Abstract
Basic fibroblast growth factor (bFGF), which plays an important role in tumour angiogenesis and progression, provides a potential target for cancer therapy. Here we screened a phage display heptapeptide library with bFGF and identified 11 specific bFGF-binding phage clones. Two of these clones had identical sequence and the corresponding peptide (referred to as P7) showed high homology to the immunoglobulin-like (Ig-like) domain III (D3) of high-affinity bFGF receptors, FGFR1 (IIIc) and FGFR2 (IIIc). The P7 peptide and its corresponding motif in D3 of FGFRs both carried negative charges and shared similar hydrophobic profiles. Functional analysis demonstrated that synthetic P7 peptides mediate strong inhibition of bFGF-induced cell proliferation and neovascularization. Our results demonstrate that the P7 peptide is a potent bFGF antagonist with strong antiangiogenetic activity, and might have therapeutic potential in cancer therapy.
Keywords: angiogenesis, basic fibroblast growth factor, phage display, tumor
Introduction
The fibroblast growth factor (FGF) family includes at least 23 members and plays an essential role in regulating the proliferation, differentiation, migration and survival of cells originated from mesoderm and neuroectoderm. FGFs exert their biological activities via interactions with a family of FGF receptors (FGFR1–4) on the cell surface [1]. The extracellular ligand binding portion of FGFRs is composed of three immunoglobulin-like (Ig-like) domains (D1, D2 and D3) [2–5]. Although a highly conserved core region and considerable homology exist between FGFs, they mediate distinct functions.
Basic FGF (bFGF) is an important member of the FGF family. Because bFGF and its receptors are abundantly expressed in malignantly transformed cells and bFGF stimulates tumour angiogenesis and progression [6–8], antagonists targeting bFGF or its receptors have been considered a potential strategy for inhibiting endothelial cell proliferation and tumour progression [9]. In order to identify peptides that can block bFGF binding to its receptors, we screened a phage display heptapeptide library with bFGF. This led to the isolation of a high-affinity bFGF-binding peptide (referred to as P7) with potent inhibitory activity against bFGF-induced cell proliferation and angiogenesis.
Materials and methods
Reagents and cell lines
Ph.D.-7™ Phage Display Peptide Library Kit and E.coli ER2738 were purchased from New England Biolabs Inc. (Beverly, MA, USA). Recombinant human bFGF was purchased from Pepro Tech Inc. (Rocky Hill, NJ, USA). Recombinant human epidermal growth factor (EGF) was prepared in our laboratory. HRP-anti-M13 mAb was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). BALB/c-derived 3T3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen Corporation, Carlsbad, CA, USA) with 10% foetal calf serum.
Biopanning of a heptapeptide phage display library with bFGF
Petri dishes (35 × 10 mm) were coated with 10 μg bFGF (in 1 ml PBS) overnight at 4°C and then blocked with bovine serum albumin (BSA) at 5 mg/ml in 0.1 M NaHCO3 for 2 hrs at room temperature. After the plates were washed 6 times (1 min. each) with 0.05% Tween-20 in PBS (0.05% PBST), the heptapeptide phage library containing 2 × 1011 clones was sequentially added and shaken gently at room temperature for 2 hrs. After washing 10 times (1 min. each) with 0.05% PBST, plate-bound phage clones were eluted with Gly-HCl buffer (pH 2.2) and neutralized with 1M Tris-HCl (pH 9.1). The eluate was amplified and purified for the next round of screening. Two additional rounds of selection were performed under more stringent conditions, in which plates were coated with a reduced amount of bFGF and shorter incubation time (5 μg for 1.5 hrs and 2.5 μg for 1 hr in the 2nd and 3rd round, respectively), and washed with a higher concentration of PBST (0.1% and 0.3% for 2nd and 3rd round, respectively) for a longer period (10 × 2 min. and 10 × 3 min. for 2nd and 3rd round, respectively). After the 3rd round of selection, the phage clones were subjected to ELISA analysis.
ELISA assay for selecting positive phages
Microtitre plates were coated at 4°C overnight with bFGF and EGF (as controls), respectively. After the plates were blocked with blocking buffer (i.e. PBS with 2% dry milk; PBSM) and washed with 0.05% PBST for three times, phage clones (1010 pfu/well) and control phage vcsM13 were added and incubated at room temperature for 1 hr. After washing 3 times with 0.05% PBST, 200 μl of horseradish peroxidase (HRP)-anti-M13 (1:5000) was added and the plates were incubated for another hour at room temperature. The plates were washed again with 0.05% PBST and substrate (50 μl/well of 3,3′,5,5′-tetramethylbenzidine; TMB) was added. The reaction was terminated 20 min. later by adding 50 μl/well of 2M H2SO4, and the absorbance was measured at 450 and 655 nm.
DNA sequencing and peptide synthesis
DNA sequences of the positive phage clones were determined performed with an automated DNA sequencer at Shanghai Sangon Company (Shanghai, China) and analysed using the BioEdit Sequence Alignment Editor software and the ProtParam programs (Ibis Biosciences, Carlsbad, CA, USA). The Ph.D.-7 Phage Display Peptide Library consists of phages bearing random heptapeptides fused to the N-terminus of the M13 phage coat protein pIII. The peptide is followed by a short spacer (Gly-Gly-Gly-Ser) and then by the wild-type pIII sequence. During panning, the N-terminus of the selected peptide sequence is free and the C-terminus is fused to the phage, having no free negatively charged carboxylate. Given that a synthetic peptide with a free C-terminus will introduce a negatively charged group at a position occupied by a neutral peptide bond, which may influence binding, a spacer sequence Gly-Gly-Gly-Ser was added to the C-terminus and the C-terminal carboxylate was amidated to block the negative charge when synthesizing the peptide corresponding to the selected sequence. The designed peptides were synthesized at SBS Genetech (Beijing, China).
Cell viability assay
BALB/c 3T3 cells were seeded in 96-well plates (5 × 103 cells/well) in DMEM containing 10% FCS and incubated for 24 hrs. The cells were washed with DMEM and cultured in DMEM with 0.4% FCS to starve the cells for 24 hrs. The medium was then replaced by DMEM containing 10 ng/ml bFGF alone, or 10 ng/ml bFGF plus serially diluted peptides. After being cultured for 48 hrs, the number of viable cells was determined by the methylthiazoletetrazolium (MTT) method as previously described [10].
Angiogenesis assay using chick embryo chorioallantoic membrane (CAM)
CAM angiogenesis assay was performed as previously described [10]. Briefly, fifteen 5-day old chicken embryos were selected and randomly divided into three groups, which were treated with 20 μl of 10 ng/ml bFGF, 20 μl of 10 ng/ml bFGF plus 1 μM synthesized peptides, or 20 μl PBS, respectively. After incubation at 37°C for 72 hrs, the blood vessel intensity was recorded.
Results
Isolation of specific bFGF-binding phage clones
Three cycles of biopanning were performed to select the specific bFGF-binding phages from the heptapeptide library. As shown in Table 1, phage recovery rate was greatly increased after each round of selection (from 6.50 × 10−4% to 4.06 × 10−3%). After three rounds of selection, the recovered phage clones were screened by ELISA to identify high-affinity bFGF-biding clones. Phage vcsM13 was used as the control in the ELISA assay. With the exception of its wild-type coat protein pIII, phage vcsM13 coat proteins are identical to those used to construct the heptapeptide library. Phage clones were considered to possess high affinity for bFGF if their O.D. values were two times greater than that of phage vcsM13. We also measured the ability of phage clones to bind EGF in order to determine binding specificities. As shown in Fig. 1, 11 clones with high affinity and specificity for bFGF were obtained from the recovered phage clones after three cycles of panning.
Table 1.
Enrichment for bFGF-binding phages
| Round | bFGF (μg) | Input phage (pfu) | Output phage (pfu) | Recovery (%) |
|---|---|---|---|---|
| 1 | 10 | 2.0 × 1011 | 1.3 × 106 | 6.50 × 10−4 |
| 2 | 5 | 3.0 × 1011 | 8.0 × 106 | 2.67 × 10−3 |
| 3 | 2.5 | 3.2 × 1011 | 1.3 × 107 | 4.06 × 10−3 |
pfu, plaque forming unit.
Fig 1.
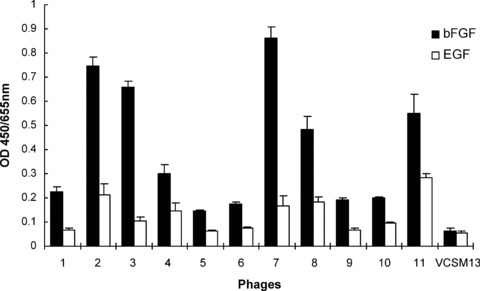
Specific binding of the positive phage clones to bFGF. The binding affinity of the 11 positive phage clones and the control vcsM13 to bFGF and EGF was determined by ELISA assay. Data presented are the mean O.D. values (±S.D.) of triplicate samples.
Sequence analysis and property prediction of bFGF-binding phage clones
The amino acid sequences of the peptides displayed on the positive phages were deduced from the DNA sequences and analysed using the BioEdit and ProtParam programs (Table 2). Phage clone No. 7 (P7) and P11 shared identical sequences P5 and P7 showed the highest sequence similarity to bFGF high-affinity receptors FGFR1 (IIIc) and FGFR2 (IIIc). P5 contains 3 amino acids of the authentic FGFR1 (IIIc) D3 sequence aa 312–318 (KTAGVNT) and 4 amino acids of the authentic FGFR2 (IIIc) D3 sequence aa 313–319 (KAAGVNT). P7 contains 5 amino acids of both the authentic FGFR1 (IIIc) D3 sequence aa 255–261 (PILQAGL) and the authentic FGFR2 (IIIc) D3 sequence aa 256–262 (PILQAGL).
Table 2.
Properties of peptides displayed by specific bFGF-binding phages
| Heptapeptide | lone | Sequence | Similarity | Theoretical pI | GRAVY |
|---|---|---|---|---|---|
| P1 | 1 | RKPGKPV | 0.0060901 | 11.17 | −1.671 |
| P2 | 2 | TLHSAQA | 0.0048721 | 6.40 | −0.114 |
| P3 | 3 | HNRPRNN | 0.0060901 | 12 | −3.471 |
| P4 | 4 | RHTHRSH | 0.0048721 | 12 | −2.817 |
| P5 | 5 | TAPGVST | 0.0073082 | 5.19 | 0.257 |
| P6 | 6 | NLTLAWR | 0.0036541 | 9.75 | −0.029 |
| P7, P11 | 7, 11 | PLLQATL | 0.0073082 | 5.52 | 1.057 |
| P8 | 8 | HTTHMYL | 0.0060827 | 6.92 | −0.486 |
| P9 | 9 | WSAPVPN | 0.0048721 | 5.52 | −0.343 |
| P10 | 10 | SFRPTPP | 0.0036541 | 9.47 | −1.143 |
pI, isoelectric point; GRAVY, grand average of hydropathicity.
In the physiological condition, P7 carries negative charges. Since the motif PILQAGL (pI 5.96) in D3 of FGFR1 (IIIc) and FGFR2 (IIIc) also carries negative charges, it is likely that P7 may bind to bFGF via electrostatic interaction. However, because P5 carries negative charges and its homologous epitopes in D3 of FGFR1 (IIIc) (aa 312–318; pI 8.75) and FGFR2 (IIIc) (aa 313–319; pI 8.75) have positive charges, P5 cannot interact with bFGF by mimicking the electrostatic interaction of the bFGF-binding motifs of FGFRs. Moreover, P7 is hydrophobic with a grand average of hydropathicity (GRAVY) of 1.057, which is similar to that of its corresponding motifs in D3 of FGFR1 (IIIc) and FGFR2 (IIIc) (GRAVY, 1.200). The mean hydrophobicity profile of P7 calculated using the Kyte & Doolittle scale is also similar to those of its corresponding motifs in D3 of FGFR1 (IIIc) and FGFR2 (IIIc) (Fig. 2). P5 is also hydrophobic (GRAVY, 0.257), but its corresponding motifs in D3 of FGFR1 (IIIc) (GRAVY, −0.457) and FGFR2 (IIIc) (GRAVY, −0.100) are hydrophilic, suggesting that P5 binding to bFGF in ELISA assay was not mediated by hydrophathic interactions. Taken together, these data suggest that the candidate peptide P7 may bind bFGF via both electrostatic and hydrophathic interactions and therefore may have a greater potential to interrupt bFGF binding to its receptors than other identified heptapeptides do.
Fig 2.
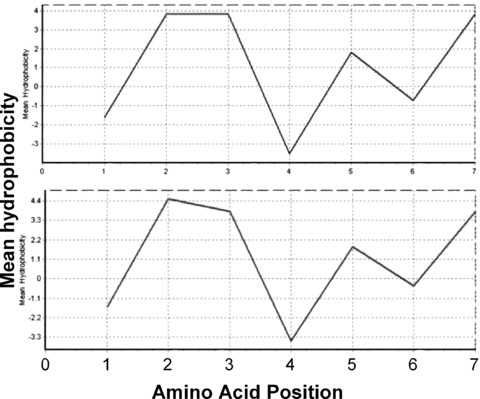
Kyte and Doolittle scale mean hydrophobicity profile of the P7 peptide. The hydrophobicity profile was calculated using the Kyte & Doolittle scale. Shown are mean hydrophobicity profile of P7 (top) and the corresponding motif in D3 of FGFR1 (IIIc) and FGFR2 (IIIc) (bottom).
Heptapeptide-library-derived peptide P7 inhibits the mitogenic activity of bFGF
The ability of P7 to block the biological activity of bFGF was first assessed by measuring its inhibitory effect on bFGF-stimulated proliferation of mouse fibroblast cells. As shown in Fig. 3, synthetic P7 peptides mediated strong inhibition of BALB/c 3T3 cell proliferation in a dose-dependent manner. The inhibitory effect was clearly detected in the cultures with as low as 0.0625 μM of P7 peptides, and a 50% inhibition (IC50) of cell proliferation was observed at about 1 μM.
Fig 3.
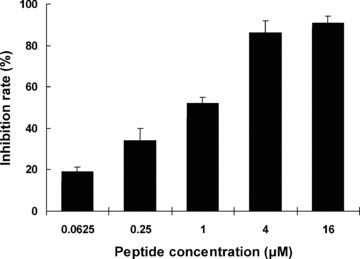
Inhibition of mitogenic activity of bFGF by the synthesized P7 peptides. BALB/c 3T3 cells were incubated with 10 ng/ml bFGF alone or 10 ng/ml bFGF plus P7 at the indicated concentrations, and cell proliferation was determined 48 hrs later. The inhibition rate was calculated as described in the ‘Methods’ section, and data are presented as the mean (±S.D.) of 3 independent experiments performed in triplicate.
Heptapeptide-library-derived peptide inhibits angiogenesis in vivo
We next determined the ability of the P7 peptide to inhibit angiogenesis in vivo in a chick embryo CAM neovascularization model [10]. Consistent with the known angiogenic activity of bFGF [1], chick embryos treated with bFGF showed significantly enhanced CAM neovasculerization compared to those treated with PBS (P < 0.01; Fig. 4). However, bFGF-induced angiogenesis was completely blocked by treatment with 1-μM synthetic P7 peptides. The number of CAM blood vessels in chick embryos treated with bFGF plus P7 peptides was markedly reduced compared to those treated with bFGF alone (P < 0.01) and became indistinguishable from the PBS controls.
Fig 4.
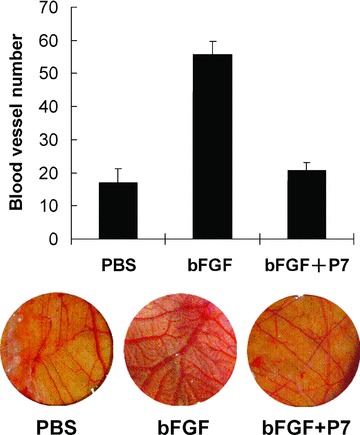
Synthesized P7 peptides inhibit bFGF-induced angiogenesis in chick embryo CAM. Data shown are blood vessel numbers (mean ± S.D.) in a 30-mm diameter of CAM (top; n= 5 per group) and representative pictures of CAM from each group (bottom).
Discussion
Tumour vascularization is closely related to tumour growth and metastasis. Therefore, antiangiogenetic therapy is a promising approach for cancer therapy [11, 12]. Various angiogenic factors including vascular endothelial growth factor (VEGF), bFGF, hepatocyte growth factor and placenta growth factor have been identified. Among these, VEGF and bFGF were found to be highly important in promoting tumour angiogenesis, and their expression was reported to correlate with vascularity and malignancy of astrocytomas, meningiomas, fibrous histiocytoma and pituitary tumours [13, 14]. VEGF promotes tumour angiogenesis through its potent mitogenic effect on the vascular endothelial cells. In addition to its direct activity in stimulating the proliferation of endothelial cells and tumour cells, bFGF can also synergistically enhance the mitogenic effect of VEGF. It has been reported that inhibition of bFGF-induced angiogenesis by anti-bFGF can effectively inhibit tumour cell growth in vivo[15].
The phage displaying technology has been widely used in identifying peptides with desirable biological properties. In the present study, we screened a phage-displayed heptapeptide library to identify bFGF antagonists, and obtained 11 phage clones that selectively bind bFGF. Sequence analysis showed that two of these clones (P7 and P11) shared identical sequences, but no consensus motif was identified in the rest of the clones. Alignment of the selected peptide sequences with the primary sequences of bFGF high-affinity receptors, FGFR1 (IIIc) and FGFR2 (IIIc), revealed high sequence homology between P7 and FGFRs. Five amino acids of P7 were identical to its corresponding motif in D3 of FGFR1 (IIIc) and FGFR2 (IIIc) [2, 3, 16]. Moreover, P7 and the corresponding motif in D3 of FGFR1 (IIIc) and FGFR2 (IIIc) both carry negative charges and share similar hydrophobic profiles.
Given that D3 is involved in ligand biding of FGFR1 (IIIc) and FGFR2 (IIIc) [2, 3, 16] and both electrostatic and hydropathic complementarities are important in peptide interactions [17–22], our results suggest that P7 may have the capability to bind bFGF and block the biological activity of bFGF by interrupting its interactions with FGFR1 and FGFR2. Consistent with this possibility, we found that P7 peptides bind with high affinity and specificity to bFGF. Functional analysis demonstrates that synthetic P7 peptides mediate strong inhibition of bFGF-stimulated cell proliferation and angiogenesis. It has been reported that peptides binding to FGFR1 identified by screening phage-epitope libraries with anti-bFGF mAbs have the ability to block high-affinity binding of bFGF to FGFR1, resulting in inhibition of vascular endothelial cell proliferation [9]. Since P7 has the potential to block the interaction of bFGF with multiple FGFRs, it may mediate a stronger antiangiogenetic effect than peptides that bind a single FGFR. Taken together, our results indicate that P7 is a potent bFGF antagonist with strong antiangiogenetic effects. Further characterization of the interaction between the P7 peptide and bFGF may lead to the development of a novel approach towards suppressing tumour angiogenesis.
Acknowledgments
The work was supported by a grant from the Science Foundation of Zhejiang Province of China (No. Z205755), the Program of New Century Excellent Talents in University (Li, XK), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (Li, XK) and the 5010 Project of Wenzhou Medical College.
References
- 1.Dailey L, Ambrosetti D, Mansukhani A, et al. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–47. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Plotnikov AN, Hubbard SR, Schlessinger J, et al. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell. 2000;101:413–24. doi: 10.1016/s0092-8674(00)80851-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Kan M, Xu J, et al. Ligand-specific structural domains in the fibroblast growth factor receptor. J Biol Chem. 1995;270:10222–30. doi: 10.1074/jbc.270.17.10222. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family: the complete mammalian fgf family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh BK, Igarashi M, Eliseenkova AV, et al. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc Natl Acad Sci. 2003;100:2266–71. doi: 10.1073/pnas.0436500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rusnati M, Presta M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr Pharm Des. 2007;13:2025–44. doi: 10.2174/138161207781039689. [DOI] [PubMed] [Google Scholar]
- 7.Cronauer MV, Schulz WA, Seifert H-H, et al. Fibroblast growth factors and their receptors in urological cancers: basic research and clinical implications. Eur Urol. 2003;43:309–19. doi: 10.1016/s0302-2838(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 8.Gross JL, Herblin WF, Dusak BA, et al. Effects of modulation of basic fibroblast growth factor on tumor growth in vivo. J Natl Cancer Inst. 1993;85:121–31. doi: 10.1093/jnci/85.2.121. [DOI] [PubMed] [Google Scholar]
- 9.Yayon A, Aviezer D, Safran M, et al. Isolation of peptides that inhibit binding of basic fibroblast growth factor to its receptor from a random phage-epitope library. Proc Natl Acad Sci. 1993;90:10643–7. doi: 10.1073/pnas.90.22.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Jiang L, Wang Y, et al. Inhibition of angiogenesis by a novel small peptide consisting of the active fragments of platelet factor-4 and vasostatin. Cancer Lett. 2007;256:29–32. doi: 10.1016/j.canlet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Shahi PK, Pineda IF. Tumoral angiogenesis: review of the literature. Cancer Invest. 2008;26:104–8. doi: 10.1080/07357900701662509. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 13.Bian XW, Du LL, Shi JQ, et al. Correlation of bFGF, FGFR-1 and VEGF expression with vascularity and malignancy of human astrocytomas. Anal Quant Cytol Histol. 2000;22:267–74. [PubMed] [Google Scholar]
- 14.Tsunenari I, Yamate J, Sharma D, et al. Expressions of vascular endothelial growth factor and basic fibroblast growth factor in tumors induced by two different cloned cell lines established from transplantable rat malignant fibrous histiocytoma. J Vet Med Sci. 2000;62:699–705. doi: 10.1292/jvms.62.699. [DOI] [PubMed] [Google Scholar]
- 15.Hori A, Sasada R, Matsutani E, et al. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991;51:6180–4. [PubMed] [Google Scholar]
- 16.Gray TE, Eisenstein M, Shimon T, et al. Molecular modeling based mutagenesis defines ligand binding and specificity determining regions of fibroblast growth factor receptors. Biochemistry. 1995;34:10325–33. doi: 10.1021/bi00033a002. [DOI] [PubMed] [Google Scholar]
- 17.Tsang KY, Diaz H, Graciani N, et al. Hydrophobic cluster formation is necessary for dibenzofuran-based amino acids to function as .beta.-sheet nucleators. J Am Chem Soc. 1994;116:3988–4005. [Google Scholar]
- 18.Peczuh MW, Hamilton AD. Peptide and protein recognition by designed molecules. Chem Rev. 2000;100:2479–94. doi: 10.1021/cr9900026. [DOI] [PubMed] [Google Scholar]
- 19.Schmuck C, Heil M. Peptide binding by one-armed receptors in water: screening of a combinatorial library for the binding of Val-Val-Ile-Ala. Chembiochem. 2003;4:1232–8. doi: 10.1002/cbic.200300613. [DOI] [PubMed] [Google Scholar]
- 20.Fassina G, Cassani G, Gnocchi P, et al. Inhibition of interleukin-2/p55 receptor subunit interaction by complementary peptides. Arch Biochem Biophys. 1995;318:37–45. doi: 10.1006/abbi.1995.1201. [DOI] [PubMed] [Google Scholar]
- 21.Fassina G, Consonni R, Zetta L, et al. Design of hydropathically complementary peptides for Big Endothelin affinity purification. Int J Pept Protein Res. 1992;39:540–8. doi: 10.1111/j.1399-3011.1992.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Zhang Q, Huang Y, et al. Quartz crystal microbalance biosensor for recombinant human interferon-[beta] detection based on antisense peptide approach. Analytica Chimica Acta. 2007;590:91–7. doi: 10.1016/j.aca.2007.03.022. [DOI] [PubMed] [Google Scholar]


