Abstract
Background
Currently there is no an appropriate model to study intestinal mucosal atrophy in vivo that preserves the nutritional status of the organism.
Materials and Methods
We created a defunctionalized segment of jejunum via a dead-end Roux-en-Y anastomosis in rats. We compared tissue morphometric parameters in the intestinal mucosa of the defunctionalized bowel to that of the mucosa proximal and distal to the anastomosis. We further measured ERK activation within the mucosa as well as sucrase-isomaltase and DPPIV levels as markers of intestinal mucosal differentiation by Western blotting of mucosal scrapings.
Results
Three days after anastomosis, the defunctionalized bowel exhibited decreased diameter and thickness of both the mucosa and the fibromuscular layer compared with adjacent bowel in continuity for luminal nutrient flow or with bowel from control animals. Sucrase-isomaltase and DPPIV levels were also decreased. Furthermore, mucosal ERK activation, assessed as the ratio of phosphorylated to total ERK, was also reduced. Animal weights did not differ between bypassed and control animals.
Conclusions
Deprivation of nutrient flow in a segment of bowel by defunctionalizing Roux-en-anastomosis produces mucosal atrophy as indicated by altered histology, differentiation marker expression, and ERK signaling, in animals that are otherwise able to maintain enteral nutrition.
Keywords: Atrophy, ERK, Differentiation, Sucrase-isomaltase, DPPIV
Introduction
The intestinal mucosa atrophies during fasting and in patients with ileus or sepsis who are not eating. Total parenteral nutrition (TPN) of laboratory animals has been used as a model to study mucosal atrophic processes (1, 2). Although TPN induces intestinal atrophy (3), it also has profound effects on intraepithelial cytokine signaling (4) and mucosal immune defense (5, 6) that may be independent of the mucosal atrophy itself. TPN also promotes ion transfer and permeability across the intestine in experimental animals (7, 8) that may change the pharmacokinetics or effects of drugs being tested for use in patients with mucosal intestinal pathology.
In addition to growth factors and intraluminal nutrients, repetitive mechanical deformation engendered by peristalsis or villous motility also modulates intestinal epithelial biology (9-11). In vitro, repetitive deformation stimulates the proliferation of intestinal epithelial cells on collagen substrates, modulates differentiation marker expression, and activates p38 and JNK signaling in intestinal Caco-2 cells in response to repetitive deformation (12). In vivo, repetitive deformation alone stimulates intracellular signaling within small intestinal or colonic mucosa in anesthetized rats (13). Suppressed contractility and diminished luminal nutrient flow reduce metabolism and initiate mucosal atrophy (1). In vitro, Extracellular signal-Regulated Kinases (ERK) signaling critically mediates the effects of repetitive deformation on human Caco-2 intestinal epithelial cells (14). However, the signaling mechanisms involved in mucosal atrophy have not been characterized in vivo. We hypothesized that creating a blind Roux en-Y anastomosis in rat jejunum will lead to mucosal atrophy and reduction of function in the defunctionalized intestinal segment which is deprived of luminal flow, while still permitting normal enteral nutrition and preserving normal biology in other segments of the intestine in the same animal.
Methods
Animals and surgical procedures information
All experiments were approved by the University Laboratory Animal Resources at Michigan State University.3 month old female Wistar rats were purchased from Charles River (Wilmington, MA) and were housed using a 12 hour light/12 hour dark cycle. Diets and water were provided ad libitum. Control animals were maintained on regular diet until one day before harvest. Sham and anastomosis operated rats were kept on liquid diets for two days before surgery and sacrificed three days postoperatively. After surgery, each animal was maintained on liquid diet for three days. An additional group of animals was kept for 30 days after defunctionalizing anastomosis and samples were then analyzed morphologically. Sham-operated animals served as controls.
Roux-en-Y anastomosis
To create a defunctionalized segment of jejunum in continuity with the remainder of the bowel, we performed a defunctionalizing Roux-en-Y anastomosis in rats through a midline laparotomy. The jejunum was divided 1 cm from the ligament of Treitz using 5-0 silk sutures (Ethicon, Inc., Somerville, NJ). The proximal jejunum (proximal limb) was anastomosed to the distal jejunum (distal limb) three cm more distally than the original transection with a side to side anastomosis using running 7-0 vicryl sutures (Figure 1A-B). In control operated animals the abdominal wall was opened and the intestine was manipulated and measured. In sham-operated animals, the small intestine was transected twice in the jejunum at sites 4 centimeters apart, at sites equivalent to those in which the experimental defunctionalizing Roux-en-Y anastomosis transections were performed. However the bowel was then reconstructed in continuity with two end to end anastomoses constructed with 7-0 vicryl suture.The abdominal cavity was closed using a running 5-0 vicryl suture. The skin was closed by using surgical staples.
Figure 1. Defunctionaled intestine changes morphology within three days after surgery.
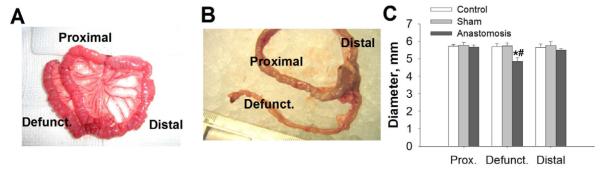
A. General view of the small intestine of rat after creation of defunctionalized limb in small intestine of rat. Jejunum was divided and was transposed to the distal part of the jejunum approximately three cm from the transaction point, where it was anastomosed in a Roux-en-Y end to site fashion, thereby creating a defunctionalized section of jejunum, B. General view of intestine at the time of harvest. Dissected intestinal segments three days after anastomosis surgery. C. Diameters of intestinal segments of defunctionalized (defunct) Roux-en-Y anastomosis vs sham operated animals (control) (n=8, p<0.05). * - significantly different from proximal and distal limb of same group, # - significantly different from area representing defunctionalized region of intestine in Control or Sham operated animals.
Morphometric analysis
Samples of the intestinal segments from the proximal, distal and Roux limbs were fixed in 10 % formalin for 24 hours and embedded in paraffin. Step sections (4.0 m thick) were prepared from all of the blocks and stained with hematoxylin and eosin (H&E). Sections were visualized and photographed on a Nikon Microphot-FXA (Nikon Microphot-FXA). Villous height and thickness of the fibromuscular layer was measured on transverse sections stained with hematoxylin and eosin using ImageJ software (NIH, Bethesda, MD) each compared with bowel proximal and distal to the defunctionalized segment from the same animal and with specimens from sham-operated animals at the same sites.
Protein isolation and Western blotting analysis
Mucosal scrapings from target intestinal segments after sacrifice and harvest were immediately immersed in ice cold lysis buffer. Tissue was homogenized using BulletBlender (Next Advance, Averill Park, NY) and then centrifuged at 15,000g for 10 min at 4°C and resolved by SDS-PAGE as previously d escribed (14). Membranes were blotted for sucrase-isomaltase, Dipeptidyl peptidase-4 (DPPIV), Phospho-ERK Thr 202, Tyr204 or total ERK with appropriate antibody (Cell Signaling Technology, Danvers, MA) as previously described (15). Membranes were then re-probed with antibodies specific for β-Actin and appropriate fluorophore conjugated secondary antibody as a loading control. Bands were visualized using Odyssey imaging system (Licor, Lincoln, NE) and analyzed with the Kodak Image Station 440CF. All exposures of probed membranes used for densitometric analysis were within the linear range.
Data analysis
Values are reported as group means ± standard error of the mean (SEM) of the non-transformed data. The differences between control and experimental groups were compared using paired or unpaired t-tests as appropriate. Prior to analysis, all data were checked to ensure they fit a normal distribution. Orthogonal contrast comparisons for the effect of surgical procedures on sucrase-isomaltase, DPPIV and ERK signaling were conducted (Control vs. Sham and Anastomosis; Sham vs, Anastomosis) as previously described (16). Differences between means were considered significant at p < 0.05.
Results
Body weight and intestine at harvest
Body weight did not differ in experimental animals at three days after surgery compared to sham-operated controls (208.3±2.7 g vs. 213.5± 8.7 g). Three days after the surgery, the proximal and distal limbs contained chyme, the anastomosis was patent (Figure 1A-B), and the defunctionalized limb was empty.
Loss of function changes histomorphometry of small intestine
Three days after surgery, the diameter of the defunctionalized intestine was significantly reduced compared to either the control, sham-operated controls or to the bowel previously immediately proximal or distal to the segment that had been defunctionalized and now immediately proximal or distal to the anastomosis (p<0.05, Figure 1B-C). Hematoxlin-eosin-staining confirmed parallel alterations in histology. The thickness of the mucosal layer was significantly decreased (p<0.05) primarily due to villous shortening, whereas the thickness of stromal layer was not changed (Figure 2A-B). Total thickness of intestinal wall of defuncionalized limb was significantly reduced compared to control (p<0.05, Figure 1C). The ratio of the thickness of the intestinal mucosa to the thickness of the fibromuscular layer did not differ among defunctionalized, proximal, and distal jejunum. In rats maintained for 30 days after defunctionalizing anastomosis, the defunctionalized bowel diameter was decreased 34.2±8.39% and the mucosal villous height reduced by 34.7±4.7% compared to the adjacent proximal bowel (p<0.05 for each, data not shown). In contrast, thickness of the fibromuscular layer in the defunctionalized gut increased 15.65±1.2% at 30 days compared to the proximal bowel. (p<0.05 for each, data not shown).
Figure 2. Histological parameters of small intestine of Roux-en-Y anastomosis three days after surgery.
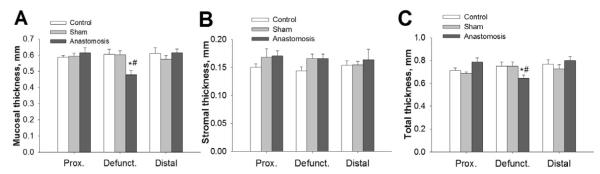
A. Mucosal thickness of defunctionalized (defunct) Roux-en-Y intestinal wall anastomosis vs sham-operated animals (control) (n=6-8, p<0.05). B. Stromal thickness of Roux-en-Y intestinal wall anastomosis vs sham operated animals (control). (n=6-8, p<0.05). C. Total thickness of Roux-en-Y intestinal wall anastomosis vs sham operated animals (control). (n=6-8, p<0.05). * - significantly different from proximal and distal limb of same group, # - significantly different from area representing defunctionalized region of intestine in Control or Sham operated animals.
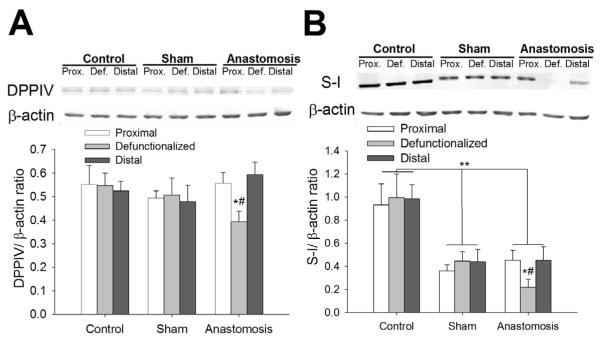
Defunctionalization is associated with reduced brush border enzyme levels
The level of DPPIV protein was 65.2%±7.9% a3nd 59.2%±9.4%, lower in the defunctionalized intestinal mucosa than in the mucosa of the proximal or distal bowel respectively (n=6, p<0.05, Figure 3A). Similarly sucrose-isomaltase protein was 61.7%±8.8% and 63.8%±8.3% lower in the defunctionalized intestinal mucosa than in the mucosa of the proximal or distal bowel respectively (n=6, p<0.05, Figure 3B). Sucrase-isomaltase and DPPIV were also lower in the defunctionalized mucosa than in the same segment of bowel from control or sham-operated animals (n=3-6, p<0.05, Figure 3A-B). Levels of DPPIV and sucrase-isomaltase were not different among segments of intestine in sham operated animals. The intake of the liquid diet for five days as well as surgical procedures reduced average level of sucrase-isomaltase in sham and anastomossis operated group (p<0.05, figure 3C).
Figure 3. Level of brush border enzymes is reduced in defunctionaled intestine compared with proximal or distal limbs or sham operated animals.
Levels of DPPIV (A) or surcrase-isomaltase (SI) (B) were lower in the defunctionalized (defunct) intestinal mucosa three days after anastomosis compared to sham-operated controls or proximal (prox) or distal bowel in the same animals (p<0.05, n=3-7). * - significantly different from proximal and distal limb of same group, # - significantly different from area representing defunctionalized region of intestine in Control or Sham operated animals, **,- significantly different comparison of Control vs Sham and Anastomosis groups (p<0.05).
Defunctionalization is associated with reduced intracellular signaling
Levels of phosphorylated and total ERK was detected by western blot and compared to β-actin levels. ERK activation (pERK/ERK relative to β-actin) was reduced by 61.±10.1% and 46.7±13.9% at 3 days vs. proximal or distal gut (p<0.05, Figure 4).
Figure 4. ERK signaling changes in defunctionaled intestine comparing with proximal or distal limbs or sham-operated animals.
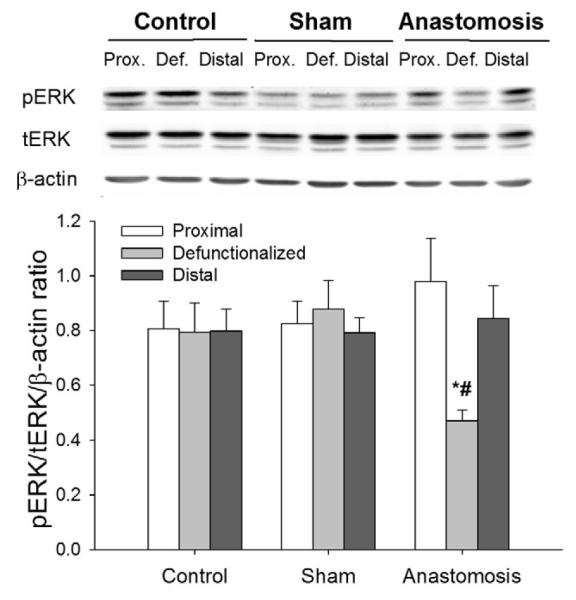
The proportion of phosphorylated ERK was significantly reduced in defunctionalized (Def.) limb of intestine on the third day after anastomosis compared to sham-operated controls or proximal (Prox.) or distal bowel in the same animals (p<0.05, n=4-7). * - significantly different from proximal and distal limb of same group, # - significantly different from area representing defunctionalized region of intestine in Control or Sham operated animals.
Discussion
Intestinal mucosal atrophy is a common problem in patients undergoing prolonged periods of fasting during ileus, sepsis, or starvation. The flow of luminal contents modulates intestinal mucosal structure and enterocyte function (1, 17). In the absence of luminal nutrients, mucosal epithelial cells lose their function, reduce their absorptive capacity, and slow their proliferation (7, 18, 19). Changes in cell and tissue architecture and loss of intestinal epithelial integrity lead to an impaired histostructure, increased permeability, and failure of the intestinal mucosal barrier.
In this work, we present an experimental model that allows the study of intestinal mucosal atrophy without the neurohumoral changes or technical difficulties that accompany total parenteral nutrition. We generated a defunctionalized segment of the jejunum lacking nutrient flow but still preserved the ability of the animal to be nourished enterally. Loss of function due to reduced interaction of luminal contents with mucosa initiates morphological and biochemical atrophy of the intestinal mucosa that correlates with decreased ERK signaling. In a previous preliminary study, we attempted to study the impact of defunctionalization on mucosal healing after ulcer formation in mice undergoing Roux-en-Y anastomoses (20) but these observations were confounded by the smaller diameter of the mouse intestine that led to partial obstruction and dilation of the defunctionalized intestinal segment not observed here in the slightly larger rat model.
The ability of deprivation of contact with luminal nutrients to induce atrophy is consistent with previous observations that the intestinal mucosa changes morphologically in stimuli. Intestinal villi increase height during intensive nutrient absorption during lactation (21) or after small intestine resection (22). Conversely, the mucosa atrophies in patients subjected to starvation, exhibiting reduced villous height and crypt length and impaired intestinal permeability (23). Prolonged loss of function in the intestine over long period of time was associated with even more pronounced morphologic changes 30 days after surgery. Similarly, in rats villous height in jejunum is reduced after 8 days on TPN (1). The defunctionalization model may be of interest to laboratories that do not have access or resources for rodent TPN or that wish to avoid the possible systemic confounding effects of TPN such as increased permeability (8) (less likely to be a problem in our model because only a small segment of the gut exhibits mucosal atrophy) and systemic defects of cell defense (24). In addition, the defunctionalizing anastomosis model permits direct paired comparisons of mucosal biology in atrophic and non-atrophied mucosa in the same animal in adjacent segments of bowel, which may be experimentally useful for some purposes.
Why small intestinal mucosal atrophy occurs in fasting or starving patients is an interesting and poorly understood question. Although the small bowel mucosa generally derives most nutrition from luminal rather than circulatory nutrients in normal function, total parenteral nutrition should be able to provide the most relevant nutrients to the mucosa by perfusion in a manner similar to that by which other organs are perfused. The hypothesis that mucosal atrophy reflected a relative glutamine deficiency not treated by conventional total parenteral nutrition solutions has not been substantiated in human studies (17). Our data clearly demonstrates that the mucosal atrophy seen with TPN is not a direct consequence of TPN itself or of a neurohumoral response to an overall absence of nutrients within the entire bowel. There may be something special about the local presence of luminal nutrients, either based upon apical vs. basal cell sensors or the existence of concentration gradients across the mucosa that is sensed by the gut epithelium.
We have previously demonstrated that physical forces like repetitive deformation (9) or pressure (25, 26) can stimulate intestinal epithelial proliferation and regulate intestinal epithelial differentiation in vitro. Interestingly, both the mitogenic and differentiating properties of repetitive deformation appear independent of the effects of glutamine (27). Luminal nutrients stimulate both peristalsis (28) and villous motility (29). Moreover, as the luminal contents are relatively non-compressible, passage of luminal contents through the bowel deforms the villi and the mucosa by a combination of pressure and shear stress. (30). Increases in extracellular pressure can also stimulate intestinal epithelial proliferation (20). It therefore becomes attractive to hypothesize that at least one of the factors contributing to mucosal atrophy in the present model is alterations in the physical forces associated with luminal nutrition that repetitively deform the mucosa. Such forces similarly stimulate cell proliferation in the cardiovascular system (31), the lung (32, 33) and other tissues (34). Additionally in this model of intestinal atrophy in defunctionalized limb atrophic processes are limited to a small section of intestine, thus one would not expect to have systemic effects from increased bacterial translocation due to increased intestinal wall permeability as is observed in the TPN model (8). Our model differs from and may be more extreme than pure human starvation in that starvation may nevertheless have gastric, biliary and pancreatic secretions flowing through the lumen of the gut. However, in critically ill fasting patients in the hospital with nasogastric tubes gastric secretions and some biliary and pancreatic secretions are diverted proximally out the tube and less pass through the bowel lumen, so this scenario may more closely resemble our current model.
The expression level and activity of brush border enzymes and intestinal epithelium-specific functional proteins are conventionally used to assess the differentiation of intestinal epithelial cells (35) . The brush-border enzyme DPPIV is amplitude-dependently activated by repetitive strain in human Caco-2 intestinal epithelial cells (11) or murine enterocytes (36). DPPIV was reduced during azathioprine-induced gastroenteritis and small bowell villus atrophy(37). Also DPPIV activity decrease in intestine of starved for three days rats (38) but not in rats after TPN (39). Sucrase-isomaltase is also expressed in differentiated enterocytes, and its level is reduced in the atrophic intestinal mucosa of methotrexate-treated rats (40). Here, we observed that defunctionalization of a jejunal segment reduced levels of these differentiation markers within three days after surgery. Our results suggest that jejunal sucrase-isomaltase may be more sensitive to the change of diet and jejunal transection than DPPIV levels or ERK signaling, independently of defunctionalization. This reduction in brush border enzymes appears to correlate with histological evidence of atrophy in the defunctionalized rat intestinal mucosa.
ERK activation is a potent mitogenic stimulus that can also induce intestinal epithelial differentiation, activated by a variety of growth factors (41) and cytokines (42). Repetitive deformation also stimulates proliferation and regulates enterocytic differentiation via a complex signal pathway (10) that involves tyrosine kinase and PKC activation and downstream ERK-mediated signaling (12). These observations are consistent with our present observation that ERK signaling is decreased in the atrophic defunctionalized mucosa in vivo. The extent to which this decrease in ERK activity actually influences the biochemical and morphological mucosal atrophy we observed awaits further study beyond the scope of this report.
In summary, the absence of interaction with luminal chyme changes intestinal morphology in a defunctionalized loop of rat intestine. Morphologic evidence of mucosal atrophy correlated with a reduction in mucosal differentiation and ERK signaling. Formation of a defunctionalizing Roux-en-Y anastomosis may be an appropriate model to investigate mucosal atrophy and the effects of luminal chyme interactions with the bowel in intact otherwise enterally-fed rodents.
Acknowledgments
Supported in part by a VA Merit Review Award (MDB) and NIH RO1 DK067257
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Ekelund M, Kristensson E, Ekblad E. Total parenteral nutrition causes circumferential intestinal atrophy, remodeling of the intestinal wall, and redistribution of eosinophils in the rat gastrointestinal tract. Dig Dis Sci. 2007;52:1833–1839. doi: 10.1007/s10620-006-9678-z. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G833–841. doi: 10.1152/ajpgi.00030.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meurling S, Roos KA. Gut structure changes in rats on continuous and intermittent complete parenteral nutrition. Acta Chir Scand. 1981;147:451–457. [PubMed] [Google Scholar]
- 4.Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, et al. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002;236:226–234. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51-42. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Gumucio DL, Teitelbaum DH. Intestinal specific overexpression of interleukin-7 attenuates the alternation of intestinal intraepithelial lymphocytes after total parenteral nutrition administration. Ann Surg. 2008;248:849–856. doi: 10.1097/SLA.0b013e31818a1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Feng Y, Sun X. Teitelbaum DH Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:338–346. doi: 10.1111/j.1749-6632.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003;31:1118–1125. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 9.Gayer CP, Craig DH, Flanigan TL, Reed TD, Cress DE, et al. ERK regulates strain-induced migration and proliferation from different subcellular locations. J Cell Biochem. 2010;109:711–725. doi: 10.1002/jcb.22450. [DOI] [PubMed] [Google Scholar]
- 10.Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009;21:1237–1244. doi: 10.1016/j.cellsig.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;168:476–488. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G75–87. doi: 10.1152/ajpgi.2001.280.1.G75. [DOI] [PubMed] [Google Scholar]
- 13.Basson MD, Coppola CP. Repetitive deformation and pressure activate small bowel and colonic mucosal tyrosine kinase activity in vivo. Metabolism. 2002;51:1525–1527. doi: 10.1053/meta.2002.36303. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology. 2006;131:1179–1189. doi: 10.1053/j.gastro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Craig DH, Zhang J, Basson MD. Cytoskeletal signaling by way of alpha-actinin-1 mediates ERK1/2 activation by repetitive deformation in human Caco2 intestinal epithelial cells. American journal of surgery. 2007;194:618–622. doi: 10.1016/j.amjsurg.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovalenko PL, Zhang Z, Yu JG, Li Y, Clinton SK, et al. Dietary vitamin d and vitamin d receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev Res (Phila) 2011;4:1617–1625. doi: 10.1158/1940-6207.CAPR-11-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nose K, Yang H, Sun X, Nose S, Koga H, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res. 2010;30:67–80. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodlad RA, Plumb JA, Wright NA. Epithelial cell proliferation and intestinal absorptive function during starvation and refeeding in the rat. Clin Sci (Lond) 1988;74:301–306. doi: 10.1042/cs0740301. [DOI] [PubMed] [Google Scholar]
- 19.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 20.Flanigan TL, Owen CR, Gayer C, Basson MD. Supraphysiologic extracellular pressure inhibits intestinal epithelial wound healing independently of luminal nutrient flow. Am J Surg. 2008;196:683–689. doi: 10.1016/j.amjsurg.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenberger LM, Trier JS. Changes in gastrin levels, food intake, and duodenal mucosal growth during lactation. Am J Physiol. 1979;237:E98–105. doi: 10.1152/ajpendo.1979.237.1.E98. [DOI] [PubMed] [Google Scholar]
- 22.Dowling RH. Small bowel adaptation and its regulation. Scand J Gastroenterol Suppl. 1982;74:53–74. [PubMed] [Google Scholar]
- 23.Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, et al. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care. 1999;14:73–77. doi: 10.1016/s0883-9441(99)90017-5. [DOI] [PubMed] [Google Scholar]
- 24.Hermsen JL, Gomez FE, Sano Y, Kang W, Maeshima Y, et al. Parenteral feeding depletes pulmonary lymphocyte populations. JPEN J Parenter Enteral Nutr. 2009;33:535–540. doi: 10.1177/0148607109332909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh MF, Woo RK, Gomez R, Basson MD. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif. 2004;37:427–441. doi: 10.1111/j.1365-2184.2004.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanigan TL, Craig DH, Gayer CP, Basson MD. The effects of increased extracellular deformation, pressure, and integrin phosphorylation on fibroblast migration. J Surg Res. 2009;156:103–109. doi: 10.1016/j.jss.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murnin M, Kumar A, Li GD, Brown M, Sumpio BE, et al. Effects of glutamine isomers on human (Caco-2) intestinal epithelial proliferation, strain-responsiveness, and differentiation. J Gastrointest Surg. 2000;4:435–442. doi: 10.1016/s1091-255x(00)80025-6. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen HN, Winograd R, Domingues GR, Lammert F. Postprandial transduodenal bolus transport is regulated by complex peristaltic sequence. World J Gastroenterol. 2006;12:6008–6016. doi: 10.3748/wjg.v12.i37.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Womack WA, Mailman D, Kvietys PR, Granger DN. Neurohumoral control of villous motility. Am J Physiol. 1988;255:G162–167. doi: 10.1152/ajpgi.1988.255.2.G162. [DOI] [PubMed] [Google Scholar]
- 30.Miftakhov RN, Wingate DL. Biomechanics of small bowel motility. Med Eng Phys. 1994;16:406–415. doi: 10.1016/1350-4533(90)90007-u. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem. 1999;274:25273–25280. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem. 2010;285:29336–29347. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi LS, Marsh HM, Basson MD. Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am J Physiol Cell Physiol. 2007;292:C1701–1713. doi: 10.1152/ajpcell.00529.2006. [DOI] [PubMed] [Google Scholar]
- 34.Webb K, Hitchcock RW, Smeal RM, Li W, Gray SD, et al. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J Biomech. 2006;39:1136–1144. doi: 10.1016/j.jbiomech.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, et al. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc Res Tech. 2000;49:394–406. doi: 10.1002/(SICI)1097-0029(20000515)49:4<394::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Yuan L, Yu Y, Sanders MA, Majumdar AP, Basson MD. Schlafen 3 induction by cyclic strain regulates intestinal epithelial differentiation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G994–G1003. doi: 10.1152/ajpgi.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler TR, Fernandez-Estivariz C, Gu LH, Fried MW, Leader LM. Severe villus atrophy and chronic malabsorption induced by azathioprine. Gastroenterology. 2003;124:1950–1957. doi: 10.1016/s0016-5085(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 38.Poullain MG, Cezard JP, Marche C, Macry J, Roger L, et al. Effects of dietary whey proteins, their peptides or amino-acids on the ileal mucosa of normally fed and starved rats. Clin Nutr. 1991;10:49–54. doi: 10.1016/0261-5614(91)90081-m. [DOI] [PubMed] [Google Scholar]
- 39.Ihara T, Tsujikawa T, Fujiyama Y, Ueyama H, Ohkubo I, et al. Enhancement of brush border membrane peptidase activity in rat jejunum induced by starvation. Pflugers Arch. 2000;440:75–83. doi: 10.1007/s004240000275. [DOI] [PubMed] [Google Scholar]
- 40.Verburg M, Renes IB, Meijer HP, Taminiau JA, Buller HA, et al. Selective sparing of goblet cells and paneth cells in the intestine of methotrexate-treated rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1037–1047. doi: 10.1152/ajpgi.2000.279.5.G1037. [DOI] [PubMed] [Google Scholar]
- 41.Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furler RL, Uittenbogaart CH. Signaling through the P38 and ERK pathways: a common link between HIV replication and the immune response. Immunol Res. 2010;48:99–109. doi: 10.1007/s12026-010-8170-1. [DOI] [PubMed] [Google Scholar]