5–24 months). While Longhi (2009) tested infants as young as three months old, Longhi measured infants’ movement during the context of mother–infant interactions, while in the present study, steps were taken to avoid mothers influencing their infants’ movement. Our attempts to isolate the audiovisual stimuli created a comparatively asocial experience for the infants in our study – an experience that might be fairly uncharacteristic of how musical behavior typically develops but, nevertheless, one that might be increasing in frequency in Western society due to frequent exposure to child- and infant-oriented media (particularly screen-based media). If the mothers in our study had encouraged their infants to move to the music as part of a more naturalistic mother–infant interaction, we believe we might have found greater amounts of rhythmic movement.
Another difference between Longhi’s (2009) study and ours is that the music infants listened to in Longhi’s study were songs sung by the infants’ own mothers, rather than recorded, non-lyrical music like that used in the present study. Mazokopaki and Kugiumutzakis’ (2009) results may also demonstrate an effect of familiarity; while the infants in their study demonstrated rhythmic movement even when isolated from their mothers, infants were tested at home (in a familiar, comfortable setting) and with traditional infant-directed songs. Thus the songs used in these two studies were presumably familiar to the infants, and infants likely had previous informal rhythmic training, such as bouncing, swaying, or rocking to these songs. As Gerry et al. (2010) and Phillips-Silver and Trainor (2005) have demonstrated, rhythmic training can influence infants’ perception of auditory rhythm. Perhaps the infants in the present study failed to demonstrate rhythmic movement to music due to the lack of rhythmic training to the songs used and a lack of familiarity with the music they listened to. Fifteen infants (31%) had previously viewed the Baby Einstein™ Mozart DVD, but even for this significant minority, it seems likely that the lullabies and other songs sung by their mothers (or other family members) would be more familiar to those infants than the songs used in our study.
Despite the methodological differences between the present study and Longhi’s (2009), Mazokopai and Kugiumutzakis’ (2009), and Zentner and Eerola’s (2010) studies, the lack of rhythmic movement in the present study is somewhat surprising given the progressive increase in stereotyped movement during early infancy, and the peak for rhythmic movement occurring between 5.5 and 9.7 months (Thelen, 1979). However, the average age of the infants in the present study (5.00 months) was younger than the lower end of the range at which rhythmic movement peaks; thus, while we may expect rhythmic movement to be present at this age, it may not be especially prevalent. Accordingly, while infants of all ages in Zentner and Eerola’s (2010) study demonstrated significant amounts of rhythmic movement to music, the results of the youngest infants (ages 5–7 months) conformed less. In Experiment 1, the amount of rhythmic movement the youngest infants produced was less than the amount of rhythmic movement produced by older infants, and in Experiment 2, the youngest infants moved just as much to infant-directed speech as they did to music (a result contrary to the results for older infants and toddlers; Zentner & Eerola, 2010). Thus it may be that the infants in the present study were too young to reliably produce rhythmic movement in the presence of music.
As mentioned previously, despite infants’ general lack of rhythmic movement, most infants did move noticeably during the study. Results indicated a main effect of order, with infants moving more to the second song they heard, and a main effect of song, with more movement to Andante, but there was no interaction between song and order. Examining the role of visual information, infants moved less when there was visual information present (the Baby Einstein™ Mozart DVD playing on the screen) than when there was no visual information present (the black and white static). Additionally, we found less movement when infants showed focused attention toward the monitor than when they were not paying attention. Last, we found that, when visual information was present, infants moved significantly less when they directed attention toward the monitor than when they were not paying attention. These results suggest that, in the case of music, visual stimuli are dominant to auditory stimuli in infancy. This finding is consistent with most of the literature on visual dominance in adults – though interestingly, not with the studies suggesting auditory dominance when adults are required to produce a rhythmic movement (Patel et al., 2005; Repp & Pinel, 2002, 2004). Auditory dominance in the case of rhythmic movements may be the product of auditory-motor neural pathways, a subject of recent research (Doupe, Perkel, Reiner, & Stern, 2005; Warren, Wise, & Warren, 2005; Zatorre, Chen, & Penhune, 2007). Despite the potential role of these pathways in motor movements in response to auditory stimuli, and the earlier development of the auditory system than the visual system in infants (Lewkowicz, 1988), the results from the present study support Lewkowicz’s (1994) finding of visual dominance in infancy.
Like our results regarding the overall prevalence of rhythmic movement, methodological differences may also be the source of our results regarding visual dominance. Whereas the present study used complex musical stimuli, the studies suggesting auditory dominance in adults used a series of identical, computer-generated tones (Patel et al., 2005; Repp and Penel, 2002, 2004). In their study of attention and visual dominance, when Sinnett et al. (2007) simplified their auditory stimuli by reducing the number of distractor stimuli, they found a reduction in (though not elimination of) visual dominance. Their explanation for this finding was that the presence of few auditory distractors freed up attentional resources; attention that would have been directed toward processing numerous distractors could now be further directed toward the auditory stimuli of interest. This increase in auditory attention therefore attenuated the effect of (already high) attention toward visual stimuli, and decreased the effect of visual dominance (Sinnett et al., 2007). Because the auditory stimuli used in the present study were fairly complex, it seems likely that no additional attentional resources were available to infants to direct toward the auditory stimuli. Without an increase in attention to the auditory stimuli, the visual stimuli would have remained prepotent.
In order to further explore sensory dominance in infancy, future studies might examine infants’ movement in the presence of rhythmic visual stimuli, both with and without auditory stimuli present. Bacher and Robertson (2001) examined the relationship between movement and visual attention in infancy in the absence of auditory stimuli, and found an inverse relationship between infants’ movement and visual attention. The visual stimuli used in their experiments, however, were largely stationary, so any movements infants produced could be assumed to be spontaneous, rather than elicited by the visual stimuli. Mazokopaki and Kugiumutzakis (2009), conversely, compared infants’ rhythmic movement both in the presence and absence of music. While they found more movement in the musical (vs. the ‘no music’) condition, they also found that the movement expressed in each condition was qualitatively different. These results suggest that caution should be taken when making comparisons between movements made in, and outside of, the presence of auditory stimuli. While the results of these two studies suggest that both visual and auditory stimuli influence infants’ movement, these results cannot address the issue of sensory dominance due to the unimodal stimuli used. Future studies comparing movement in conditions both with and without visual and auditory stimuli could suggest several things: movement in the presence of rhythmic visual stimuli alone would further suggest visual dominance, but a comparative lack of movement without auditory stimuli would suggest an important role for auditory-motor neural pathways in infant movement production. While infants in the present study demonstrated little rhythmic movement, it is plausible that the movements they demonstrated were developmental precursors to rhythmic movements which, in turn, may be developmental precursors to movements in synchrony with music (Zentner & Eerola, 2010). Because rhythmic movement in adults appears to be governed by auditory dominance, it may be that the developmental progression from arrhythmic movements to movements synchronized to music may reflect a developmental transition from visual dominance in infancy to auditory dominance in childhood and adulthood. Further studies examining sensory dominance and rhythmic movements in childhood may elucidate this issue.
Acknowledgments
We would like to thank Dr. Aniruddh Patel for his generous conceptual assistance and helpful discussions. We also gratefully acknowledge the assistance of Jamie Crotinger, Cindy Gutierrez-Barrazza, Ashley O’Hearn, Valeria Olsson, Melissa Stauble, the Mother-Infant Study staff and undergraduates, and the mothers and infants who participated in this study.
Funding
The project described was supported by Award Number SC1HD060887 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health
Biographies
Gin Morgan is a postdoctoral fellow in psychology at New Mexico State University. She received her doctorate in psychology from Columbia University.
Cynthia M. Killough is a graduate student in psychology at New Mexico State University. She received her BA in psychology from New Mexico State University.
Laura A. Thompson is Professor of Psychology at New Mexico State University. She received her doctorate in psychology from the University of California, Santa Cruz.
Footnotes
While nearly every infant in our study produced noticeable movement, infants’ seated position may have restricted their movement more than if they were observed in a reclined or lying position.
One-minute segments were used because they were deemed long enough to capture representative movement behaviors, but short enough to avoid boredom or fussiness. We analyzed the average movement level of a subset of infants to determine how movement levels early in each song compared to movement levels later in each song. Because movement levels significantly declined as the song progressed (t(9) = 3.12, p = .01), we analyzed only one minute of each song, beginning soon after the onset of the song.
Due to a few irregularities in the onset of the video recording, video footage of the first 1-2 seconds of stimulus exposure was not available for some infants. To obtain comparable musical segments for between-subjects analyses, all videos were therefore coded beginning approximately five seconds after the onset of the song.
It might be argued that movement coded as 3 is qualitatively, rather than quantitatively, different from movement coded as 1 or 2. To address this issue, we repeated all of our analyses, changing observations originally coded as 3 (rhythmic movement) to 2 (not rhythmic, but easily visible, movement). For each analysis, our findings were replicated with the new coding scheme.
This lack of voluntary movement could have been caused by a number of factors, including internal or external distractions, or movement (such as repositioning) by the mother.
We also calculated results excluding only cases in which there were no observations (e.g., if an infant always directed attention toward the monitor when visual information was present, those data were excluded, but that infants’ data for when the visual information was absent were still analyzed, assuming the infant was observed both attending to, and not attending to, the monitor). The results from these analyses also confirmed the role of attention; infants moved less when they directed attention to the monitor, regardless of whether visual information was present (no visual information, t(42) = 2.61, p (two-tailed) < .05; visual information, t(41) = 5.08, p (two-tailed) < .001).
References
- Bacher LF, Robertson SS. Stability of coupled fluctuations in movement and visual attention in infants. Developmental Psychobiology. 2001;39:99–106. doi: 10.1002/dev.1034. [DOI] [PubMed] [Google Scholar]
- Brown S, Merker B, Wallin NL, Merker B, Brown S. An introduction to evolutionary musicology. In: Wallin NL, editor. The origins of music. MIT Press; Cambridge, MA: 2000. pp. 3–24. [Google Scholar]
- Campbell DW, Eaton WO. Sex differences in the activity level of infants. Infant and Child Development. 1999;8:1–17. [Google Scholar]
- Colavita FB. Human sensory dominance. Perception and Psychophysics. 1974;16:409–412. [Google Scholar]
- Colavita FB, Weisberg D. A further investigation of visual dominance. Perception and Psychophysics. 1979;25:345–347. doi: 10.3758/bf03198814. [DOI] [PubMed] [Google Scholar]
- Denac O. A case study of preschool children’s musical interests at home and at school. Early Childhood Education Journal. 2008;35(5):439–444. [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends in Neurosciences. 2005;28(7):353–363. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Drake C, Jones MR, Baruch C. The development of rhythmic attending in auditory sequences: attunement, referent period, focal attending. Cognition. 2000;77:251–288. doi: 10.1016/s0010-0277(00)00106-2. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychological Review. 1993;100(3):363–406. [Google Scholar]
- Gerry DW, Faux AL, Trainor LJ. Effects of Kindermusik training on infants’ rhythmic enculturation. Developmental Science. 2010;13:545–551. doi: 10.1111/j.1467-7687.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- Goswami U. Cognitive development: The learning brain. Psychology Press; New York, NY: 2008. [Google Scholar]
- Hannon EE, Trehub SE. Tuning in to musical rhythms: Infants learn more readily than adults. Proceedings of the National Academy of Sciences. 2005;102:12639–12643. doi: 10.1073/pnas.0504254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D, Reiner M. Sensory dominance in combinations of audio, visual, and haptic stimuli. Experimental Brain Research. 2009;193:307–314. doi: 10.1007/s00221-008-1626-z. [DOI] [PubMed] [Google Scholar]
- Kirschner S, Tomasello M. Joint drumming: Social context facilitates synchronization in preschool children. Journal of Experimental Child Psychology. 2009;102(3):299–314. doi: 10.1016/j.jecp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Koppen C, Levitan CA, Spence C. A signal detection study of the Colavita visual dominance effect. Experimental Brain Research. 2009;196:353–360. doi: 10.1007/s00221-009-1853-y. [DOI] [PubMed] [Google Scholar]
- Kravitz H, Boehm JJ. Rhythmic habit patterns in infancy: Their sequence, age of onset, and frequency. Child Development. 1971;42:399–413. [PubMed] [Google Scholar]
- Levitin DJ. This is your brain on music: The science of a human obsession. Dutton; New York, NY: 2006. [Google Scholar]
- Lewkowicz DJ. Developmental changes in infants’ bisensory response to synchronous durations. Infant Behavior and Development. 1986;9:335–353. [Google Scholar]
- Lewkowicz DJ. Sensory dominance in infants: 1. Six-month-old infants’ response to auditory-visual compounds. Developmental Psychology. 1988;24(2):155–171. [Google Scholar]
- Lewkowicz DJ. Infants’ response to temporally based intersensory equivalence: The effect of synchronous sounds on visual preferences for moving stimuli. Infant Behavior and Development. 1992;15:297–324. [Google Scholar]
- Lewkowicz DJ. Limitations on infants’ response to rate-based auditory-visual relations. Developmental Psychology. 1994;30:880–892. [Google Scholar]
- Longhi E. ‘Songese’: Maternal structuring of musical interaction with infants. Psychology of Music. 2009;37(2):195–213. [Google Scholar]
- Lukas S, Philipp AM, Koch I. Switching attention between modalities: Further evidence for visual dominance. Psychological Research. 2010;74:255–267. doi: 10.1007/s00426-009-0246-y. [DOI] [PubMed] [Google Scholar]
- Masataka N. Preference for infant-directed singing in 2-day-old hearing infants of deaf parents. Developmental Psychology. 1999;35:1001–1005. doi: 10.1037//0012-1649.35.4.1001. [DOI] [PubMed] [Google Scholar]
- Katerina Mazokopaki, Giannis Kugiumutzakis. Infant rhythms: Expressions of musical companionship. In: Malloch S, Trevarthen C, editors. Communicative musicality: Exploring the basis of human companionship. Oxford University Press; Oxford: 2009. pp. 185–208. [Google Scholar]
- McAuley JD, Jones MR, Holub S, Johnston HM, Miller NS. The time of our lives: Life span development of timing and event tracking. Journal of Experimental Psychology: General. 2006;135:348–367. doi: 10.1037/0096-3445.135.3.348. [DOI] [PubMed] [Google Scholar]
- Nettl B. An ethnomusicologist contemplates universals in musical sound and musical culture. In: Wallin NL, Merker B, Brown S, editors. The origins of music. MIT Press; Cambridge, MA: 2000. pp. 462–472. [Google Scholar]
- Patel AD, Iversen JR, Chen Y, Repp BH. The influence of metricality and modality on synchronization with a beat. Experimental Brain Research. 2005;163:226–238. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- Phillips-Silver J, Trainor LJ. Feeling the beat: Movement influences infant rhythm perception. Science. 2005;308:1430. doi: 10.1126/science.1110922. [DOI] [PubMed] [Google Scholar]
- Posner MI, Nissen MJ, Klein RM. Visual dominance: An information-processing account of its origins and significance. Psychological Review. 1976;83(2):157–171. [PubMed] [Google Scholar]
- Provasi J, Bobin-Begue A. Spontaneous motor tempo and rhythmical synchronisation in 2½-and 4-year-old children. International Journal of Behavioral Development. 2003;27(3):220–231. [Google Scholar]
- Repp BH, Penel A. Auditory dominance in temporal processing: New evidence from synchronization with simultaneous visual and auditory sequences. Journal of Experimental Psychology: Human Perception and Performance. 2002;28(5):1085–1099. [PubMed] [Google Scholar]
- Repp BH, Penel A. Rhythmic movement is attracted more strongly to auditory than to visual rhythms. Psychological Research. 2004;68:252–270. doi: 10.1007/s00426-003-0143-8. [DOI] [PubMed] [Google Scholar]
- Shatz M. On mechanisms of language acquisition: Can features of the communicative environment account for development? In: Wanner E, Gleitman LR, editors. Language acquisition: the state of the art. Cambridge University Press; Cambridge: 1982. pp. 102–127. [Google Scholar]
- Shenfield T, Trehub SE, Nakata T. Maternal singing modulates infant arousal. Psychology of Music. 2003;31(4):365–375. [Google Scholar]
- Sinnett S, Spence C, Soto-Faraco S. Visual dominance and attention: The Colavita effect revisited. Perception & Psychophysics. 2007;69(5):673–686. doi: 10.3758/bf03193770. [DOI] [PubMed] [Google Scholar]
- Spelke ES. Perceiving bimodally specified events in infancy. Developmental Psychology. 1979;15(6):626–636. [Google Scholar]
- Spelke ES, Born WS, Chu F. Perception of moving, sounding objects by 4-month-old infants. Perception. 1983;12:719–732. doi: 10.1068/p120719. [DOI] [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behaviour. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Thorpe LA, Trehub SE. Duration illusion and auditory grouping in infancy. Developmental Psychology. 1989;25(1):122–127. [Google Scholar]
- Trainor LJ. Infant preferences for infant-directed versus noninfant-directed playsongs and lullabies. Infant Behavior and Development. 1996;19:83–92. [Google Scholar]
- Trehub SE. Infants’ perception of musical patterns. Perception & Psychophysics. 1987;41:635–641. doi: 10.3758/bf03210495. [DOI] [PubMed] [Google Scholar]
- Trehub SE. Human infants’ perception of auditory patterns. International Journal of Comparative Psychology. 1990;4:91–110. [Google Scholar]
- Trehub SE, Nakata T. Emotion and music in infancy [Special Issue] Musicae Scientiae. 2001-2002:37–61. [Google Scholar]
- Trehub SE, Trainor LJ. Singing to infants: Lullabies and play songs. Advances in Infancy Research. 1998;12:43–77. [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Spence C. Is visual dominance modulated by the threat value of visual and auditory stimuli? Experimental Brain Research. 2009;193:197–204. doi: 10.1007/s00221-008-1608-1. [DOI] [PubMed] [Google Scholar]
- Warren JE, Wise RJS, Warren JD. Sounds do-able: Auditory-motor transformations and the posterior temporal plane. Trends in Neurosciences. 2005;28(12):636–643. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Winkler I, Haden GP, Ladinig O, Sziller I, Honing H. Newborn infants detect the beat in music. Proceedings of the National Academy of Sciences. 2009;106(7):2468–2471. doi: 10.1073/pnas.0809035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: Auditory-motor interactions in music perception and production. Nature Reviews Neuroscience. 2007;8:547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- Zentner M, Eerola T. Rhythmic engagement with music in infancy. Proceedings of the National Academy of Sciences. 2010;107(13):5768–5773. doi: 10.1073/pnas.1000121107. [DOI] [PMC free article] [PubMed] [Google Scholar]