Abstract
Docetaxel has been used as first-line chemotherapy in advanced non-small cell lung carcinoma (NSCLC), but further extensive and effective application is prevented by drug resistance. MicroRNAs (miRNAs) have recently been identified as important posttranscriptional regulators, which are involved in various biological processes. The aim of this study was to identify microRNA expression profiles involved in the development of docetaxel resistance in NSCLC. Here, microarray chip technology was employed to identify miRNA expression profiles in docetaxel-resistant human NSCLC cell line (SPC-A1/docetaxel). Then, the changes of miRNAs expression (>2-fold compared with control SPC-A1 cell line) were testified by quantitative real-time RT-PCR (qRT-PCR) assay. Furthermore, the potential target genes regulated by selected miRNAs were analysed by various target prediction tools. The expression of a total of 52 miRNAs showed significant difference between SPC-A1/docetaxel cells and control SPC-A1 cells (P < 0.01). Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). Potential target genes controlled by six selected miRNAs were divided into four groups according to various functions: apoptosis and proliferation (71 genes), cell cycle (68 genes), DNA damage (26 genes) and DNA repair (59 genes). The expression of a few target genes in SPC-A1/docetaxel and SPC-A1 cells were further confirmed by qRT-PCR and Western blot. Taken together, the identification of microRNA expression profiles in docetaxel-resistant NSCLC cells could provide a better understanding of mechanisms involved in drug sensitivity or resistance, which would be helpful to develop novel strategies for targeted therapies in chemorefractive NSCLC patients.
Keywords: microRNA profile, non-small cell lung carcinoma, chemoresistance, docetaxel
Introduction
Non-small cell lung carcinoma (NSCLC) represents the most frequent type of lung cancer, which is one of the most common cancers in the world [1]. NSCLC is composed primarily of adenocarcinoma, squamous cell carcinoma and to a lesser extent large-cell lung cancer. Non-small cell histology accounts for 70–80% of lung cancer diagnoses and the disease is generally diagnosed at an advanced stage. Although various treatment options were extensively explored in clinic, it has been proved that systemic chemotherapy can provide improvement in both survival and quality of life for patients with advanced NSCLC [2]. The taxanes (paclitaxel and docetaxel) are novel microtubule-stabilizing agents, which have become an integral part of several commonly used chemotherapy regimens in NSCLC [3]. However, the development of intrinsic or acquired resistance to taxanes remains the greatest obstacle to the successful treatment of NSCLC patients.
Therefore, it is needed to identify and understand the diverse mechanisms of chemoresistance so as to develop effective strategies to overcome the docetaxel resistance of NSCLC. Genomic and proteomic studies have yielded a wealth of novel insights into molecular targets and mechanisms of cancer chemosensitivity and resistance. Up to now, a number of mechanisms of resistance to antimicrotubule agents have been described, including the multidrug resistance gene, oncoprotein 18 (stathmin) gene, mitotic serine/threonine kinases (Aurora-A), inhibitors of apoptosis protein (survivin), alteration in tubulin dynamics and differences in β-tubulin isotype expression [4–9]. However, the mechanism of docetaxel resistance is very complicated and still unclear, so it needs to be further elucidated.
The changes of chemoresistant gene expression include DNA, mRNA and protein levels, but the levels of mRNA and the encoded proteins are often not proportional, which can have a number of causes, among them, post-transcriptional regulation by microRNAs (miRNAs). miRNAs are small non-coding RNAs of 21 to 25 nucleotides that negatively modulate protein expression [10]. miRNAs are involved in several biological processes, such as development, proliferation, apoptosis and differentiation [11, 12]. Moreover, many studies have shown that aberrant microRNA expression is correlated with malignant transformation and tumour development [13, 14]. Thus, we demonstrated that miRNAs might play important roles in mediating chemosensitivity and resistance of human tumours. In order to identify miRNAs and determine their roles in mediating docetaxel resistance in NSCLC, we employed a microRNA array to detect a distinctive miRNA expression pattern of docetaxel-resistant NSCLC cell line (SPC-A1/docetaxel) different from that of docetaxel-sensitive NSCLC cell line (SPC-A1). Then, we employed bioinformatics methods for the analysis of miRNA expression arrays to find the target genes regulated by miRNAs, and further analyse their functions associated with chemoresistance of tumours. The expression of a few target genes were chosen to be confirmed by quantitative real-time RT-PCR (qRT-PCR) and Western blot assays. Taken together, the miRNA signature of docetaxel resistance could be helpful to develop novel strategies for targeted therapies in chemorefractive NSCLC patients.
Materials and methods
Cell lines and cell culture
The human lung adenocarcinoma cell line (SPC-A1) was purchased from Shanghai Institute of Cell Biology (Shanghai, China). The docetaxel-resistant lung adenocarcinoma cell line (SPC-A1/docetaxel) was established and preserved in our lab. All cell lines were cultured in RPMI 1640 (GIBCO-BRL, Grand Island, NY, USA) medium supplemented with 10% foetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin in humidified air at 37°C with 5% CO2.
MicroRNA microarray
Total RNA from lung adenocarcinoma cell line (SPC-A1) and corresponding docetaxel-resistant lung adenocarcinoma cell line (SPC-A1/docetaxel) was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The quality and quantity of the RNA samples were assessed by standard electrophoresis and spectrophotometer methods. miRNA microarray analysis was performed by LC Sciences (Houston, TX, USA). Microarrays utilized μParaflo™ microfluidic chip technology and optimized RNA hybridization probes with a detection limit of <100 attomole that was cross referenced to the Sanger miRNA database (Release 10.0, microrna.sanger.ac.uk/) after normalization. The data from the microarray was collected and analysed in accordance to the MIAME guidelines. Differential miRNA expression was determined using a two-sided t-test on a single miRNA basis. Differentially detected signals were generally accepted as true when the ratios of the P-value were <0.01 and were then selected for cluster analysis.
qRT-PCR analysis of miRNAs expression
qRT-PCR assay was performed on two samples. Using the TaqMan MicroRNA Assay Kit (Applied Biosystems, Foster City, CA, USA), which utilizes miRNA-specific primers, we reverse-transcribed 10 μg of each RNA sample according to the manufacturer’s instructions. The resulting cDNA was semi-quantitatively amplified in 45 cycles on an ABI 7500 Real-Time PCR System, using TaqMan Universal PCR Master Mix No Amp Erase UNG and Taqman MicroRNA Assays for hsa-miR-192, hsa-miR-200b, hsa-miR-194, hsa-miR-424, hsa-miR-30e*, hsa-miR-212 and 18s rRNA (Applied Biosystems). Each qRT-PCR assay was performed at least three times.
miRNAs target prediction
Candidate miRNAs which changed more than 2-fold were picked for target prediction. Potential miRNA target among genes negatively correlated with miRNA expression was determined using the publicly available miRanda (http://www.microrna.org) and TargetScan (http://www.targetscan.org) algorithms. The secondary structure of potential target transcripts was explored using the web-based RNA analyser tool (http://wb2x01.biozentrum.uni-wuerzburg.de/). The genes predicted as candidate miRNAs targets and those selected on the basis of gene ontology were aligned by their gene names, and genes appearing in both lists were chosen and listed.
qRT-PCR analysis of target genes
Total RNA was isolated and reverse-transcribed from two cell samples. qRT-PCR assay was performed to detect β-actin expression that was used to normalize the amount of cDNA for each sample. β-actin primers were as follows: sense 5′-TGACGGGGTCACCCACACTGTGCCCATC-3′; reverse, 5′-CTAGAAGCATTTGCGGTGGACG-3′. Equal amounts of cDNA from each sample were amplified using the following primers to detect the expression of target genes: ABCC3 (GenBank Y17151) sense 5′-GTCACCCCCTTGGTGGTGGG-3′, reverse 5′-ACCACCATCTGG-GATCTGTC-3′; Bcl-2 (GenBank NM_000657) sense 5′- CGGGAGCTGTGGGCGCCGCG-3′, reverse 5′-TCCCGGTTGACGCTCTCCAC-3′; BIK (GenBank NM_001197) sense 5′-ATGTCTG-AAGTAAGACCCCTCTC-3′, reverse 5′-AGCAGCGCCAGCAGCAGCAG-3′; PRKCA (GenBank NM_002737) sense 5′-TGAGGCGAAGAACGTGCAC-3′, reverse 5′-TTTCAATTTGAATGTAAAG-G-3′; Aurora-A (GenBank NM_003600) sense 5′-GGACCGATCTAAAGAAAACTG-3′, reverse 5′-TTTCAGGTGCCGATGGCAGG-3′; ERBB2IP (GenBank Accession No. BC144075) sense 5′-TTTACACAAACTGAGTTTGC-3′, reverse 5′-GGCACTTCCGTGAATTCGTTAC-3′. Two independent experiments were performed in triplicate and PCR products were measured using an ABI PRISM 7700 sequence detection system and analysed with ABI PRISM 7000 SDS software (Applied Biosystems). The mRNA expression of target genes was normalized by that of β-actin mRNA.
Western blot analysis of target genes
Cells were harvested by suspension in 50 μl lysis buffer (50 mM Tris-HCl pH 8.0, 1 mM ethylenediaminetetraacetic acid pH 8.0, 5 mM DTT, 2% SDS) on ice for 30 min, and the resulting lysates were cleared by centrifugation. Proteins were resolved by 10% SDS-PAGE and electroblotted onto nitrocellulose membrane, blocked by 5% skim milk, and probed with mouse anti-PRKACB, anti-CASP2, anti-Bcl-2, anti-PRKCA, anti-TP53, anti-ERBB2IP and anti-β-tubulin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following incubation with horseradish peroxidase-conjugated goat antimouse IgG (Sigma, St. Louis, MO, USA). The bands were visualized by chemiluminescence using a chemiluminescence kit (Invitrogen).
Statistical analysis
All numerical data from qRT-PCR assays were analysed after standard curves were derived for each miRNA of interest. Statistical analysis was performed with Student’s t-test, and P < 0.05 was considered statistically significant. Each experiment was performed in triplicate and individual samples were run in triplicate.
Results
Main experimental procedure
In the present study, experiments and bioinformatics methods were integrated to create a global view of specific miRNAs associated with docetaxel resistance of NSCLC, thus allowing their targets and functions to be identified (Fig. 1).
Fig 1.
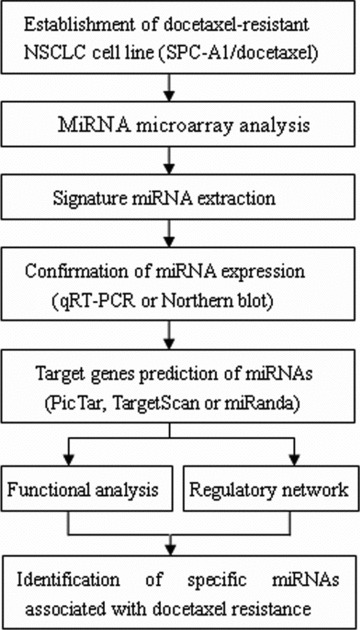
Schematic strategy used to analyse docetaxel resistance-associated miRNAs in SPC-A1/docetaxel cell line.
Analysis of the quality of RNA isolated from SPC-Al and SPC-A1/docetaxel cells
The quality of total RNA was analysed through denaturing agarose gel electrophoresis and UV spectrometer. The results indicated that the RNA isolated from SPC-Al and SPC-A1/docetaxe cells showed clear bands of 28SrRNA and 18SrRNA (Fig. 2). The values of OD260/OD280 of each sample were 2.02 and 2.01, respectively, and the values of OD260/OD230 of each sample were 2.06 and 2.09, respectively. All these results showed that the resulting high-quality RNA was suitable for microRNA microarray, RT-PCR and reverse transcription followed by quantitative real-time PCR.
Fig 2.
Denaturing agarose gel electrophoresis of RNA isolated from cell samples. (1) SPC-A1; (2) SPC-A1/docetaxel.
miRNA expression profiles in lung adenocarcinoma cell line (SPC-A1) and docetaxel-resistant lung adenocarcinoma cell line (SPC-A1/docetaxel)
To identify the changes of miRNA expression profile between lung adenocarcinoma cell line (SPC-A1) and docetaxel-resistant lung adenocarcinoma cell line (SPC-A1/docetaxel), miRNA microarray analysis was carried out. The miRNA expression profile of two lung adenocarcinoma cell lines showed that 53 out of 470 human miRNAs were differentially expressed between SPC-A1 cell line and SPC-A1/docetaxel cell line (Fig. 3). In the fold-change analysis (Table 1), 6 of the 52 flagged miRNAs in SPC-A1/docetaxel cell line showed at least a 2-fold change in expression level compared to the control SPC-A1 cell line, while there were 46 miRNAs that showed <2-fold change in expression level.
Fig 3.
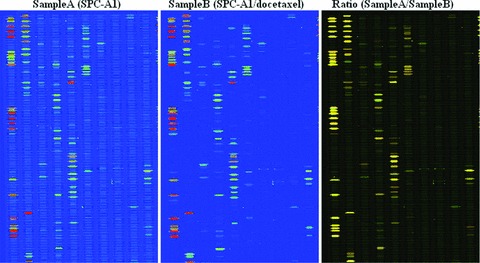
Microarray chip analysis of miRNAs expression in both docetaxel-resistant NSCLC cell line (SPC-A1/docetaxel) and control NSCLC cell line (SPC-A1).
Table 1.
miRNA list (differentially expressed transcripts with P-value < 0.01)
| miRNA profiles Up-regulation miRNA name | Fold change | Down-regulation miRNA name | Fold change |
|---|---|---|---|
| has-miR-192a | 4.77 | has-miR-200ba | 4.18 |
| has-miR-424a | 2.72 | has-miR-194a | 3.60 |
| has-miR-98a | 2.63 | has-miR-212a | 2.43 |
| has-miR-30e* | 1.42 | has-miR-1268 | 1.36 |
| has-miR-181b | 1.33 | has-miR-132 | 1.12 |
| has-miR-148a | 1.26 | has-miR-638 | 1.07 |
| has-miR-365 | 1.04 | has-miR-1246 | 0.93 |
| has-miR-454 | 0.98 | has-miR-22 | 0.88 |
| has-miR-374b | 0.92 | has-miR-923 | 0.87 |
| has-miR-1280 | 0.88 | has-miR-100 | 0.83 |
| has-miR-26b | 0.79 | has-miR-423–5p | 0.70 |
| has-miR-125a-5p | 0.77 | has-miR-193a-5p | 0.68 |
| has-miR-720 | 0.73 | has-miR-152 | 0.65 |
| has-miR-1308 | 0.71 | has-miR-10a | 0.54 |
| has-miR-30a* | 0.63 | has-miR-125b | 0.52 |
| has-miR-30b | 0.62 | has-miR-151–5p | 0.52 |
| has-miR-320d | 0.55 | has-miR-21 | 0.47 |
| has-miR-320b | 0.48 | has-miR-7b | 0.34 |
| has-miR-20b | 0.45 | has-miR-27b | 0.32 |
| has-miR-224 | 0.45 | has-miR-15b | 0.27 |
| has-miR-30a | 0.44 | has-miR-16 | 0.27 |
| has-miR-30c | 0.38 | has-miR-7a | 0.26 |
| has-miR-17 | 0.36 | has-miR-7c | 0.26 |
| has-miR-320c | 0.34 | ||
| has-miR-let-7e | 0.32 | ||
| has-miR-106a | 0.31 | ||
| has-miR-7g | 0.27 | ||
| has-miR-1826 | 0.26 | ||
| has-miR-191 | 0.24 |
miRNAs representing >2-fold expression change, compared with SPC-A1.
Different molecules (isomerides) in a molecular family.
Confirmation of miRNA microarray data by qRT-PCR
To confirm the miRNA microarray data, we used Taqman MicroRNA Assay Kit (Appiled Biosystems) to perform real-time qRT-PCR analyses of the expression levels of miR-192, 200b, 194, 424, 98 and 212 in both SPC-A1 cell line and SPC-A1/docetaxel cell line. It is generally accepted that gene-expression levels should be normalized by a carefully selectable stable internal control gene. To validate the presumed stable expression of a given control gene, prior knowledge of a reliable measure to normalize this gene in order to remove any non-specific variation is needed. For each sample, the expression values were normalized to the 18s rRNA gene (the most stable housekeeping gene), and we calculated the expression levels relative to SPC-A1 cell line. The expression pattern found in the arrays was confirmed through this additional analysis. As compared with SPC-A1 cell, miR-200b, three miRNAs (miR-200b, 194 and 212) were significantly down-regulated in docetaxel-resistant lung adenocarcinoma cell line (SPC-A1/docetaxel), while three miRNAs (miR-192, 424 and 98) were significantly up-regulated docetaxel-resistant lung adenocarcinoma cell line (SPC-A1/docetaxel). For those candidate miRNAs, the qRT-PCR analysis showed similar patterns of up- or down-regulation to those shown in the results of microarray analysis (Fig. 4). However, the magnitude of the fold changes in expression differed somewhat between what was shown in microarray analysis and what was shown in qRT-PCR analysis. Thus, miRNA expression profiles show significant difference between SPC-A1 and SPC-A1/docetaxel (P < 0.01), suggesting that miRNAs may play an important role in the development of acquired chemoresistance of docetaxel in NSCLC.
Fig 4.
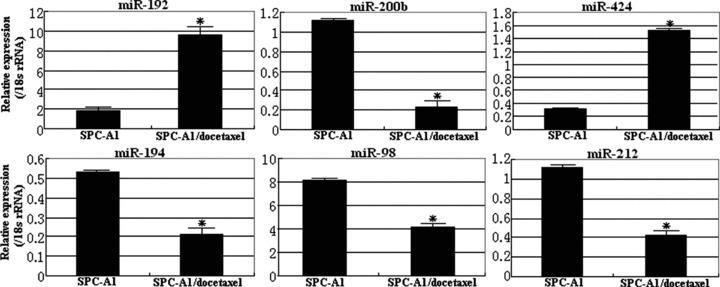
Validation of microarray analysis data by qRT-PCR for candidate miRNAs associated with docetaxel resistance of NSCLC cells. The expression levels of six miRNAs (miR-200b, 194, 212, miR-192, 424 and 98) were determined by qRT-PCR. Each qRT-PCR assay was performed at least three times, *P < 0.01.
Prediction of miRNA targets
Identification of potential target genes of miRNAs associated with docetaxel resistance of lung adenocarcinoma is essential to investigate their biological functions. Recent studies have shown that miRNAs can regulate expression of their target genes by decreasing mRNA stability, in addition to translational inhibition. Candidate miRNAs, which showed at least a 2-fold change, were picked for target prediction and analysed with three target prediction tools, which were TargetScan 5.1 on the Whitehead Institute for Biomedical Research website (http://www.targetscan.org/), miRBase Targets Release Version v5 in the Enright Lab on the Wellcome Trust Sanger Institute website (http://microrna.sanger.ac.uk/targets/v5/) and miRNA Target Database-ncRNA.org (http://www.microrna.org/microrna/home.do). By analysis of above three databases, human genes which were known to be involved in cell proliferation, apoptosis, cell cycle and DNA damage or repair were selected from the Gene Ontology website (http://www.geneontology.org/). Then, those genes predicted to be targets of the candidate miRNAs were chosen and listed in Table 2. All data might provide the foundation for further analysis of mechanisms of docetaxel resistance in NSCLC.
Table 2.
Global target genes prediction of miRNAs
| Putative targets of miRNAs and their functions | ||||
|---|---|---|---|---|
| miRNA name | Apoptosis and proliferation | Cell cycle | DNA damage | DNA repair |
| miR-192 | BAG1, BCL2L11, CUL3, CUL5, CYCS, DHCR24, DYRK2, RCC3, IGF1, MALT1, MAPK1, NGFR, NTRK2, PERP, PRKACB | ABCC3, APPL1, BCAT1, CCND2, CDC7, CUL3, CUL5, TRIP13, SFTPB, RNF8, RBL2, RB1, RAD1, E2F6, KLK10 | DYRK2, XPA, ERCC3, MYO6, RAD1, | RNF8, PRNP, ATP2C1, BCAT1, CEBPG, DBT, IDS, NAT5, MAN2A2, SMC5, |
| miR-200b | APAF1, CBX4, BCL2, DLC1, HMOX1, MALT1, NOTCH1, PTEN, SORT1, XRCC2 | AHR, BTRC, CDC14A, CDK2, CDK6, CLASP1, CTSB, E2F3, ETS1, FOXN3, ITGB1, NBN, | DYRK2, NBN, HMOX1, MRPS35, NPM1 | UHRF1, UBE2V1, UBE2N, TTC5, SHPRH, RRM2B, MMP19, MMP16, ERCC8, |
| miR-424 | IGF1R, BCL2L2, TRAF1, TLR1, SOX9, SMAD3, SMAD7, PIK3R1, PIK3R2, KRAS, IGF1R, BIK, CD28 | E2F3, E2F7, CDK6, CDC23, BIRC5, APP, ABL1, MLH3, LATS2, KATNB1,HNF4A, HMGA2 | XPC, TIPIN, RAD9A, RAD23B, PHLDA3, | ACACA, AGPAT2, ACSL1, MMP24, RNF8, MMP3, SSRP1, UBE2B, UNG, WDR33 |
| miR-194 | APC, FADD, TRAF6, SPPL3, SORT1, SIRT1, PRKRA, PRKCA, IL6R | CDK6, E2F6, DDX11, VPS36, TLK1, RAD51, SORT1, PPT1, HGSNAT | CEP63, WRN, GTF2H1, SIRT1, SP100 | RBX1, ESCO2, MGEA5, PIGN, RAD51L, PPAT, CUL4B, BCCIP, ATM |
| miR-98 | TP53, TNFSF10, TEX261, SARM1, MAP3K1, FAS, FASLG, CASP3, BCL2L1, TBX5, RNF7, HGF | E2F2, HMGA2, MAPK4, USP6, STAG3, PARD6B, IDS, E2F6, AURKA, AURKB, ABCB9 | CEP63, RAD1, ERCC6, TP53, RAD9A, | ACSL6, AGPAT3, DCT, BCAT1, RNF8, SMUG1, ENPP4, POLQ, NAT12, HDHD1A, ALDOC |
| miR-212 | APAF1, ERBB2IP, APC, API5, BCL10, BNIP2, BRCA1, DEDD, HIP1, KRAS, PML, SMAD3, TNFRSF10B | ZWINT, TEX11, RB1, PSMD12, NEDD9, MIS12, MAPK3, FBXO31, EP300, ARL3, GAS7, PSME3 | BRCA1, DYRK2, MSH2, SIRT1, NBN | ASF1A, CSRP2BP, FUCA2, MMP16, PURB, SMC6, EPHX2, NONO, RAD50, HEXB, IDS |
Confirmation of the expression of a few target genes by qRT-PCR and Western blot assays
qRT-PCR or Western blot assays were performed to detect the expression of a few potential target genes (ABCC3, Bcl-2, BIK, PRKCA, Aurora-A and ERBB2IP) at both transcriptional and translational levels. As shown in Fig. 5, the mRNA and protein expression levels of ABCC3 and BIK genes in SPC-A1/docetaxel cells were significantly lower than those in SPC-A1 cells (P < 0.05), while the mRNA and protein expression levels of Bcl-2, PRKCA and Aurora-A genes in SPC-A1 cells were significantly higher than those in SPC-A1 cells (P < 0.05). Although the mRNA expression level of ERBB2IP gene showed no difference between SPC-A1/docetaxel cell and SPC-A1 cell (P > 0.05), the protein expression level of ERBB2IP gene in SPC-A1/docetaxel cell was significantly higher than that in SPC-A1 cell. Therefore, the expression levels of miRNAs showed inverse correlation with the protein expression levels of their potential target genes. MiRNAs inhibit target gene expression, by interacting with target mRNAs at specific sites in order to induce cleavage of the message RNA or inhibit translation, therefore, the regulation of potential target genes by miRNAs might take place at pre-translation or translation stage, which finally induces the inhibition of protein expression.
Fig 5.
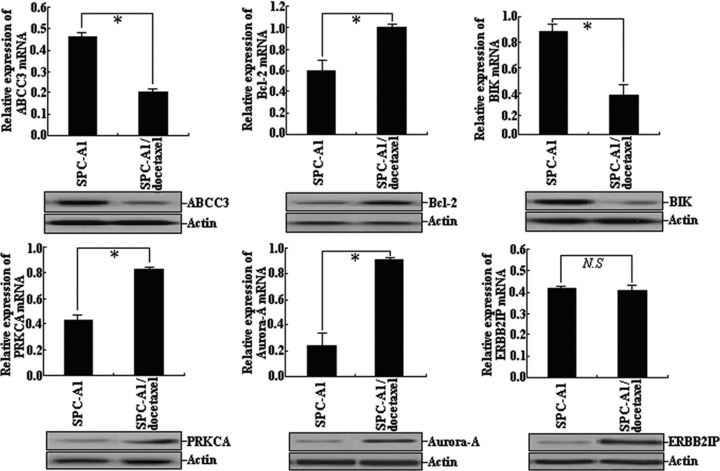
Confirmation of the expression of a few potential target genes (ABCC3, Bcl-2, BIK, PRKCA, Aurora-A and ERBB2IP) by qRT-PCR and Western blot assays. Each assay was performed at least three times.*P < 0.05, N.S., P > 0.05.
Discussion
Non-small-cell lung cancer (NSCLC) is a major global health problem. Despite improvement in diagnosis and treatment, over 60,0000 patients still die of lung cancer annually in China. Current treatment options for patients with NSCLC depend on the stage of disease and mainly include surgery, chemotherapy, radiation therapy, immunotherapy and targeted therapies [15, 16]. In some cases, surgery, radiation therapy and chemotherapy will be used together to treat NSCLC. Surgery may be used to treat early or localized lung cancer. However, most NSCLC patients are diagnosed with locally advanced or metastatic disease, at which point, surgery may no longer be an option. In that case, radiation therapy and medications may be used to treat the advanced NSCLC. Although there are a number of medications available to treat advanced NSCLC, the standard first-line treatment option for the advanced disease is still chemotherapy [17].
Presently, some of the commercially approved chemotherapy drugs and targeted therapies, including anti-angiogenic therapies used to treat advanced NSCLC are as follows: cisplatin, carboplatin, docetaxel or paclitaxel, gemcitabine, erlotinib or pemetrexed [18–23]. Antitubulin agents have played, and will continue to play, a significant role in NSCLC chemotherapy. Taxanes bind preferentially to microtubules leading to stabilization, which are involved in the formation of mitotic spindles during M phase of the cell cycle [24]. Docetaxel, one the first two cytotoxics of the taxane family, is highly active and frequently used for adjuvant therapy after resection of localized NSCLC and in combination with radiation for locally advanced NSCLC and treatment of patients with advanced NSCLC. Unfortunately, like most chemotherapeutic agents, the clinical success of docetaxel was limited by the insurgence of cellular resistance [25]. Elucidating the complex mechanisms of docetaxel resistance is crucial to better understanding of docetaxel and its use in the clinical setting. It has been reported that multiple factors underlying docetaxel resistance include mutations in both α and β tubulin, differing β-tubulin isotype compositions, P-glycoprotein (Pg) overexpression and increased microtubule dynamics associated with altered microtubule-associated protein (MAP) expression [26]. To further explore drug resistant mechanism of docetaxel and provide theoretical support for drug resistant reversal induced by docetaxel, a docetaxel-resistant variant of human lung adenocarcinoma cell line SPC-A1 (SPC-A1/docetaxel) was previously established and preserved by our lab. SPC-A1/docetaxel cell line was established after 14 months’ induction by continuously exposing human lung adenocarcinoma cell line SPC-A1 to docetaxel whose dose was gradually increased. The incipient concentration of docetaxel was 0.008 μg/l, and SPC-A1/docetaxel cell line stably grew into 5.0 μg/l docetaxel at last. MTT assay showed index of drug resistance of SPC-A1/docetaxel was 13.20 to docetaxel, while cell doubling time of SPC-A1/docetaxel cells and SPC-A1 cells were 35.1 and 27.4 hrs, respectively [27]. The establishment of docetaxel-resistant NSCLC cell culture model provided foundation for research on its drug resistant mechanisms.
miRNAs are short non-coding RNAs of 19–24 nt, that regulate gene expression in multi-cellular organisms. A lot of researches have indicated that miRNAs play an important role in carcinogenesis and tumour progression including NSCLC [28]. It is likely, therefore, that they can also modulate sensitivity and resistance to anticancer drugs in substantial ways. While altered expression of miRNAs in primary human NSCLCs has been used for tumour diagnosis and prognosis [29], the potential involvement of miRNAs in induction of drug resistance, particularly, in docetaxel resistance has not been investigated. Therefore, in this study, high-throughput miRNA microarray technology with 470 miRNAs selected from miRBase was used to detect miRNA expression in parental SPC-A1 cells and its counterparts made resistant to docetaxel (SPC-A1/docetaxel). Microarray data showed that 52 miRNAs showed significant difference between SPC-A1 cell line and SPC-A1/docetaxel cell line (P < 0.01). We also found 29 up-regulated and 23 down-regulated miRNAs in SPC-A1/docetaxel. In this analysis, 6 miRNAs in SPC-A1/docetaxel cells, respectively, showed >2-fold changes compared to control cells. Although not all miRNAs show similar regulation patterns in docetaxel-resistant NSCLC cells, we identified many miRNAs with at least 2-fold expression changes for further research. Next, to confirm the expression of six miRNAs identified in the microarray analysis, we performed qRT-PCR assay. The qRT-PCR analysis of miRNAs (miR-200b, 194, 212, miR-192, 424 and 98) was shown in Fig. 2. For all six miRNAs, the qRT-PCR experiment confirmed their down or up-regulation, although the magnitude of the changes in the expression levels was found to be different between the microarray and qRT-PCR analyses.
Up to now, there have no reports about the correlation between above six miRNAs and drug resistance of NSCLC. To elucidate the regulatory mechanisms of those miRNAs associated with docetaxel resistance of NSCLC, we employed various bioinformatical methods to identify potential target genes of those miRNAs. Then, those target genes were divided into four groups according to different functions (The Gene Ontology website: http://www.geneontology.org/): apoptosis and proliferation (71 genes), cell cycle (68 genes), DNA damage (26 genes) and DNA repair (59 genes), which have been reported to be associated with drug resistance of human tumours (Table 2). Computational predictions suggest that one miRNA can target many target mRNAs and each target mRNA also can be targeted by many miRNAs, which has been proved by other research groups. For example, in our analysis, we found that Bcl-2 gene might be not only a potential target gene controlled by miR-200b but also a potential target gene controlled by miR-424. Thus, there should be not a one-to one relationship but a one-to-many or many to one relationship between miRNAs and target genes. Recently, many studies show that functional miRNA-binding sites can lie in the coding regions or 5′-UTRs of endogenous mRNAs. Therefore, the number of target genes regulated by miRNAs should be larger than what was analysed by bioinformatics. A few potential target genes were chosen to be further confirmed by qRT-PCR and Western blot assays. Although the expression levels of miRNAs show inverse correlation with the protein expression levels of their potential target genes, further studies need to provide experimental proof implicating testifying potential regulatory relationship between miRNAs and predicted target genes.
Concluding remarks
Taken together, our experimental data showed that alteration of miRNA expression profile in docetaxel-resistant NSCLC cell line might deregulate drug resistance-related genes and would provide potential mechanisms that underlie in the docetaxel resistance of NSCLC and provide a novel molecular therapeutic target for chemorefractive NSCLC therapy.
Acknowledgments
This study was supported by Jiangsu Province Postdoctor Foundation and we are grateful to every one of the Department of Medical Oncology for their sincere help and technical support.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jalal SI, Ademuyiwa FO, Hanna NH. The role of maintenance chemotherapy in advanced non small cell lung cancer. Curr Opin Oncol. 2009;21:110–5. doi: 10.1097/CCO.0b013e328322cf49. [DOI] [PubMed] [Google Scholar]
- 3.Edelman MJ, Gandara DR. Promising new agents in the treatment of non-small cell lung cancer. Cancer Chemother Pharmacol. 1996;37:385–93. doi: 10.1007/s002800050402. [DOI] [PubMed] [Google Scholar]
- 4.Ding S, Chamberlain M, McLaren A, et al. Cross-talk between signalling pathways and the multidrug resistant protein MDR-1. Br J Cancer. 2001;85:1175–84. doi: 10.1054/bjoc.2001.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alli E, Bash-Babula J, Yang JM, et al. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–9. [PubMed] [Google Scholar]
- 6.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 7.Meng H, Tanigawa N, Hao CY, et al. Chemoresponse to docetaxel correlates with expression of the survivin splicing variants in patients with gastric cancer. Hepatogastroenterology. 2007;54:1934–40. [PubMed] [Google Scholar]
- 8.Risinger AL, Giles FJ, Mooberry SL. Microtubule dynamics as a target in oncology. Cancer Treat Rev. 2009;35:255–6. doi: 10.1016/j.ctrv.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamath K, Wilson L, Cabral F, et al. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280:12902–7. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 10.Chekanova JA, Belostotsky DA. MicroRNAs and messenger RNA turnover. Methods Mol Biol. 2006;342:73–85. doi: 10.1385/1-59745-123-1:73. [DOI] [PubMed] [Google Scholar]
- 11.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2007;96:R40–4. [PubMed] [Google Scholar]
- 12.Maatouk D, Harfe B. MicroRNAs in development. Sci World J. 2006;6:1828–40. doi: 10.1100/tsw.2006.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–79. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Weinberg RA. MicroRNAs in malignant progression. Cell Cycle. 2008;7:570–2. doi: 10.4161/cc.7.5.5547. [DOI] [PubMed] [Google Scholar]
- 15.Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;12:587–96. doi: 10.1188/08.CJON.587-596. [DOI] [PubMed] [Google Scholar]
- 16.Gkiozos I, Charpidou A, Syrigos K. Developments in the treatment of non-small cell lung cancer. Anticancer Res. 2007;27:2823–7. [PubMed] [Google Scholar]
- 17.Bernstein ED, Herbert SM, Hanna NH. Chemotherapy and radiotherapy in the treatment of resectable non-small-cell lung cancer. Ann Surg Oncol. 2006;13:291–301. doi: 10.1245/ASO.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Besse B, Le Chevalier T. Adjuvant or induction cisplatin-based chemotherapy for operable lung cancer. Oncology. 2009;23:520–7. [PubMed] [Google Scholar]
- 19.Chiappori A, Simon G, Williams C, et al. Phase II study of first-line sequential chemotherapy with gemcitabine-carboplatin followed by docetaxel in patients with advanced non-small cell lung cancer. Oncology. 2005;68:382–90. doi: 10.1159/000086979. [DOI] [PubMed] [Google Scholar]
- 20.Belani CP TAX 326 Study Group. Docetaxel in combination with platinums (cisplatin or carboplatin) in advanced and metastatic non-small cell lung cancer. Semin Oncol. 2002;29:4–9. doi: 10.1053/sonc.2002.34255. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-TerrÈs C, Tisaire JL, Kobina S, et al. Cost-minimisation analysis of three regimens of chemotherapy (docetaxel-cisplatin, paclitaxel-cisplatin, paclitaxel-carboplatin) for advanced non-small-cell lung cancer. Lung Cancer. 2002;35:81–9. doi: 10.1016/s0169-5002(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 22.Carrión RP, Gracián AC, Hernandez PS. Erlotinib as a single agent in select subsets of patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2007;8:425–8. doi: 10.3816/CLC.2007.n.026. [DOI] [PubMed] [Google Scholar]
- 23.Longo-Sorbello GS, Chen B, Budak-Alpdogan T, et al. Role of pemetrexed in non-small cell lung cancer. Cancer Invest. 2007;25:59–66. doi: 10.1080/07357900601130748. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5:S3–6. [PubMed] [Google Scholar]
- 25.Geney R, Ungureanu M, Li D, et al. Overcoming multidrug resistance in taxane chemotherapy. Clin Chem Lab Med. 2002;40:918–25. doi: 10.1515/CCLM.2002.161. [DOI] [PubMed] [Google Scholar]
- 26.McGrogan BT, Gilmartin B, Carney DN, et al. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 27.SUN Hai, GENG Jian, JIN Jie, et al. Establishment and characterization of a docetaxel-resistant variant of human lung adenocarcinoma cell line SPC-A1. China Oncol. 2007;17:283–7. [Google Scholar]
- 28.Tong AW. Small RNAs and non-small cell lung cancer. Curr Mol Med. 2006;6:339–49. doi: 10.2174/156652406776894554. [DOI] [PubMed] [Google Scholar]
- 29.Raponi M, Dossey L, Jatkoe T, et al. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 2009;69:5776–83. doi: 10.1158/0008-5472.CAN-09-0587. [DOI] [PubMed] [Google Scholar]


