Abstract
The use of foetal liver cells (FLC) in the context of hepatic tissue engineering might permit efficient in vitro expansion and cryopreservation in a cell bank. A prerequisite for successful application of bioartificial liver tissue is sufficient initial vascularization. In this study, we evaluated the transplantation of fibrin gel-immobilized FLC in a vascularized arterio-veno-venous (AV)-loop model. FLC were isolated from embryonic/foetal (ED 16) rat livers and were enriched by using magnetic cell sorting (MACS). After cryopreservation, FLC were labelled by pkh-26. Cells were transplanted in a fibrin matrix into a subcutaneous chamber containing a microsurgically created AV-loop in the femoral region of the recipient rat. The chambers were explanted after 14 days. Subcutaneous implants without an AV-loop and cell-free implants served as controls. Fluorescence microscopy of the constructs was used to identify pkh-26+- donor cells. Characterization was performed by RT-PCR and immunhistology (IH) for CK-18 and CD31. Transplantation of FLC using the AV-loop permitted a neo-tissue formation in the fibrin matrix. A high-density vascularization was observed in the AV-loop constructs as shown by CD31 IH. Viable foetal donor cells were detected which expressed CK-18. FLC can be successfully used for heterotopic transplantation. Fibrin matrix permits rapid blood vessel ingrowth from the AV-loop and supports engraftment of FLC. It is therefore an appropriate environment for hepatocyte transplantation in combination with microsurgical vascularization strategies. Transplantation of fibrin gel-immobilized FLC may be a promising approach for the development of highly vascularized in vivo tissue-engineering-based liver support systems.
Keywords: foetal transplantation, neo-vascularization, liver cell transplantation, hepatic tissue engineering, AV loop
Introduction
Liver transplantation is a well-established procedure that represents a curative therapy for many liver diseases, which lead to liver failure in end-staged disease [1]. However, these procedures are limited by donor organ shortage, high costs, and the lifelong need of immunosuppression [2]. Therefore, cell transplantation concepts using isolated liver cells are under experimental evaluation for the development of an alternative treatment [3].
Cell transplantation has some advantages over organ transplantation: One donor organ could be used for many candidates. Cell transplantation is also thought to be less immunogenic, since transplanted allogenic cells could be immunomodulated prior to implantation or even autologous cells might be utilized following in vitro modification [3]. Cell transplantation might be an option for patients with metabolic diseases since not the complete organ needs to be replaced and the deficient metabolic function could be replaced or at least supported by only a small portion of hepatocytes [4]. In previous studies, we identified several foetal liver cell (FLC) progenitors during rat liver development [5]. A high-yield isolation method for foetal hepatic cells was established by using magnetic cell sorting (MACS) [6]. In vitro data showed a high growth potential of foetal hepatic cells, which was highest when cells were isolated from early foetal livers. Furthermore, hepatic differentiation potential of foetal hepatic cells has been shown in vitro[7]. Recently, foetal rat liver cells have been successfully transplanted into the portal vein for repopulation of diseased livers in the rat model [8]. Therefore, FLC seemed to be ideal candidates as a novel cell resource for hepatocyte transplantation and tissue engineering of the liver.
The transplantation of cells seeded on three-dimensional matrices into the peritoneal cavity has shown several advantages of a tissue-engineering approach [9]: It permits the transplantation of a cell number equivalent to a whole liver [10], the transplantation of genetically altered cells [11] and long-term engraftment of transplanted cells [12]. A highly stimulative effect of the matrix due to a three-dimensional orientation of the cells could be demonstrated for hepatocytes in vitro[13]. Furthermore, the modification of the three-dimensional matrices by coating with extracellular matrix molecules or by immobilization of growth factors could further enhance the engraftment and function of the transplanted cells [14, 15]. Fibrin gel allows as a two-component in situ setting matrix the minimal invasive application of different cell types of tissues. This approach of injectable tissue engineering has been successfully used for transplantation of hepatocytes [16, 17]. Proliferation and differentiation as well as function of hepatocytes transplanted outside the liver are strongly dependent on appropriate hepatotrophic stimulation [18]. A major limitation, however, is that the initial engraftment efficiency of the transplanted hepatocytes is severely limited within the tissue-engineered constructs. Thus, a sufficient initial vascularization seems to be a crucial step to get a highly efficient engraftment of the transplanted cells. For any clinical application of hepatocyte transplantation, the development of a vascularized three-dimensional cell-matrix construct for hepatocyte transplantation would be desirable that provides the clues for appropriate cell differentiation and a rapid high-density vascularization. Recent improvements in the generation of pre-vascularized bone, fatty tissue and muscle structures are promising and may be transferable to the situation in hepatic tissue engineering [19–23]. One of the main problems in hepatocyte transplantation – the vascularization and oxygenation of hepatocytes – may be solved by such a technique of tissue engineering.
The combination of FLC with efficient vascularization techniques might be a promising approach towards transplantation of large volumes of bioartificial liver tissues. In this study, we evaluated the transplantation of fibrin gel-immobilized FLC in a vascularized transplantation model using an arterio-veno-venous (AV)-loop in a proof-of principal rat model.
Materials and methods
Animals
Foetuses of pregnant female Lewis (Charles-River, Sulzfeld, Germany) rats were used as organ donors. Recipients were female Lewis rats (250–400 g). Animals were housed at the veterinary care facility of the University of Leipzig Medical Center and the University of Erlangen Medical Center, submitted to a 12-hr day/night cycle and had access to water and standard rat chow ad libitum. All experiments were approved by the animal care committee of the Saxonian Ministry of internal affairs and the Bavarian Ministry of Health. German regulations for the care and use of laboratory animals were followed at all times.
Isolation and processing of foetal livercells for transplantation
FLC were isolated from embryonic/foetal day (ED) 16 rat livers by collagenase digestion and following MACS depletion of OX-43/44 positive haematopoietic cells as described previously [5, 6]. After isolation, approximately 4.0 × 106 cells were suspended in 1.0 ml Cryo-Save I (C.C.Pro, Neustadt, Germany) and stored frozen at –80°C before use. Cells were thawed in a water bath at 37°C and washed twice in Williams Medium E (Gibco BRL Invitrogen, Karlsruhe, Germany). Viability of foetal cells after thawing was 91 ± 4%, as assessed by trypan-blue test. Labelling of foetal hepatocytes before transplantation was performed using the PKH26-labeling kit (Sigma) as described elsewhere [17]. In brief, the cell pellet was mixed with 500 μl pkh26-dye (diluted 1:50 in Dilutent C, Sigma PKH26-labeling kit, Sigma-Aldrich, Munich, Germany) and was incubated for 5 min. at room temperature. Labelling reaction was stopped by adding 10 ml foetal calf serum (FCS; Sigma-Aldrich, St. Louis, MO, USA). Labelled foetal hepatocytes were washed twice in Williams-Medium E prior to transplantation.
For assessing the quality of the cells after cryopreservation, cells were seeded on collagen type I (1.2 μg/cm2; Sigma-Aldrich) coated 48-well plates (BD Bioscience, San Jose, CA, USA). Initial 2.5 × 105 cells were seeded per well in 1 ml culture medium. Culture medium was a foetal hepatocyte medium (FHM) containing Williams Medium E without L-glutamine (Gibco Invitrogen, Karlsruhe, Germany) supplemented with 50 mg/l L-glutamine (Gibco), 100 IU/l penicillin/streptomycin (Gibco), 20 mM/l HEPES (Gibco), 20 mM sodium pyruvate (Gibco), 0.2 mg/ml magnesium-sulphate (Gibco), 6.5 nM dexamethasone (Sigma), 10 ng/ml epidermal growth factor (Gibco), 20 mU/ml insulin (Gibco), 10% FCS (Sigma) and 0.1% bovine serum albumin (Sigma). Cells were counted under an inverse microscope at culture days 1, 3, 7 and 10. Cell recovery rates during culture were calculated and were given in percentage of initially seeded cells. Cytospins of the cultured cells were drawn and an immunohistochemical staining for CK-18 was performed as described in the following text.
The fibrin matrix was prepared using a clinically approved fibrin gel (Tissuecol, Baxter, Unterschleiβheim, Germany) with a fibrinogen concentration of 10 mg/ml, a thrombin concentration of 50 IE/ml and an aprotinin concentration of 1500 K.I.E/ml diluted in 40 mM CaCl2 as described previously [17]. Foetal hepatocytes (4.0 × 106) from the pellet were resuspended in 400 μl of the fibrinogen component.
For seeding the cell/fibrinogen mixture was applied together in the transplantation chamber, the AV vessel loop was placed within the cell/fibrinogen suspension and 200 μl of the thrombin solution was added for matrix aggregation.
Animal procedures, AV-loop constructionand cell transplantation
Twenty adult female Lewis (Charles-River) rats (250–400 g) were used as recipients. The foetal hepatocyte transplantation and explantation of the constructs were performed under isofluorane (Baxter) inhalational anaesthesia. The microsurgery was performed under an operational microscope (Karl Zeiss, Jena, Germany). A single shot antibiotic prophylaxis (0.2 ml Tardomycal Comp III, Bayer, Leverkusen, Germany) was administered before the operation. In 10 recipients an AV-loop was created as described previously [19]. In brief, after exposition of the right-sided femoral vessels a 20-mm vein graft was harvested from the right femoral vein. An AV-loop was created by interposition of the vein graft between the left-sided femoral artery and the left-sided femoral vein using two interrupted non-resorbable 11–0 micro-sutures. The AV-loop was placed into a sterile cylindrical Teflon chamber (inner diameter 10 mm, height 6 mm). Four plastic rods prevented dislocation of the loop after implantation. Then the injectable cell/matrix suspension (group A, n= 5) or the plain fibrin matrix (group B) was filled in the chamber and the AV-loop was inserted into the matrix. After adding the second matrix component, the matrix aggregated, the chamber was closed and the skin was closed by sutures in layers. For subcutaneous control groups, the chambers containing either matrix/cell mixtures (group C, n= 5) or matrix components alone (group D, n= 5) were transplanted in a subcutaneous pocket underneath the skin at the back of the animals with the opening facing the muscle layer. The constructs were explanted after 14 days under isofluorane inhalational anaesthesia. Before explantation, a laparotomy was performed and the animals were administered 100 ml heparin solution (100 IU/ml) and then 30 ml India ink solution (India ink [50% v/v India ink, Rohrer, Leipzig, Germany] in 5% gelatin [Roth, Karlsruhe, Germany] and 4% mannitol [Neolab, Heidelberg, Germany]) via the abdominal aorta. After harvesting the transplantation chambers, the neo-tissue was divided and processed for histological and PCR-analysis.
RNA isolation and RT-PCR analysis
RNA was extracted using the Invisorb Spin Cell-RNA™ Mini-kit (Invitek, Berlin, Germany) according to the manufacturer’s instructions. RNA was stored at –80°C. RT of extracted RNA was performed using the bulk First-strand c-DNA synthesis kit (Amersham, Freiburg, Germany). The primers for GAPDH and CK-18 were published previously [6]. The cDNA was stored at –20°C. PCR with cDNA was performed using primers for CK-18 and GAPDH. For the PCR reaction 7 μl cDNA-template was mixed with 5 μl 10 × PCR-buffer, 1 μl 10 mM dNTP’s, 1.5 μl 50 mM MgCl2, 1 μl primers (50 ng/μl) and 1 μl polymerase (Ampli-Taq., Gibco) for each probe. PCR was carried out in a programmable Biometra Uno-Thermobloc (Biometra, Göttingen, Germany) with following conditions: 94°C for 10 min. and then 30 (GAPDH, CK-18) cycles each comprising denaturation for 1 min. at 94°C, annealing for 1 min. at 60°C, and extension for 1 min. at 72°C. After the PCR was completed reaction tubes were kept for 4 min. at 72°C and then 4°C. Negative controls routinely used for each set of primers included control without template. Samples were analysed on 1% agarose gels. The size of the PCR-fragments was estimated using a 100-base-pare ladder (Gibco BRL).
Cryosection and immunohistochemistry
Specimen (explants) were directly frozen in Tissuetec and stored at –80°C. Frozen specimen were cut at a cryomicrotome at 10-μm thickness and fixation was done with methanol at –20°C for 5 min., and aceton at 4°C for 15 sec. Furthermore, frozen cytospins of cell cultures were also processed for immunostaining. Immunohistochemical analysis was performed using mouse monoclonal antibodies (mAb) specific for CK-18 (ICN Cappel, Aurora, OH, USA), or monoclonal anti-rat CD31 (Dako, Hamburg, Germany). CK-18 and CD31 staining was performed using the alkaline phosphatase-antialkaline phosphatase (APAAP) technique. Antibodies were differently diluted (1:10). Secondary marking was done with rabbit-antimouse IgG mAb diluted 1:50. Slides were then incubated with mouse-APAAP-complex and the alkaline phosphate substrate, New Fuchsin, was used for staining. Slides were counterstained with haematoxylin.
Results
Growth and differentiation of FLC before and after cryopreservation
Cell recovery rates of Thy1-negative FLC isolated from ED 16 cultured in FHM were 180 ± 27% at day 3, 285 ± 37% at day 7 and 346 ± 17% at day 10 in primary cultures; Cell recovery after cryopreservation were 91 ± 6% at day 3, 189 ± 25% at day 7 and 229 ± 11% at day 10 in primary cultures, respectively (Fig. 1A). Immunocytochemical staining showed CK-18-positive cells at days 1 and 3 in cultures before and after cryopreservation (Fig. 1B).
Fig 1.
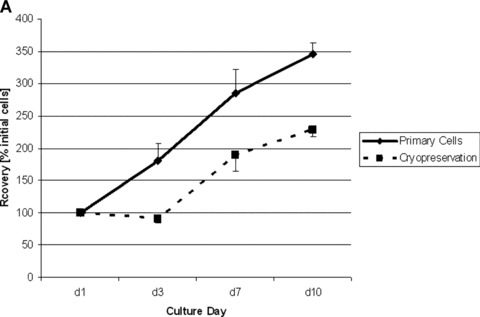
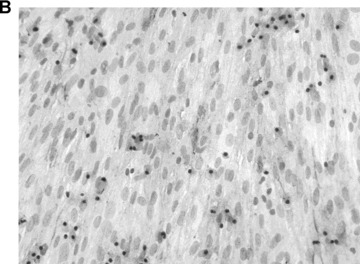
Growth and differentiation of ED 16 foetal liver cells (FLC) before and after cryopreservation in vitro. (A) Cell recovery (percentage initially attached cells) during culture period before and after cryopreservation showed little loss of growth potential of cryopreserved FLC. (B) CK-18 staining 10 × 10. CK-18 positive cells were found in cultures of FLC after cryopreservation at day 3 in culture.
Matrix aggregation and blood vessel ingrowth
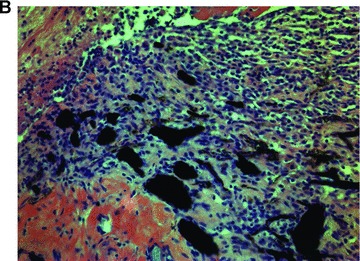
Viable and labelled FLC could be easily seeded within the subcutaneous chamber by injection of a cell/fibrinogen suspension. The AV-vessel loop generated by microsurgery was placed under direct vision in a circle at the periphery of the subcutaneous chamber. After adding the thrombin component, the matrix aggregated and the vessel-loop was embedded in the cell-containing gel (Fig. 2A). After explantation, the AV-vessel loop was identified under the microscope. A CD31 staining showed an intact endothelium within the arterious portion of the AV-vessel loop (Fig. 2B). Furthermore, a highly vascularized neo-tissue was observed within the fibrin matrix 14 days after transplantation, as shown by CD31 immunhistology (IH) (Fig. 2C). Functional capillaries could also be visualized by India ink labelling (Fig. 2B). There was no difference between both AV loop groups in terms of distribution and density of newly grown blood vessels.
Fig 2.
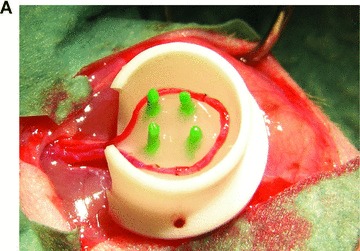
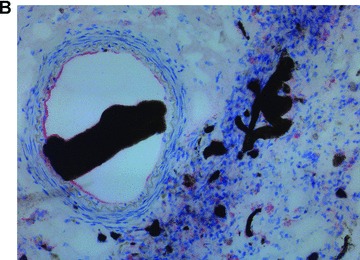
Neo-vascularization within the explanted neo-tissue at day 14 after transplantation. (A) Transplantation situs of the arterio-veno-venous vessel loop within the subcutaneous chamber loaded with FLC in the fibrin-matrix (4×). (B) CD31 staining 10 × 10. The staining for the endothelial cell marker CD31 showed an intact endothelial layer in the central AV-loop vessel. (C): CD31 staining 10 × 10. The CD31 staining confirmed an intact endothelium of numerous small capillaries formed 14 days after transplantation within the fibrin-matrix in the neo-tissue.
Foetal liver cell transplantationwithin the fibrin matrix
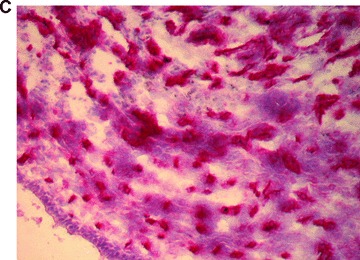
In total, five animals were transplanted with the foetal liver cell–fibrin gel construct within the AV-loop model (group A). At autopsy there was no evidence of any complication related to the surgical procedure. At time of explantation there were no signs of any severe side effects, the subcutaneous chambers appeared intact, and there were no adhesions or signs of infection. After 2 weeks, the fibrin matrix was still visible in the subcutaneous chambers. Transplanted cells could be detected by their positive pkh-26 staining using fluorescence microscopy (Fig. 3A) mainly in proximity of the major vascular axis. The cells formed three-dimensional cell conglomerates within the fibrin matrix (Fig. 3B, haematoxylin and eosin staining). Microscopically, the cells showed a characteristic polygonal cell shape with a high density of cell-to-cell contacts. The nuclei are prominent with nucleoli (Fig. 3C, haematoxylin and eosin, detail in higher magnification Fig. 3D). In addition, a close relationship of three-dimensional tissue formation with the vascularized areas in the transplant was observed (Fig. 2B, India ink staining). In groups B and D no pkh26-positive cells could be seen at any time. However, the aggregation of the matrix and ingrowth of fibrous tissue was observed from the opening of the transplantation chamber. Furthermore, also the ingrowth of capillaries was observed, however in a low density and limited only to the opening of the chamber.
Fig 3.
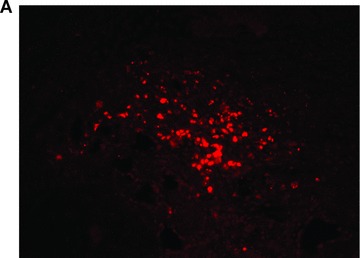
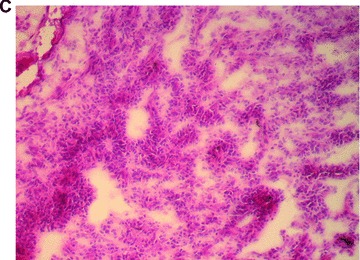
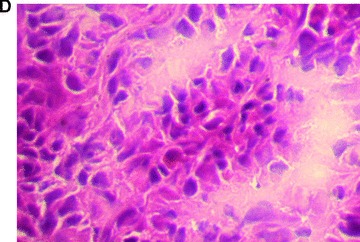
Morphology of engrafted foetal liver cells (FLC) in the explanted neo-tissue at day 14 after transplantation. (A) Fluorescence microscopy revealed the pkh+ transplanted FLC within the neo-tissues (10 × 4). (B) Haematoxylin and eosin staining (10 × 4) of a serial section showed the engraftment of cell groups in close relationship to the neo-capillarization (seen by India ink staining). (C) Haematoxylin and eosin staining 10 × 10. Higher magnification of cell groups demonstrated that polygonal shaped cells with prominent nuclei formed a neo-tissue within the fibrin matrix in vicinity to the central AV-loop vessel. (D) Haematoxylin and eosin staining, detail of 3C (10 × 20). Foetal hepatocytes formed a three-dimensional tissue permitting high cell density and cell-to-cell contacts within the matrix in the neo-tissue.
Differentiation markers of transplanted FLC within the AV-loops
IH staining demonstrated engraftment of CK-18 positive FLC within the transplanted subcutaneous chamber (Fig. 4A, CK-18 IH). Although only a small number of CK-18 positive cells could be detected, RT-PCR analysis showed CK-18 mRNA expression within the transplanted tissues after 14 days and thus confirmed the results of the CK-18 staining (Fig. 4B, agarose gel with CK-18 mRNA signal). Expression of CK-18 mRNA was not detected in cell-free controls (groups B and D) and in groups with FLC transplanted in the subcutaneous chambers (group C).
Fig 4.
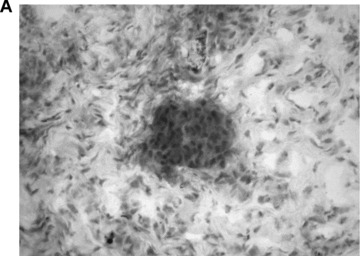
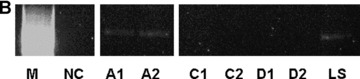
Liver-specific CK-18 expression in the explanted neo-tissue at day 14 after transplantation. (A) Immunohistochemistry for CK-18 showed specific expression of this hepatocytic marker in the transplanted foetal liver cell groups (FLC, magnification 10 × 20). (B) RT-PCR analysis of specific gene expression within the neo-tissues confirmed CK-18 mRNA expression within the AV-loop transplantation group 14 days after transplantation (A1, A2). Legend: NC: Negative control without template; LS: Adult hepatocytes; Group A (A1, A2): transplantation of FLC in AV-loop; Group C (C1, C2): FLC transplanted in subcutaneous chamber; Group D (D1, D2): Subcutaneous groups without FLC.
Discussion
In this study, we investigated engraftment and differentiation of fibrin gel-immobilized FLC in a vascularized tissue-engineering model. The high density and functionality of capillaries grown into the fibrin matrix after transplantation was demonstrated by India ink injection. CD31 is an endothelial marker, which was expressed within the main vessel loop as well as within the capillaries showing an intact endothelial differentiation of the neo-vascularization.
Thus, the in vivo data of our study indicate that the AV-loop transplantation model provides a beneficial environment for the rapid high-density neo-vascularization which was regularly around the axis of the central AV-loop vessel in the three-dimensional neo-tissue. Cells formed a three-dimensional neo-tissue, which was located well in the vicinity of the capillary network within the transplantation construct. However, large areas with no detectable cell engraftment were present. The cell loss may be due to a sub-optimal nutrition and oxygenation of the transplanted cells in areas with a larger distance to the central AV-loop blood vessel. Limited supply for the cells in the centre of three-dimensional high-density cultures under static conditions in cultures as well as in three-dimensional transplanted tissues is a well-known problem [24]. Importantly, in this study the transplantation of the cells and the creation of the central vascular axis were performed at the same time. After 14 days a high-density axial microvasculature could be detected within the three-dimensional cell/matrix constructs. The transplantation efficiency may be dramatically improved by a capillary network formation prior to transplantation of tissue-specific cells. This pre-vascularization strategy will be employed in future studies aiming on generation of larger volumes of vascularized liver tissue since the high-density intrinsic axial neo-vascularization achieved by the AV-loop technology seems to be a very promising novel approach towards sufficient engraftment and long-term viability of transplanted hepatocytes. Even implantation of in vivo grown units of axially vascularized bioartificial liver tissue into the portal circulation following microsurgical anastomosis of the pedicle vessels might be technically feasible. This setting would eventually allow hepatotrophic stimulation of the bioartificial liver tissue for optimal growth and differentiation of the transplanted cells.
In our transplantation model we could observe sufficient engraftment of FLC within the fibrin matrix in the AV-loop, which formed a three-dimensional neo-tissue. A significant proportion of the engrafted cells showed a polygonal shape, a big nucleus with prominent nucleoli which fitted well with the morphology of immature liver cells. Furthermore, we were able to demonstrate the expression of the liver epithelial cell-specific cytokeratin 18 by immunohistochemistry and RT-PCR in the engrafted cells, though the number of animals in the cell transplantation group was low. Although a certain number of the transplanted cells showed a cell-specific differentiation towards hepatocytic cells within the transplant constructs, some of the transplanted cells displayed a less differentiated phenotype. This phenomenon might be related to a lack of hepatotrophic stimulation at the heterotopic transplantation site [25]. Induction of hepatocytic differentiation in larger proportions of the transplanted FLC is subject of ongoing research in our laboratory. Co-transplantation of other cell types which potentially induce differentiation, e.g. pancreatic islets, or application of known hepatotrophic substances like insulin or HGF using modern drug release systems are conceivable scenarios [18].
In a previous study, we showed that the injection method using a fibrin in situ setting matrix is technically feasible without significant safety problems or serious side effects [17]. Furthermore, as our histological examination directly after transplantation showed, fibrin glue organized into a three-dimensional fibre network. The three-dimensional orientation of the cell-matrix mixture might add a further beneficial stimulus for tissue formation and function of the transplanted cells, as demonstrated for the transplantation of hepatocytes [16, 17]. In addition, own in vitro data showed the expression of hepatocyte-specific markers in cultures of adult hepatocytes within a three-dimensional fibrin matrix even under static conditions over the whole observation period [17]. Thus, fibrin matrix, which is already being used as a carrier for transplantation of adult hepatocytes in several experimental settings, is apparently also applicable for transplantation of FLC and might support differentiation into mature hepatocytes.
The use of hepatic stem cells may become important for the improvement of tissue-engineering approaches and cell-based therapeutic strategies for liver diseases. Experimental treatment of liver diseases by repopulation of diseased livers with healthy hepatocytes [26], or hepatocyte transplantation [4], tissue engineering of the liver [27] and ex vivo gene therapy have achieved promising results in animal models [28]. For such approaches hepatic stem cells may provide several advantages over hepatocytes: (i) Stem cells could be easily expanded, e.g. during a culture period, and (ii) transduction of stem cells may result in the expansion of ‘cured’ daughter cells [29]. In a study of foetal liver cell transplantation intraportally for liver repopulation, a hepatic differentiation potential even of early FLC (ED 14) was shown [8]. In this study, we used FLC from ED16. The cells were used after cryopreservation. Our data in cell cultures showed no significant impairment of cell growth in vitro after cryopreservation. Furthermore, we demonstrated the expression of the liver cell-specific CK-18 in cultures of FLC before and after cryopreservation. In our previous study, we also were able to demonstrate the expression of the liver cell-specific markers AFP and albumin in cultures of FLC [7]. These cells should possess a secure differentiation potential towards mature hepatocytes, furthermore, as shown by own experiments, these cells can be easily expanded in cultures [7]. Thus, FLC showed the possibility of cell banking by cryopreservation in this study. However, the use of foetal cells may be more challenging considering the hepatocyte-specific differentiation in comparison to adult hepatocytes. Hence, the enormous growth potential recommends these cells as advantageous compared with adult hepatocytes regarding a limited cell/organ resource.
In summary, FLC can be successfully used for heterotopic hepatocyte transplantation. Fibrin matrix is an appropriate environment for hepatocyte transplantation and permits rapid blood vessel ingrowth for the supply of the transplanted cells in an AV-loop transplantation model. Therefore, the combination of the use of FLC with a high growth potential in combination with a successful three-dimensional transplantation model using the AV-loop technology providing a high-density capillary network seems to be a novel and very promising approach for further development of a tissue-engineered implantable liver support system for a future clinical use.
Acknowledgments
The authors would like to thank Mrs. G. Scholz, Mrs. B. Roth and the medical students Mr. D. Schultze and Mrs. K. Straβburger for technical assistance.
References
- 1.Keeffe EB. Liver transplantation: current status and novel approaches to liver replacement. Gastroenterology. 2001;120:749–62. doi: 10.1053/gast.2001.22583. [DOI] [PubMed] [Google Scholar]
- 2.Harper AM, Rosendale JD. The Unos OPTN waiting list and donor registry: 1988–1996. Clin Transpl. 1996;10:69–90. [PubMed] [Google Scholar]
- 3.Malhi H, Gupta S. Hepatocyte transplantation: new horizons and challenges. J Hepatobiliary Pancreat Surg. 2001;8:40–50. doi: 10.1007/s005340170049. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–9. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 5.Fiegel HC, Park JH, Lioznov MV, et al. Characterization of cell types during rat liver development. Hepatology. 2003;37:148–54. doi: 10.1053/jhep.2003.50007. [DOI] [PubMed] [Google Scholar]
- 6.Fiegel HC, Kluth J, Lioznov MV, et al. Hepatic lineages isolated from developing rat liver show different ways of maturation. Biochem Biophys Res Comm. 2003;305:46–53. doi: 10.1016/s0006-291x(03)00662-4. [DOI] [PubMed] [Google Scholar]
- 7.Fiegel HC, Bruns H, Höper C, et al. Cell growth and differentiation of different hepatic cells isolated from fetal rat liver in vitro. Tissue Eng. 2006;12:123–30. doi: 10.1089/ten.2006.12.123. [DOI] [PubMed] [Google Scholar]
- 8.Oertel M, Menthena A, Chen YQ, et al. Comparison of hepatic properties and transplantation of Thy1+ and Thy1- cells isolated from embryonic day 14 rat fetal liver. Hepatology. 2007;46:1236–45. doi: 10.1002/hep.21775. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.Uyama S, Kaufmann PM, Takeda T, et al. Delivery of whole liver-equivalent hepatocyte mass using polymer devices and hepatotrophic stimulation. Transplantation. 1993;55:932–5. doi: 10.1097/00007890-199304000-00044. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert JC, Takada T, Stein JE, et al. Cell transplantation of genetically altered cells on biodegradable polymer scaffolds in syngeneic rats. Transplantation. 1993;56:423–7. doi: 10.1097/00007890-199308000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Kneser U, Kaufmann PM, Fiegel HC, et al. Long-term differentiated function of heterotopically transplanted hepatocytes on three dimensional polymer matrices. J Biomed Mater Res. 1999;47:494–503. doi: 10.1002/(sici)1097-4636(19991215)47:4<494::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann PM, Heimrath S, Kim BS, et al. Highly porous polymer matrices as a three-dimensional culture system for hepatocytes. Cell Transplant. 1997;6:463–8. doi: 10.1177/096368979700600505. [DOI] [PubMed] [Google Scholar]
- 14.Mooney DJ, Park S, Kaufmann PM, et al. Biodegradable sponges for hepatocyte transplantation. J Biomed Mater Res. 1995;29:959–65. doi: 10.1002/jbm.820290807. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Cusick RA, Browne F, et al. Local delivery of basic fibroblast growth factor increases both angiogenesis and engraftment of hepatocytes in tissue-engineered polymer devices. Transplantation. 2002;73:1589–93. doi: 10.1097/00007890-200205270-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gwak SJ, Choi D, Paik SS, et al. Stable hepatocyte transplantation using fibrin matrix. Biotechnol Lett. 2004;26:505–8. doi: 10.1023/b:bile.0000019558.99209.84. [DOI] [PubMed] [Google Scholar]
- 17.Bruns H, Kneser U, Holzhuter S, et al. Injectable liver: a novel approach using fibrin gel as a matrix for culture and intrahepatic transplantation of hepatocytes. Tissue Eng. 2005;11:1718–26. doi: 10.1089/ten.2005.11.1718. [DOI] [PubMed] [Google Scholar]
- 18.Kneser U, Kaufmann PM, Fiegel HC, et al. Heterotopic hepatocyte transplantation utilizing pancreatic islets co-transplantation for hepatotrophic stimulation: morphologic and morphometric analysis. Pediatr Surg Int. 1999;15:168–74. doi: 10.1007/s003830050547. [DOI] [PubMed] [Google Scholar]
- 19.Kneser U, Polykandriotis E, Ohnolz J, et al. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 20.Polykandriotis E, Arkudas A, Horch RE, et al. Autonomously vascularized cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med. 2007;11:6–20. doi: 10.1111/j.1582-4934.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach AD, Arkudas A, Tjiawi J, et al. A new approach to tissue engineering of vascularized skeletal muscle. J Cell Mol Med. 2006;10:716–26. doi: 10.1111/j.1582-4934.2006.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kneser U, Stangenberg L, Ohnolz J, et al. Evaluation of processed bovine cancellous bone matrix seeded with syngenic osteoblasts in a critical size calvarial defect rat model. J Cell Mol Med. 2006;10:695–707. doi: 10.1111/j.1582-4934.2006.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;188:745–53. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann PM, Sano K, Uyama S, et al. Heterotopic hepatocyte transplantation: assessing the impact of hepatotrophic stimulation. Transplant Proc. 1994;26:2240–1. [PubMed] [Google Scholar]
- 26.Grompe M. Liver repopulation for the treatment of metabolic diseases. J Inherit Metab Dis. 2001;24:231–44. doi: 10.1023/a:1010375203539. [DOI] [PubMed] [Google Scholar]
- 27.Fiegel HC, Lange C, Kneser U, et al. Fetal and adult liver stem cells for liver regeneration and tissue engineering. J Cell Mol Med. 2006;10:577–87. doi: 10.1111/j.1582-4934.2006.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raper SE. Hepatocyte transplantation and gene therapy. Clin Transplant. 1995;9:249–54. [PubMed] [Google Scholar]
- 29.Feldmann G. Liver transplantation of hepatic stem cells: potential use for treating liver diseases. Cell Biol Toxicol. 2001;17:77–85. doi: 10.1023/a:1010954020488. [DOI] [PubMed] [Google Scholar]


