Abstract
Bone fractures, where the innate regenerative bone response is compromised, represent between 4 and 8 hundred thousands of the total fracture cases, just in the United States. Bone tissue engineering (TE) brought the notion that, in cases such as those, it was preferable to boost the healing process of bone tissue instead of just adding artificial parts that could never properly replace the native tissue. However, despite the hype, bone TE so far could not live up to its promises and new bottom-up approaches are needed. The study of the cellular interactions between the cells relevant for bone biology can be of essential importance to that. In living bone, cells are in a context where communication with adjacent cells is almost permanent. Many fundamental works have been addressing these communications nonetheless, in a bone TE approach, the 3D perspective, being part of the microenvironment of a bone cell, is as crucial. Works combining the study of cell-to-cell interactions in a 3D environment are not as many as expected. Therefore, the bone TE field should not only gain knowledge from the field of fundamental Biology but also contribute for further understanding the biology of bone. In this review, a summary of the main works in the field of bone TE, aiming at studying cellular interactions in a 3D environment, and how they contributed towards the development of a functional engineered bone tissue, is presented.
Keywords: Bone, tissue engineering, cell-to-cell interactions, co-cultures, osteogenesis, vascularization, osteoblast, chondrocyte, osteoclast, stem cells
Introduction
Bone tissue is a highly specialized tissue that has as main function the structural support of the body. Moreover, it also functions as a mineral reservoir of the body, a protection for the internal organs and is involved in motion and load bearing. Taking in account the functional importance of this tissue, one may infer that any bone injury poses a high impact on the quality of life of an individual. In the United States alone, there are about 8 million bone fractures/year of which 5–10% represent cases of healing delay or non-union fractures [1, 2] that require the enhancement of the bone tissue innate regenerative capacity. In order to tackle these issues, tissue engineering (TE) presents itself as a phenomenal tool. TE has been defined [3] as an interdisciplinary area that combines the knowledge of the engineering and life sciences fields for the creation of functional constructs that improve, maintain or restore the function of a given tissue. The current TE paradigm encompasses the application of three basic elements: appropriate cells, a 3D polymeric matrix that supports cell growth, and growth factors that provide cells an adequate chemical environment [4, 5]. Nonetheless, in order to apply these principles to such a special tissue like bone it is imperative to be sensitive to bone biology aiming at understanding how its correct function is achieved. Clearly, fundamental in this process is to recognize the complex biochemical environment that surrounds cells in bone tissue and within which cellular interactions play a pivotal role. This review focuses on the importance of cell-to-cell interactions in the context of bone tissue engineering first by looking at basic bone biology and then at what has been done to study those interactions. Finally, several co-culture models are analysed in the frame of the different strategies used in bone TE.
Bone biology
In the adult skeleton, bone tissue presents two different architectural forms: the trabecular bone [6–9], with 50–90% porosity, represents approximately 20% of the skeleton and can be found in the metaphysys of long bones and in vertebral bodies; and the cortical bone [6–9], an almost solid form of bone with a low porosity that represents 80% of the skeleton.
Osteoblasts, osteocytes and osteoclasts are the three main cell types that can be found in bone tissue, having each one of these defined functions crucial for bone homeostasis [10–14]. Osteoblasts, anchorage-dependent cells, are highly responsive to mechanical and chemical stimulus that are relayed through multiple cell-to-matrix and cell-to-cell interactions [15, 16]. These interactions are mediated through specific receptors and transmembranous proteins such as integrins, cadherins and connexins leading to bone’s extracellular matrix production and mineralization [15–19]. As matrix is deposited and calcified, some osteoblasts become entrapped in it and achieve their fully differentiated state, becoming osteocytes. These are the most abundant cells in bone. They are smaller and rounder than osteoblasts and present a high number of filopodia that permit homotypic connections as well as interactions with bone-linning osteoblastic cells. These connections form a 3D network that is believed [13, 20, 21] to function as a mechanical stimuli transduction system and as a general regulator of bone homeostasis. Nonetheless, osteoclasts are also seen as critical players in this regulatory process. They are multinucleated and highly specialized cells, derived from the haematopoietic lineage, that have the function of resorbing bone by creating a tight seal resorption pit at the bone surface [22–24]. In these pits, osteoclasts create an acidic environment and secrete lytic enzymes that become activated at low pH.
The exact mechanisms involved in bone homeostasis are still far from being well understood; however, two routes are currently accepted to be responsible for skeletal formation and maintenance: bone modelling and bone remodelling [25–27]. The modulation of skeleton geometry during growth, in order to reach the optimal geometry to fulfil the requirements of bone function, is referred to as bone modelling [25–27]. This process progresses by selective bone resorption or formation at specific sites. Osteoclastic activity is regulated independently of osteoblastic activity, i.e. bone formation never occurs where bone is being resorbed [25–27]. In opposition, in bone remodelling [25–27], osteoclastic activity is strictly coupled with osteoblastic activity. Bone forming and bone resorbing activities occur in a coordinated manner, so that the amount of produced bone balances the amount of resorbed bone [27]. This coupling of bone formation and bone resorption is spatially enclosed within specialized anatomic structures called basic multicellular units (BMUs) [27–30]. These temporary structures, mainly formed by osteoblasts and osteoclasts, exert their action in three sequential phases that, overall, constitute a bone remodelling cycle: activation (of remodelling activity in the target area), resorption and formation [27–30]. It is currently accepted [27, 29, 30] that BMUs progress through the bone in a 3D way, forming tunnels in the cortical bone or trenches in the trabecular bone. Spatial control of the BMUs, in terms of target area selection and movement, is thought to be controlled by the osteocytes [27, 29, 30] since the sensitivity of these cells to mechanical stimulus in bone tissue, as well as their organization in a network represent significant properties for the perfect conduction of the bone remodelling process [27, 29, 30]. Bone vasculature is also decisive in the remodelling process. In addition to the demonstration of an intimate relation between new blood vessels and osteogenesis [31–33], it was already proved that every BMU is located in the vicinity of a blood vessel [34], which grows at the same rate the BMU advances [34, 35]. These facts indicate the crucial role of vasculature in bone remodelling and consequently the importance of vascular cells such as endothelial cells and pericytes. The most likely role of blood vessels as a source of biochemical signals and cells, and as a major player in the coupling of bone resorption and formation during bone remodelling is explored elsewhere [36].
The relevance of cell–cell interactions in bone tissue engineering
Taking in consideration the biology of bone tissue, it is quite clear that the understanding of the cellular interactions that regulate the homeostasis and regeneration of this remarkable tissue is essential to a successful TE strategy. The study of these cellular interactions in vitro relies on co-culture systems, a tremendously useful methodology where two or more cell types are cultured at the same time. It increases the complexity of typical cell culture systems, allowing the in vitro settings to closely mimic the in vivo environment. 2D co-culture systems have been extensively used by cell biologists to study cell interactions as an attempt to understand specific cellular mechanisms and pathways. In this part of this review, the importance of cell-to-cell interactions and how these are studied in the bone TE field are discussed and summarized in Fig. 1.
Fig 1.
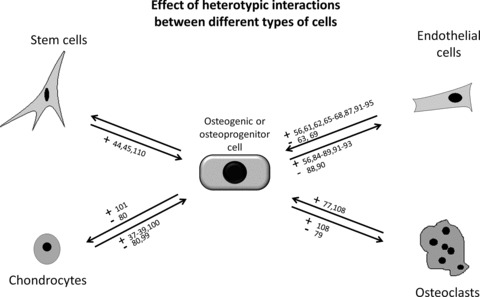
Positive (+) and negative (–) effects of cell-to-cell interactions between different cell types relevant in bone biology. Cell differentiation, function and proliferation were the reviewed parameters.
The self-renewal and multilineage differentiation abilities of stem cells (SCs) render these cells as the potential ultimate source of cells to create tissue-like constructs for TE, including bone TE. Subsequently, several studies [37–43] regarding cellular interactions relevant for bone TE involve, in addition to osteoblasts, different populations of SCs obtained from various origins and species. However, these studies have been performed mainly to assess the differentiation potential of SCs towards the osteogenic lineage by establishing several co-culture systems. Mature cells like chondrocytes [37–39], macrophages [40], endothelial cells [42] or myeloma cells [41] have all been proven to promote the osteogenic differentiation of mesenchymal SCs (MSCs), either by direct or indirect interactions. Co-culture with osteoblasts was also reported as a method to direct the differentiation of embryonic stem cells (ESCs), either from mouse or human origin, into the osteogenic lineage [44, 45]. However, a major issue that many tissue engineers fail to address is the atypical osteoblastic function of osteoblastic cells in culture when compared with osteoblasts in vivo. This is reflected on the quality of bone formed by osteoblasts in vitro[46–48], either in a 3D or a 2D environment, which is undoubtedly a major drawback when envisaging a possible clinical application.
Another very important issue that greatly affects the performance of a TE construct is the vascularization issue since an insufficient blood supply in implanted engineered tissues will determine their failure [49–52]. Vascularization is regarded as essential in TE in general and in bone TE in particular [53, 54]. In the usual bone TE strategy, where bone cells are seeded and cultured in scaffolds before implantation, a recurrent find points out to cell death at the bulk of the scaffold by hypoxia [49, 50, 55]. It became clear that the production of a vascular network that could perfuse the engineered constructs is essential. So far, the proposed solution relies on the creation of a blood vessel network within an engineered tissue prior to transplantation by incorporating cells that will lead to blood vessel formation within the scaffold matrix [56–59] and subsequent engraftment with the host tissue. Moreover, the extent of knowledge regarding the interactions between endothelial cells and bone forming or osteoprogenitor cells indicates the existence of reciprocal interactions between both types of cells that are essential to their normal function [60–69]. It is therefore logical that the interaction of endothelial cells and osteogenic or osteoprogenitor cells is a critical issue to be explored by bone tissue engineers.
As described in the previous section, bone modelling and remodelling processes rely in the specific crosstalk between osteoblasts and osteoclasts. It is, for example, well established that osteoblasts are deeply involved in the formation of osteoclasts as well as in their correct functioning [47, 70–74] but the effects of osteoclasts over osteoblastic function are still poorly understood. Studies done with osteoclast-deficient mice models have shown not only a lack of bone resorption activity but also deficient osteoblastic activity, which is reflected in the quality of the formed bone [75, 76]. Dai et al. [75] showed that implanting bone buds from osteoclast deficient mice into wild type mice resulted in the recovery of the buds’ normal development. This work presents a major indication that engineering bone tissue for clinical use may require the use of osteoclasts along with osteoblasts. Therefore, compelling evidence suggests that osteoclasts have functions, namely regulatory functions, other than just bone resorption, which is reviewed elsewhere [77]. Additionally, the vital importance of cell-to-cell interactions for bone homeostasis has been also reinforced by other works. Indications regarding the production of pro-osteogenic factors by osteoclasts were given in a study that proved that osteoclast-conditioned medium induced bone nodule formation by murine MC-3T3-E1 pre-osteoblasts [78]. Increasing attention has also been given to the signalling action between ephrinB2 cell surface protein, present in osteoclasts, and its receptor EphB4, present in osteoblasts [79]. It was shown that ephrinB2-EphB4 signalling is bidirectional and links two key molecular mechanisms of differentiation, one osteogenic and the other osteoclastogenic [79]. This is of major importance since it describes a mechanism where both cells influence the differentiation of one another, possibly in simultaneous, by cell–cell contact.
Additional cues to create a successful bone TE construct might be provided by the endochondral ossification process in which chondrocytes produce a cartilagineous anlage that is mineralized by osteoblasts and osteocytes later in the process. A logic analysis would consider the high probability of chemical factors that enhance osteogenic activity and mineralization being produced by chondrocytes. Nonetheless, a study by Jiang et al.[80] shows that in spite of an increase in alkaline phosphatase (ALP) activity in osteoblasts co-cultured with chondrocytes, their mineralization ability was diminished when compared to controls. This negative effect seemed however restricted to fully differentiated osteoblasts, which is in accordance with other works in the literature that describe chondrocytes as having a positive effect on the osteogenic differentiation of MSCs [37–39]. Thus, in a bone TE strategy based on the use of chondrocytes, it may be beneficial to use MSCs or other osteoblastic precursors instead of fully differentiated osteoblasts.
Therefore, creative ways to ensure the clinical success of engineered constructs are needed. The study of the interactions between cell types relevant for bone TE is a powerful tool in the design of tissue-engineered bone constructs. But how is this knowledge applied by bone tissue engineers?
Co-culture models in bone tissue engineering
Angiogenesis
As mentioned before, vascularization is a critical issue for TE constructs aiming at regenerating tissues. It has been demonstrated that the in vivo implantation of a cellularized construct without the production of an adequate vascular network leads to cell necrosis in the bulk of the construct [49–52]. Models such as the arteriovenous loop (AVL) model [81–83] propose a very good strategy to overcome this problem. In this model, an AVL is inserted in an isolation chamber together with a scaffold or matrix adequate for bone TE with the objective to induce vascularization in the latter [83]. Using this model, Arkudas and colleagues [82] injected rat osteoblasts into non-vascularized processed bovine cancellous bone (PBCB) matrices and into similar matrices that were prevascularized using the AVL method. Both matrices were then implanted sub-cutaneously in rats. The prevascularized show by far the best results in terms of osteoblasts survival and expression of osteogenic genes, thus stressing the importance of the vascularization issue in bone TE.
3D co-cultures established in scaffolds have been proposed by tissue engineers as a powerful tool to overcome this question [56, 84–95]. Two works reported that co-culturing endothelial cells with human bone marrow MSCs (hBMSCs) in poly-lactic-glycolic acid (PLGA) scaffolds increased bone formation after implantation in a critical size rat calvarial defect compared to implanted scaffolds only seeded with hBMSC [94, 95]. These studies emerged as a proof-of-concept that co-culturing endothelial cells with osteogenic or osteogenic progenitor cells in a 3D scaffold can be an adequate strategy for targeting the vascularization of tissue-engineered bone. Several other works [56, 84–95] have been reporting the co-cultivation of endothelial cells with osteogenic or osteoprogenitor cells for bone TE purposes. Rouwkema et al.[87] showed that co-culturing human umbilical vein endothelial cells (HUVECs) with hMSCs in a spheroid aggregate model up-regulated ALP expression of the hMSCs in comparison with monocultured spheroids. On the other hand, Kyriakidou et al.[91] found that co-culturing HUVECs with a human osteoblast-like cell line, MG-63, in porous poly(ɛ-caprolactone) (PCL) scaffolds under dynamic conditions favoured the proliferation of both types of cells but not their function. ALP activity and ECM production of the osteoblast-like cells did not show a significant enhancement in comparison to the controls. More interestingly, Yu et al.[92, 93] reinforced the potential of osteoblast/endothelial cell 3D co-cultures for bone tissue engineering purposes. ECs and osteoblasts differentiated from the bone marrow of BALB/c mice were co-cultured in hydroxyapatite-PCL (HA-PCL) scaffolds and implanted in a critical size bone defect in the femur of mice of the same strain [93]. The authors observed a dramatic increase in vascularization and bone formation in the co-culture groups in comparison with scaffolds only seeded with osteoblasts. In addition, necrosis was found to occur in the osteoblasts groups but not in the co-culture groups. Similar results were found by the same group in a work that was carried out under the same conditions but in rats [92]. Besides the enhanced bone formation and vascularization without signs of necrosis, the co-culture grafts presented better mechanical properties than those only seeded with osteoblasts.
A significant effort has also been applied to understand the effect of osteogenic or osteoprogenitor cells on the angiogenic potential of endothelial cells. Wenger et al.[90] established a 3D collagen-based co-culture system of HUVECs and human osteoblasts (hOB). In this model, cells were grown in spheroid aggregates (the result of cell’s self-aggregation in non-adhering conditions), either homogeneous, only with HUVECs, or heterogeneous, HUVECs plus hOB, and then embedded in collagen gels. It was verified that the presence of osteoblasts within the heterogeneous aggregates, diminished the formation of sprouts under angiogenic stimulus as compared with homogeneous spheroids suggesting an inhibitory effect of osteoblasts over endothelial cells activity. In contrast, Rouwkema et al.[87] developed a spheroid model where HUVECs were cultured with hMSCs. In this case, HUVECs were capable of forming a pre-vascular network that was further developed after in vivo subcutaneous implantation in nude mice. Using polyurethane (PU) cylindrical scaffolds, Hofmann et al.[85] performed in vitro co-cultures of HUVEC and hOB, having platelet-released growth factors (PRGF) as the only supplementation of the culture medium. Osteoblasts-seeded scaffolds were found to support cell proliferation and vessel formation by HUVECs in contrast with scaffolds loaded only with HUVECs. The contradictory results of some of these works suggest that this strategy is highly dependent on culture conditions such as cell seeding density, 2D or 3D culture and differentiation state of osteogenic cells.
The origin of endothelial cells appears also as an important issue. Stahl et al.[88] established a co-culture model similar to Wenger et al.[90] but of HUVECs and human umbilical cord blood endothelial progenitor cells (EPCs) and primary osteoblasts. The authors verified that osteoblasts inhibited the sprouting of HUVECs but not of EPCs. Similar results were reported in works that established co-cultures of human primary osteoblasts and HUVECs, human outgrowth endothelial cells (hOECs) and human dermal microvascular endothelial cells (hDMECs) in 2D conditions, 3D aggregates and 3D scaffolds [84, 89]. hDMECs were cultured in the presence and absence of hOB on porous discs of three different materials, beta-tricalcium phosphate (beta-TCP), HA, nickel-titanium (NiTi) and, additionally, on silk fibroin nets [89]. For all the materials, hDMECs only formed microcapillary-like structures in the presence of osteoblasts, independently of the angiogenic supplementation. When hOECs and HUVECs were cultured with and without osteoblasts either in 2D conditions or in a 3D spheroid aggregate co-culture model, a pre-vascular network was only formed by hOECs and in the presence of osteoblasts [84]. In accordance, Santos et al.[56] co-cultured hDMECs with primary human osteoblasts in starch-poly(3-caprolactone) (SPCL) fibre mesh scaffolds. Results showed that hDMECs self-assembled in a microcapilary-like structure (Fig. 2), with a lumen where cells where positive for collagen IV, a marker of endothelial basement membrane (Fig. 3). No such results were encountered in the control groups where hDMECs were cultured alone. Moreover, osteoblasts were found to produce more VEGF and their collagen I mRNA levels were significantly higher when in co-culture. This is a proof of osteoblast-endothelial cell bi-directional communication. Reinforcing this, similar results were reported [86] when hOB were co-cultured with hOEC in SPCL scaffolds and then subcutaneously implanted in SCID mice. The constructs with both types of cells revealed improved vascularization in comparison with constructs only seeded with hOEC. Thus, these works have proven that osteoblasts provide endothelial cells sufficient stimuli for them to form a network of micro-vessel like structures. More importantly, in some cases, the formation of that network was achieved without the addition of angiogenic factors to the culture medium [89]. This is probably the best demonstration on how co-cultures provide a higher complexity culture system, with self-regulation, that can be of much use in TE approaches.
Fig 2.
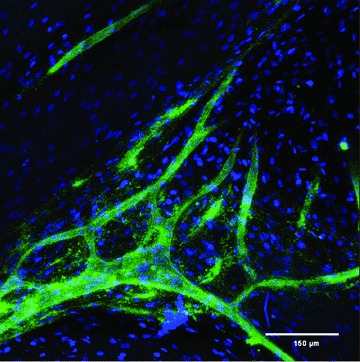
Distribution and organization of hDMECs and hOBs in co-culture on SPCL fibre mesh scaffolds after 35 days of culture. In order to distinguish between the two cell populations, samples were stained for PECAM-1(CD31; green fluorescence, endothelial-specific) and nuclei (blue fluorescence, both hOBs and hDMECs). (This picture is a kind gift of Marina I. Santos.)
Fig 3.
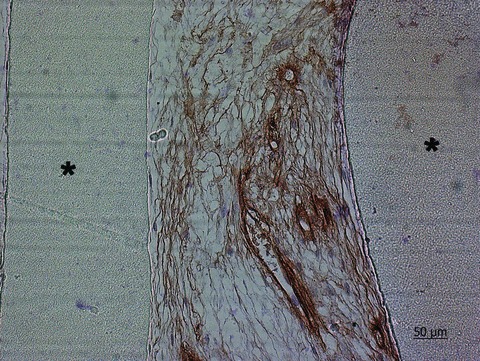
Collagen IV immunohistochemical staining of thin-section of hDMECs and hOBs in co-culture on SPCL fibre meshes after 35 days of culture. Nuclei were counterstained with Mayer’s haematoxylin. ‘*’ identifies the scaffold material. (This figure is a kind gift of Marina I. Santos.)
Overall, these approaches represent the growing awareness of bone tissue engineers to the significance of working on the vascularization issue prior to bone tissue formation, which is a natural evolution taking in account the (lack of) results delivered by typical TE approaches so far.
Osteochondral strategies
There are conditions where cartilage defects progress to the underlying sub-chondral bone, affecting, at the same time, bone and cartilage tissues [96]. In these cases, the application of osteochondral TE constructs is seen as a valid solution to regenerate both bone and cartilage tissues [97, 98]. To date, the proposed osteochondral TE constructs consist on co-culturing osteogenic and chondrogenic cells in 3D supports [99–101]. However, there are few works in the literature using osteochondral constructs that address bone formation, focusing most of them instead on the cartilage part.
Spalazzi et al.[101] studied the interactions between bovine osteoblasts and chondrocytes in poly-lactide-co-glycolide and bioactive glass (PLGA-BG) composite scaffolds and films. The authors observed that in comparison with control groups, only seeded with osteoblasts or chondrocytes, co-cultured constructs enhanced the ability of chondrocytes to maintain their normal morphology for a longer time period. The presence of osteoblasts and respective layered matrix also increased chondrocyte proliferation and matrix production. Similar results were observed by Mahmoudifar et al.[100] after co-culturing, under dynamic conditions, human foetal chondrocytes and osteoblasts in PLGA scaffolds. The presence of osteoblasts was found to improve cartilage formation in terms of glycosaminoglycans (GAG) content and total collagen deposition in comparison with no osteoblasts-containing controls. In contrast, the authors did not find significant benefit, in terms of cartilage formation, in co-culturing the chondrocytes with either bone chips or cartilage chips as possible sources of growth factors. Although the authors acknowledge that mineralization occurred in the osteoblast seeded part of the scaffold, no further analysis, like for the work by Spalazzi et al.[101], were performed concerning the osteogenic performance of the construct. The effect of chondrocytes over osteogenic cells in an osteochondral construct was explored in a work by Cao et al.[99]. Human osteogenic cells, previously differentiated form stromal cells of the iliac crest, were co-cultured with human chondrocytes, obtained from the rib cartilage, in PCL scaffolds. The presence of chondrogenic cells had a positive effect on the production of the early osteogenic marker ALP but a negative effect on the expression of the later marker, osteocalcin. Nevertheless, the mineralization of the osteochondral construct was supported by the osteogenic cells. In contrast, Jiang et al.[80] reported that culturing bovine chondrocytes and osteoblasts in micromass culture had a negative effect both on the chondrocyte and osteoblastic phenotype. Moreover, mineralization in the co-cultures was significantly diminished in comparison with monoculture controls.
These results demonstrate that chondrocytes and osteoblasts can modulate both cell types’ phenotype in tissue-engineered constructs. However, how will tissue engineers manipulate these interactions, either by controlling the culture conditions or the 3D scaffold, in order to obtain suitable osteochondral contructs, is still to be seen.
Macrophages, monocytes and osteoclasts
Macrophages, which are key players in an inflammatory process within the periprosthetic milieu, produce several cytokines and other soluble factors that affect all the other neighbouring cells [102–104] and have been often associated with implant failure. In this sense, co-cultures of monocytes and macrophages, with osteoblasts have been used in the biomaterial field as a way to elucidate the macrophages biological response to orthopaedic biomaterials particulates in a more complex system that includes bone-forming cells, osteoblasts [105–107]. The translation of the results from these works into the TE field is scarce; however, co-culturing monocytes/macrophages or monocyte/macrophages-derived osteoclasts and bone cells in 3D biodegradable matrices might be an interesting approach towards engineering high-quality bone [47]. Bone tissue engineers have not explored much this approach, existing therefore few works [108, 109] that co-cultured monocytes and stromal cells in 3D scaffolds. Domaschke et al.[108] studied the in vitro remodelling of mineralized collagen I scaffolds using co-cultures of ST-2, a mouse bone marrow stromal cell line, and human monocytes. In this work, it was demonstrated that the co-cultures induced both osteoclastogenesis and osteoblastogenesis from progenitor cells of both lineages in a process, according to the authors, comparable to bone remodelling that involved the resorbing of the scaffold by the osteoclasts and matrix mineralization by the osteoblasts.
It seems obvious that exploring this model that focuses on the balance between the degradative osteoclastic and the constructive osteoblastic activities, as well as co-culturing monocytes/macrophages and bone cells to highlight the initial bone healing mechanisms, may be of extreme use for developing successful bone tissue engineering strategies.
Stem cells
Nowadays, SCs, which are capable of differentiate into the most varied lineages of cells, are seen as an exceptionally promising tool in TE. In bone TE, there has been the tendency to differentiate those cells into the osteogenic lineage using exogenous factors, before scaffold seeding, probably due to time and cost efficiency. Nonetheless, SCs differentiation can be also directed towards the osteogenic lineage in situ using co-culture systems in a 3D environment. Kim et al.[110] showed that human ESCs committed to the osteogenic lineage by co-culture with primary cells from bone explants and seeded in poly(D,L-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds were able to support new bone formation in an immunodeficient mouse model. Although not presenting the controls with hESCs not subjected to co-culture with the bone primary cells, this work demonstrates the potential of the use of co-cultures to direct the fate of SCs. There is no doubt that using 3D co-cultures to induce the differentiation of SCs towards the osteogenic lineage will be a potentially successful path for future bone tissue engineers.
Future directions
The study of cellular interactions can provide not only the understanding of the major drawbacks that tamper bone TE evolution but also the solution to overcome those obstacles. The jump from 2D cultures to 3D cultures in the TE field was an acknowledgement that culture systems needed to emulate more closely in vivo systems. In fact, it was suggested that many of the lessons brought by 3D research in TE could be adopted to other field such as cancer research [111].
To manipulate cellular function through the use of co-cultures is nothing less than trying to mimick what happens in vivo. As this is one of the precepts of TE it should be largely embraced by tissue engineers. Combining co-cultures with 3D culture is the way to achieve higher complexity models of superior quality.
Nonetheless, the major hurdle in bone TE is and will continue to be the vascularization issue. The question ‘OSTEOGENESIS AND THE VASCULATURE: SHOULD THE SCAFFOLD COME FIRST?’ as presented by Mikos et al.[57] makes more sense than ever. The tendency in the next few years will be to make endothelial cells an indispensable component of any bone TE strategy though osteoblasts or osteoprogenitor cells will continue to be essential. Perhaps the default strategy in the future will be to co-culture, in a 3D scaffold, endothelial and osteogenic cells. Either way, co-cultures will continue to be an essential tool in basic research but if it will be so in applied research, only time will tell.
Acknowledgments
The authors acknowledge Marina I. Santos for kindly providing for the micrographs for Figs 2 and 3. Also, financial support through the PhD grant SFRH / BD / 44893 / 2008 to R.P. Pirraco by the Portuguese Foundation for Science and Technology (FCT) is acknowledged.
References
- 1.Dawson JI, Oreffo ROC. Bridging the regeneration gap: stem cells, biomaterials and clinical translation in bone tissue engineering. Arch. Biochem. Biophys. 2008;473:124–31. doi: 10.1016/j.abb.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Jordan KM, Sawyer S, Coakley P, et al. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2004;43:381–4. doi: 10.1093/rheumatology/keh045. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1:3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol. Biosci. 2004;4:743–65. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman LV, Spjut HJ, Abell MR. Bones and joints. Baltimore, MD: Williams and Wilkins; 1976. [Google Scholar]
- 7.Baron R. General principles of bone biology. 5th ed. Washington, DC: American Society of Bone and Mineral Research; 2003. pp. 1–8. [Google Scholar]
- 8.Hill PA, Orth M. Bone remodeling. J. Orthod. 1998;25:101–7. doi: 10.1093/ortho/25.2.101. [DOI] [PubMed] [Google Scholar]
- 9.Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22:2581–93. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 10.Aubin JE, Liu F. The osteoblast lineage. In: Bilezikian JPRL, Rodan GA, editors. Principles of bone biology. San Diego: Academic Press; 1996. pp. 51–68. [Google Scholar]
- 11.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–4. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 12.Mackie EJ. Osteoblasts: Novel roles in orchestration of skeletal architecture. Int. J. Biochem. Cell Biol. 2003;35:1301–5. doi: 10.1016/s1357-2725(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 13.Nijweide PJ, Burger EH, Nulend JK. The osteocyte. In: Bilezikian JPRL, Rodan GA, et al., editors. Principles of bone biology. San Diego: Academic Press; 1996. pp. 115–26. [Google Scholar]
- 14.Väänänen K. Osteoclast function: biology and mechanisms. In: Bilezikan JP, Raisz LG, Rodan GA, editors. Principles of bone biology. Vol. 1. San Diego: Academic Press; 1996. pp. 127–40. [Google Scholar]
- 15.Bennett JH, Moffatt S, Horton M. Cell adhesion molecules in human osteoblasts: structure and function. Histol. Histopathol. 2001;16:603–11. doi: 10.14670/HH-16.603. [DOI] [PubMed] [Google Scholar]
- 16.Marie PJ. Role of n-cadherin in bone formation. J. Cell. Physiol. 2002;190:297–305. doi: 10.1002/jcp.10073. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi RT. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit. Rev. Oral Biol. Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- 18.Globus RK, Moursi A, Zimmerman D, et al. Integrin-extracellular matrix interactions in connective tissue remodeling and osteoblast differentiation. ASGSB Bull.: Publ. Am. Soc. Gravit. Space Biol. 1995;8:19. [PubMed] [Google Scholar]
- 19.Stains JP, Civitelli R. Cell-cell interactions in regulating osteogenesis and osteoblast function. Birth Defects Res. Part C: Embryo Today: Reviews. 2005;75:72–80. doi: 10.1002/bdrc.20034. [DOI] [PubMed] [Google Scholar]
- 20.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–9. doi: 10.1016/s8756-3282(00)00421-x. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo C, Palazzini S, Marotti G. Morphological study of intercellular junctions during osteocyte differentiation. Bone. 1990;11:401–6. doi: 10.1016/8756-3282(90)90134-k. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki T, Hong MH, Udagawa N, et al. Expression of vacuolar h+-atpase in osteoclasts and its role in resorption. Cell Tissue Res. 1994;278:265–71. doi: 10.1007/BF00414169. [DOI] [PubMed] [Google Scholar]
- 23.Teitelbaum SL, Abu-Amer Y, Ross FP. Molecular mechanisms of bone resorption. J. Cell. Bioch. 1995;59:1–10. doi: 10.1002/jcb.240590102. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum SL, Tondravi MM, Ross FP. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J. Leukoc. Biol. 1997;61:381–8. doi: 10.1002/jlb.61.4.381. [DOI] [PubMed] [Google Scholar]
- 25.Frost HM. Intermediary organization of the skeleton. 1-2. Boca Raton: CRC; 1986. [Google Scholar]
- 26.Jee WS, Frost HM. Skeletal adaptations during growth. Triangle. 1992;31:77–88. [PubMed] [Google Scholar]
- 27.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006;8:455. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 28.Frost HM. Tetracycline-based histological analysis of bone remodeling. Calcif. Tissue Int. 1969;3:211–37. doi: 10.1007/BF02058664. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo K, Irie N. Osteoclast–osteoblast communication. Arch. Biochem. Biophys. 2008;473:201–9. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 31.Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J. Cell. Biochem. 1994;55:304–9. doi: 10.1002/jcb.240550306. [DOI] [PubMed] [Google Scholar]
- 32.Kanczler JM, Oreffo ROC. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cells Mater. 2008;15:100–14. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 33.Streeten EA, Brandi ML. Biology of bone endothelial cells. Bone Miner. 1990;10:85–94. doi: 10.1016/0169-6009(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 34.Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J. Cell. Biochem. 1994;55:273–86. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- 35.Parfitt AM. Skeletal heterogeneity and the purposes of bone remodeling: implications for the understanding of osteoporosis. In: Marcus RFD, Nelson DA, Rosen CJ, editors. Osteoporosis. 2nd ed. San Diego: Academic Press; 2008. pp. 315–29. [Google Scholar]
- 36.Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000;26:319–23. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- 37.Gerstenfeld LC, Barnes GL, Shea CM, et al. Osteogenic differentiation is selectively promoted by morphogenetic signals from chondrocytes and synergized by a nutrient rich growth environment. Connect. Tissue Res. 2003;44:85–91. [PubMed] [Google Scholar]
- 38.Gerstenfeld LC, Cruceta J, Shea CM, et al. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J. Bone Miner. Res. 2002;17:221–30. doi: 10.1359/jbmr.2002.17.2.221. [DOI] [PubMed] [Google Scholar]
- 39.Nurminskaya M, Magee C, Faverman L, et al. Chondrocyte-derived transglutaminase promotes maturation of preosteoblasts in periosteal bone. Dev. Biol. 2003;263:139–52. doi: 10.1016/s0012-1606(03)00445-7. [DOI] [PubMed] [Google Scholar]
- 40.Champagne CM, Takebe J, Offenbacher S, et al. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- 41.Karadag A, Scutt AM, Croucher PI. Human myeloma cells promote the recruitment of osteoblast precursors: mediation by interleukin-6 and soluble interleukin-6 receptor. J. Bone Miner. Res. 2000;15:1935–43. doi: 10.1359/jbmr.2000.15.10.1935. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J, Wu J, Tang R, et al. Research in use of vascular endothelial cells to promote osteogenesis of marrow stromal cells. J. Biomed. Eng. 2003;20:447–50. [PubMed] [Google Scholar]
- 43.Wang Y, Volloch V, Pindrus MA, et al. Murine osteoblasts regulate mesenchymal stem cells via wnt and cadherin pathways: mechanism depends on cell-cell contact mode. J. Tissue Eng. Regenerat. Med. 2007;1:39–50. doi: 10.1002/term.6. [DOI] [PubMed] [Google Scholar]
- 44.Buttery LDK, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 45.Hwang YS, Randle WL, Bielby RC, et al. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with hepg2-conditioned medium and modulation of the embryoid body formation period: application to skeletal tissue engineering. Tissue Eng. 2006;12:1381–92. doi: 10.1089/ten.2006.12.1381. [DOI] [PubMed] [Google Scholar]
- 46.Declercq H, Van den Vreken N, De Maeyer E, et al. Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: comparison of different isolation techniques and source. Biomaterials. 2004;25:757–68. doi: 10.1016/s0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]
- 47.Han D, Zhang Q. An essential requirement for osteoclasts in refined bone-like tissue reconstruction in vitro. Med. Hypotheses. 2006;67:75–8. doi: 10.1016/j.mehy.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Huang W, Carlsen B, Wulur I, et al. Bmp-2 exerts differential effects on differentiation of rabbit bone marrow stromal cells grown in two-dimensional and three-dimensional systems and is required for in vitro bone formation in a PLGA scaffold. Exp. Cell Res. 2004;299:325–34. doi: 10.1016/j.yexcr.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 49.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973;138:745–53. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kneser U, Kaufmann PM, Fiegel HC, et al. Long-term differentiated function of heterotopically transplanted hepatocytes on three-dimensional polymer matrices. J. Biomed. Mater. Res. 1999;47:494–503. doi: 10.1002/(sici)1097-4636(19991215)47:4<494::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 51.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J. Biomed. Mater. Res. 2000;51:376–82. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 52.Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, et al. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19:1405–12. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 53.Murphy WL, Simmons CA, Kaigler D, et al. Bone regeneration via a mineral substrate and induced angiogenesis. J. Dent. Res. 2004;83:204–10. doi: 10.1177/154405910408300304. [DOI] [PubMed] [Google Scholar]
- 54.Pelissier P, Villars F, Mathoulin-Pelissier S, et al. Influences of vascularization and osteogenic cells on heterotopic bone formation within a madreporic ceramic in rats. Plast. Reconstr. Surg. 2003;111:1932–41. doi: 10.1097/01.PRS.0000055044.14093.EA. [DOI] [PubMed] [Google Scholar]
- 55.Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002;8:529–39. doi: 10.1089/107632702760240454. [DOI] [PubMed] [Google Scholar]
- 56.Santos MI, Unger RE, Sousa RA, et al. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone–starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407–15. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–39. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mooney DJ, Mikos AG. Growing new organs. Sci. Am. 1999;280:60–7. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 59.Santos MI, Tuzlakoglu K, Fuchs S, et al. Endothelial cell colonization and angiogenic potential of combined nano-and micro-fibrous scaffolds for bone tissue engineering. Biomaterials. 2008;29:4306–13. doi: 10.1016/j.biomaterials.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 60.Brighton CT. Early histological and ultrastructural changes in medullary fracture callus. J. Bone Joint Surg. Am. 1991;73:832–47. [PubMed] [Google Scholar]
- 61.Decker B, Bartels H, Decker S. Relationships between endothelial cells, pericytes, and osteoblasts during bone formation in the sheep femur following implantation of tricalciumphosphate-ceramic. Anat. Rec. 1995;242:310–20. doi: 10.1002/ar.1092420304. [DOI] [PubMed] [Google Scholar]
- 62.Guenther HL. Endothelial cells in culture synthesize a potent bone cell active mitogen. Endocrinology. 1986;119:193–201. doi: 10.1210/endo-119-1-193. [DOI] [PubMed] [Google Scholar]
- 63.Jones AR, Clark CC, Brighton CT. Microvessel endothelial cells and pericytes increase proliferation and repress osteoblast phenotypic markers in rat calvarial bone cell cultures. J. Orthop. Res. 1995;13:553–61. doi: 10.1002/jor.1100130410. [DOI] [PubMed] [Google Scholar]
- 64.Pechak DG, Kujawa MJ, Caplan AI. Morphological and histochemical events during first bone formation in embryonic chick limbs. Bone. 1986;7:441–58. doi: 10.1016/8756-3282(86)90004-9. [DOI] [PubMed] [Google Scholar]
- 65.Villanueva JE, Nimni ME. Promotion of calvarial cell osteogenesis by endothelial cells. J. Bone Miner. Res. 1990;5:733–9. doi: 10.1002/jbmr.5650050710. [DOI] [PubMed] [Google Scholar]
- 66.Villars F, Bordenave L, Bareille R, et al. Effect of human endothelial cells on human bone marrow stromal cell phenotype: Role of vegf. J. Cell. Biochem. 2000;79:672–85. doi: 10.1002/1097-4644(20001215)79:4<672::aid-jcb150>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Villars F, Guillotin B, Amedee T, et al. Effect of huvec on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am. J. Physiol. Cell Physiol. 2002;282:775–85. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 68.Guillotin B, Bourget C, Remy-Zolgadri M, et al. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell Physiol. Biochem. 2004;14:325–32. doi: 10.1159/000080342. [DOI] [PubMed] [Google Scholar]
- 69.Thomas Meury, Sophie Verrier, Alini M. Human endothelial cells inhibit bmsc differentiation into mature osteoblasts in vitro by interfering with osterix expression. J. Cell. Biochem. 2006;98:992–1006. doi: 10.1002/jcb.20818. [DOI] [PubMed] [Google Scholar]
- 70.Bai S, Kopan R, Zou W, et al. Notch1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J. Biol. Chem. 2008;283:6509–18. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- 71.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 72.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis 1. Endocr. Rev. 2000;21:115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 73.Theoleyre S, Wittrant Y, Tat SK, et al. The molecular triad opg/rank/rankl: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–75. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Wada T, Nakashima T, Hiroshi N, et al. Rankl–rank signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Dai XM, Zong XH, Akhter MP, et al. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J. Bone Miner. Res. 2004;19:1441–51. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- 76.Sakagami N, Amizuka N, Li M, et al. Reduced osteoblastic population and defective mineralization in osteopetrotic (op/op) mice. Micron. 2005;36:688–95. doi: 10.1016/j.micron.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Karsdal MA, Martin TJ, Bollerslev J, et al. Are nonresorbing osteoclasts sources of bone anabolic activity. J. Bone Miner. Res. 2007;22:487–94. doi: 10.1359/jbmr.070109. [DOI] [PubMed] [Google Scholar]
- 78.Karsdal MA, Neutzsky-Wulff AV, Dziegiel MH, et al. Osteoclasts secrete non-bone derived signals that induce bone formation. Biochem. Biophys. Res. Commun. 2008;366:483–8. doi: 10.1016/j.bbrc.2007.11.168. [DOI] [PubMed] [Google Scholar]
- 79.Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinb2-ephb4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem. Biophys. Res. Commun. 2005;338:762–70. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 81.Polykandriotis E, Euler S, Arkudas A, et al. Regression and persistence: remodelling in a tissue engineered axial vascular assembly. J. Cell. Mol. Med. 2009;13:4166–75. doi: 10.1111/j.1582-4934.2009.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arkudas A, Beier JP, Heidner K, et al. Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng. 2007;13:1549–60. doi: 10.1089/ten.2006.0387. [DOI] [PubMed] [Google Scholar]
- 83.Kneser U, Polykandriotis E, Ohnolz J, et al. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 84.Fuchs S, Hofmann A, Kirkpatrick CJ. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577–88. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 85.Hofmann A, Ritz U, Verrier S, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–26. doi: 10.1016/j.biomaterials.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 86.Fuchs S, Ghanaati S, Orth C, et al. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2008;30:526–34. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 87.Rouwkema J, Boer JD, Blitterswijk CAV. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–93. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 88.Stahl A, Wu X, Wenger A, et al. Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: a model for bone replacement grafts. FEBS Lett. 2005;579:5338–42. doi: 10.1016/j.febslet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Unger RE, Sartoris A, Peters K, et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965–76. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 90.Wenger A, Stahl A, Weber H, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004;10:1536–47. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- 91.Kyriakidou K, Lucarini G, Zizzi A, et al. Dynamic co-seeding of osteoblast and endothelial cells on 3D polycaprolactone scaffolds for enhanced bone tissue engineering. J. Bioact. Compat. Pol. 2008;23:227–43. [Google Scholar]
- 92.Yu H, VandeVord PJ, Mao L, et al. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2008;30:508–17. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 93.Yu H, VandeVord PJ, Gong W, et al. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J. Orthop. Res. 2008;26:1147–52. doi: 10.1002/jor.20609. [DOI] [PubMed] [Google Scholar]
- 94.Kaigler D, Krebsbach PH, Wang Z, et al. Transplanted endothelial cells enhance orthotopic bone regeneration. J. Dent. Res. 2006;85:633. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 95.Kaigler D, Krebsbach PH, West ER, et al. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–7. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 96.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. J. Am. Acad. Orthop. Surg. 2006;14:90–100. doi: 10.5435/00124635-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Mano JF, Reis RL. Osteochondral defects: present situation and tissue engineering approaches. J. Tissue Eng. Regenerat. Med. 2007;1:261–73. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 98.Oliveira JM, Rodrigues MT, Silva SS, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 2006;27:6123–37. doi: 10.1016/j.biomaterials.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 99.Cao T, Ho KH, Teoh SH. Scaffold design and in vitro study of osteochondral coculture in a three-dimensional porous polycaprolactone scaffold fabricated by fused deposition modeling. Tissue Eng. 2003;9:103–12. doi: 10.1089/10763270360697012. [DOI] [PubMed] [Google Scholar]
- 100.Mahmoudifar N, Doran PM. Tissue engineering of human cartilage and osteochondral composites using recirculation bioreactors. Biomaterials. 2005;26:7012–24. doi: 10.1016/j.biomaterials.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 101.Spalazzi JP, Dionisio KL, Jiang J, et al. Osteoblast and chondrocyte interactions during coculture on scaffolds. IEEE Eng. Med. Biol. Mag. 2003;22:27–34. doi: 10.1109/memb.2003.1256269. [DOI] [PubMed] [Google Scholar]
- 102.Drees P, Eckardt A, Gay RE, et al. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat. Clin. Pract. Rheumatol. 2007;3:165–71. doi: 10.1038/ncprheum0428. [DOI] [PubMed] [Google Scholar]
- 103.Green TR, Fisher J, Matthews JB, et al. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J. Biomed. Mater. Res. 2000;53:490–7. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 104.Nakashima Y, Sun D, Trindade MCD, et al. Signaling pathways for tumor necrosis factor-a and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J. Bone Joint Surg. 1999;81:603–15. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Rodrigo A, Vallés G, Saldana L, et al. Alumina particles influence the interactions of cocultured osteoblasts and macrophages. J. Orthop. Res. 2006;24:46–54. doi: 10.1002/jor.20007. [DOI] [PubMed] [Google Scholar]
- 106.Vallés G, Gil-Garay E, Munuera L, et al. Modulation of the cross-talk between macrophages and osteoblasts by titanium-based particles. Biomaterials. 2008;29:2326–35. doi: 10.1016/j.biomaterials.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Curran JM, Gallagher JA, Hunt JA. The inflammatory potential of biphasic calcium phosphate granules in osteoblast/ macrophage co-culture. Biomaterials. 2005;26:5313–20. doi: 10.1016/j.biomaterials.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 108.Domaschke H, Gelinsky M, Burmeister B, et al. In vitro ossification and remodeling of mineralized collagen i scaffolds. Tissue Eng. 2006;12:949–58. doi: 10.1089/ten.2006.12.949. [DOI] [PubMed] [Google Scholar]
- 109.Nakagawa K, Abukawa H, Shin MY, et al. Osteoclastogenesis on tissue-engineered bone. Tissue Eng. 2004;10:93–100. doi: 10.1089/107632704322791736. [DOI] [PubMed] [Google Scholar]
- 110.Kim S, Kim SS, Lee SH, et al. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly (d, l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29:1043–53. doi: 10.1016/j.biomaterials.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 111.Hutmacher DW, Horch RE, Loessner D, et al. Translating tissue engineering technology platforms into cancer research. J. Cell. Mol. Med. 2009;13:1417–27. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


