Abstract
The colonization of the liver by colorectal cancer (CRC) cells is a complicated process which includes many stages, until macrometastases occur. The entrapment of malignant cells within the hepatic sinusoids and their interactions with resident non-parenchymal cells are considered very important for the whole metastatic sequence. In the sinusoids, cell connection and signalling is mediated by multiple cell adhesion molecules, such as the selectins. The three members of the selectin family, E-, P- and L-selectin, in conjunction with sialylated Lewis ligands and CD44 variants, regulate colorectal cell communication and adhesion with platelets, leucocytes, sinusoidal endothelial cells and stellate cells. Their role in CRC liver metastases has been investigated in animal models and human tissue, in vivo and in vitro, in static and shear flow conditions, and their key-function in several molecular pathways has been displayed. Therefore, trials have already commenced aiming to exploit selectins and their ligands in the treatment of benign and malignant diseases. Multiple pharmacological agents have been developed that are being tested for potential therapeutic applications.
Keywords: cell adhesion molecule, CD44, colorectal cancer, liver metastasis, selectin, sialyl Lewis antigen
Introduction
Colorectal cancer (CRC) is a common malignant tumour and affects about 650,000 individuals worldwide. The patients are mainly of advanced age and cases before the age of 50 are infrequent, unless for a hereditary cause. CRC is the second leading cause of cancer-related death in the ‘developed world’, killing around 205,000 individuals in Europe every year [1–3]. The prognosis and the overall life expectancy are predominantly determined by the progression of metastatic lesions and not by the primary carcinoma. The liver constitutes the main host organ for colorectal metastases and despite the progress in diagnostic modalities, more than 25% of CRC patients present with metastatic hepatic lesions at the time of initial diagnosis. Surgery remains the best therapeutic approach, although only one third are potentially resectable metastases. Curative resections may prolong survival up to 5 years in almost half of the patients. Unfortunately, if colorectal liver metastases receive no treatment, life expectancy rarely exceeds 1 year [4–6].
The development of CRC hepatic lesions is a long not completely understood process. Malignant cells at the primary site initially migrate through the endothelium of the vasculature and enter the systemic circulation (intravasation). Then, they need to survive the mechanical pressure, collisions with other cells and attacks by immune cells; some of them may reach the portal vein, which is the gateway to the hepatic sinusoids. The latter are specific capillaries which form a dense network, where important interactions among CRC and hepatic resident cells occur, including apoptosis, angiogenesis, proteolysis and adhesion [7–9].
The maintenance, promotion or disruption of cell adhesion is critical for liver colonization by CRC cells and numerous cell adhesion molecules (CAMs) are involved [10]. Selectins are present within the sinusoids, regulating CRC cell arrest and extravasation in the liver. As they are expressed on non-parenchymal hepatic cells, such as sinusoidal endothelial cells (SECs) and stellate cells, and their ligands on malignant cells, selectins stand at a molecular crossroads and play a pivotal role in CRC liver metastases [11].
Selectins
The family of selectins includes three transmembrane CAMs: E-selectins, which are expressed exclusively by endothelial cells, L-selectins present in leucocytes and P-selectins in platelets and endothelial cells. Selectin molecular structure consists of five domains: an N-terminal c-type lectin, a single epidermal growth factor like, two to nine complement binding domains, a single transmembrane and a short intracellular domain which forms a cytoplasmic tail (Fig. 1). The domain termed transmembrane anchors the molecules to the cellular surface, whereas the cytoplasmic tail supports molecular signalling processes. Notably, selectins require certain carbohydrates as connection mediators, including the P-selectin ligand glycoprotein 1 (PSGL-1) and the sialylated oligosaccharides sialyl Lewis α (sLea) and x (sLex) [12–15]. Moreover, it is well established that the fucose-generating FX enzyme is the main agent that interacts with selectin ligands, when the latter bind homologue molecules, functioning both as a reductase and an epimerase. Interestingly, terminal fucosylated glycans may present selectin ligand function in human beings. Consequently, fucosylation appears to control selectin-dependent adhesion [16, 17]. Selectins may be expressed and/or activated when certain mediators are present, such as numerous interleukins, tumour necrosis factor (TNF) or toxins [18].
Fig 1.
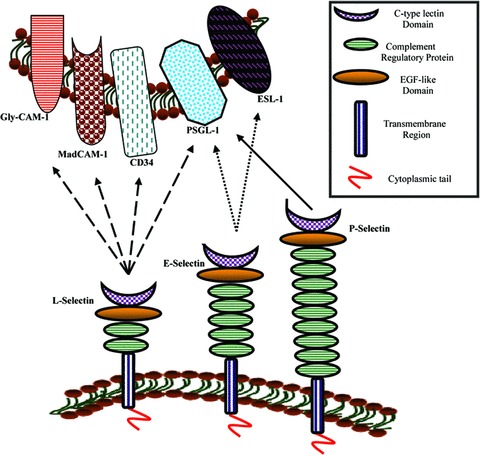
Selectins and their ligands. L-selectin ligands: Glycosylation-dependent CAM 1 (GlyCAM-1), Mucosal addressin CAM 1 (MadCAM-1), leucocyte leukosialin (CD34), P-Selectin-Glycoprotein Ligand 1 (PSGL-1). E-selectin ligands: E-Selectin Ligand 1 (ESL-1) and PSGL-1. P-selectin ligands: PSGL-1. Selectins bind their carbohydrate ligands via the c-type lectin domain in calcium-mediated manner [10, 122].
Selectins mediate tumour cell extravasation and metastasis in a similar way that they facilitate leucocyte arrest in the vasculature and migration to inflamed tissues. During the first steps of leucocyte recruitment, L-selectins expressed on T lymphocytes and other immune cells, in conjunction with P- and E-selectins on endothelial cells, interact with endothelial and leucocyte carbohydrate ligands respectively. When a primary adhesion occurs, immunoglobulin superfamily (IgSF) members, such as intercellular adhesion molecules (ICAMs) and vascular CAM 1 (VCAM-1), and integrins, are expressed to sustain a stable attachment and cellular signalling during transendothelial migration [19–21]. Following the methodology and investigating the results of leucocyte locomotion, multiple studies suggested that metastasizing CRC cells originate and maintain their adhesion to the endothelium and migrate to foreign tissue through the expression of E-, P- and L-selectins, as well as their ligands, including sLEx, sLEa and cluster of differentiation 44 variants (CD44v) [22–25]. When initial cellular bonds are successfully formed, β1 and β4 integrins in concert with IgSFCAMs are activated to further reinforce the primary selectin bonds and support signalling toward normal tissue colonization [26–28].
Importantly, in vitro studies using static models [29, 30] or flow shear stress conditions which better depict the metastatic environment [31], advocated that not only does E-selectin support primary malignant cell attachment to the endothelium, but also regulates diapedesis (the transmigration of circulating cells through the vascular endothelium) and CRC cell invasion of the hepatic parenchyma. A DNA microarray analysis revealed that E-selectin provoked gene expression alterations in metastatic CRC cells, down-regulating seven genes; the influence was 10-fold higher in comparison with primary non-metastatic cells. The cellular expression of high mobility group box 1, a chromosomal protein involved in DNA transcription and repair, was also decreased, but its free release was promoted, resulting in endothelial cell activation and E-selectin expression [32]. It was also observed that while this CAM is not present on colon cells, it is highly expressed on small blood vessels, close to metastatic colon cancer lesions [33]. Furthermore, measurements of soluble E-selectin in patients with CRC demonstrated that high serum values significantly correlated with hepatic metastases [34–36].
P-selectins play a crucial role in platelet-CRC cell interactions, as they are expressed by the former and bind to fucosylated sialylated mucin ligands of the latter. Accumulating data support that haematogenous metastasis involves platelet-malignant cell interactions. From a mechanistic point of view, platelets may form complexes with tumour cells and leucocytes and cause their arrest in the vascular wall; these cellular masses of increased volume function as emboli and are prone to entrapment in the vasculature. Subsequently, platelets appear to promote malignant cell extravasation. Also, platelets may stimulate tumour proliferation, enhance interactions with the extracellular matrix (ECM) and induce tumour growth and angiogenesis mediating the production of molecules like the platelet-derived growth factor or vascular endothelial growth factor (VEGF) [37, 38].
Experimental analysis of primary CRC tissue specimens, in comparison with secondary hepatic lesions, indicated that liver metastases were virtually deprived from P-selectin expression and leucocyte infiltration. On the contrary, primary tumours presented significantly higher levels of this CAM, as well as leucocyte intramural activity. It was concluded that P-selectin aids CRC cells to evade inflammatory reaction, promoting the metastatic process [39]. Experiments under shear flow conditions revealed that P-selectin may form the initial bonds for metastasizing cells to adhere within the vasculature [40–42]. Similar flow models showed a favourable therapeutic action of heparin against colon metastasis through blockade of P-selectin bonds [43].
Venous thromboembolism appears to affect frequently patients who receive anti-cancer treatment, compromising the quality of their life and increasing mortality. P-selectin has been identified as a reliable biomarker for this complication. Although its clinical use is still under evaluation, it appears that this CAM could be exploited in the identification of patients at high risk for venous thromboembolism, such as those under chemotherapy, who should receive thromboprophylaxis, including low molecular or unfractionated heparin [44, 45].
The in vitro experimental investigation of CRC cell kinetics in flow conditions indicated that these malignant cells interact with polymorphonuclear leucocytes and form complexes in the same order of magnitude, as they bind to platelets. Notably, L-selectin-mediated bonds appeared to be the most stable against shear stress and permitted the formation of aggregates, facilitating CRC cell arrest in the microvessels of distant organs [46]. Additionally, a synergy of P- and L-selectins during the metastatic process of colon carcinoma was observed, where P-selectins supported platelet and tumour cell interactions, while L-selectins acted in later stages of metastasis [47].
The importance of adhesion molecules in liver metastasis, including the selectins, was also experimentally highlighted through intravital microscopy. Fluorescence labelled CRC cells were injected in rodents and their circulation was observed within the liver microvasculature. It was concluded that malignant cells interacted with the sinusoidal endothelium and adhered via E- and P-selectins, ICAM-1 and VCAM-1. The role of mechanical entrapment was underestimated in these experiments, because no tumour cell arrest was observed in capillary systems with smaller diameters, such as renal, mesenteric or muscular ones. Moreover, TNF-α, a cytokine produced by stimulated Kupffer cells (KCs), appeared to promote the expression of adhesion molecules early during the metastatic process [48–50].
Carcinoembryonic antigen (CEA) may function as an auxiliary E- and L-selectin ligand and stabilize colon cells against fluid shear during their dissemination in the vasculature. Also, it was observed that CEA cooperates with CD44 variant isoforms (CD44v), which are functional ligands for P-selectin, when fluid shear stress increases. These important findings could explain the high metastatic potential of CEA-overexpressing CRC cells, through selectin-mediated molecular pathways [51].
KCs, the hepatic macrophages, present an 80 kDa CEA receptor (CEA-R), classified as β-2 adrenergic, responsible for binding and subsequent degradation of CEA [52, 53]. Experiments on murine livers, demonstrated that the stimulation of these macrophages with CEA caused the production of cytokines, such as TNF-α and IL-1β. Culture of human umbilical cord endothelial cells (HUVECs) in conditioned media from these stimulated KCs induced the expression of E-selectin by the endothelial cells and their adhesion with highly metastatic CRC cell lines [54]. The association of CEA and E-selectin through KCs and vascular endothelium is another important clue in liver metastasis research, although it should be noted that the role of KCs is not limited to cell adhesion. These cells may also exert cytotoxic activities against tumour cells or release growth factors, such as hepatocyte growth factor (HGF), and proteolytic enzymes, such as metalloproteinase 9 (MMP-9), promoting malignant cell proliferation and extravasation, as well as angiogenesis and ECM degradation [55, 56].
Selectins appear to be differentially expressed on the vascular endothelium of various tissues, as was observed for E-selectin on the rat central nervous system and the human ocular microvasculature [57, 58]. Furthermore, multiple experiments investigated E-selectin expression after TNF and IL-1 stimulation on human dermal microvascular endothelial cells and human intestinal microvascular endothelial cells in comparison with HUVECs [59–61] or on human iliac venous- and arterial venous endothelial cells [62]; they all concluded that endothelial cells of different origin express E-selectin in a dissimilar way. On the other hand, colon cancer cell lines express different selectin ligands and consequently adhere to different selectin molecules. While Colo320 cell line may link with P- and L- selectins, HT-29 only binds E-selectin and Caco-2 shows no interaction with members of the selectin family [63]. Additionally, different colon cancer cell lines present alternative adhesion kinetics to E-selectin [64]. The investigation and analysis of the preceding data concluded that selectins may play a pivotal role in the selection of the host organ for the development of distant metastases [65]. This is an interesting proposal which may substantially contribute to the explanation of the high incidence of liver colonization by CRC cells.
The association of VEGF with E-selectin was studied in murine models with sarcoma cells. It was reported that VEGF is produced by tumour cells and enhances angiogenesis through a significant up-regulation of E-selectin on vascular endothelial cells [66]. Although this interrelationship appears crucial for the development of metastases, no research data have been published on CRC. However, recent experiments on stellate cells, the liver sinusoidal fat-storing cells, showed that when activated, they express E-selectin ligand 1 (ESL-1) on their cellular membrane and that this expression could be associated with hypoxia [67]. As stellate cells may function as oxygen-sensing cells and are involved in angiogenesis, their expression of the main E-selectin ligand reveals a new cell type potentially involved in CRC liver metastasis [68, 69].
Selectins constitute a major therapeutic target for multiple maladies, such as asthma, psoriasis, endotoxemia and cancer, and various antagonists have been developed. Bimosiamose (Fig. 2) is a pan-selectin synthetic antagonist and the leading selectin inhibitor in clinical development. It has been tested in animal models and human beings for inflammatory diseases, such as asthma and chronic obstructive pulmonary disease, with favourable results and low toxicity [70–72]. Heparin also exerts anti-inflammatory as well as anti-metastatic effects, partly inhibiting L- and P-selectin binding. Although, its animal origin and heterogeneous structure limit its value and new semi-synthetic glucan sulphates were produced and administered in murine models. Phycarin sulphates were reported to block P-selectin effectively and their application as anti-inflammatory and anti-cancer drugs will be further evaluated [73–75].
Fig 2.
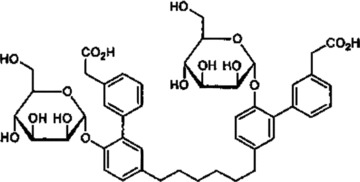
The pan-selectin antagonist bimosiamose ([hexane-1,6-diylbis [6′-(α-D-mannopyranosyloxy) biphenyl-3′,3-diyl]] diacetic acid) http://www.who.int/druginformation (WHO Drug Information Vol.15, No.3&4, 2001).
Biological engineering achieved the development of numerous anti-selectin antibodies. A promising one appears to be HuEP5C7.g2, a humanized antibody, which blocks leukaemia cell binding to E- and P-selectin positive cells and possesses favourable pharmacokinetic properties, with long circulation half-life [76, 77]. Moreover, synthetic ligands for selectin binding were in vitro tested and gold colloid particles presented high values of L- and P-selectin immobilization with no cytotoxic effect; their further assessment on animal models is on the way [78]. In general, while the inhibition of selectins appears a promising treatment for several diseases, it is still under evaluation mainly in animal models, a limited number of pharmaceutical compounds have reached small clinical trials and no reliable data exist for solid tumours, such as CRC and its metastases.
Selectin ligands
Sialyl Lewis antigens
The Lewis blood group includes multiple structurally similar carbohydrates present on erythrocytes, but also in other different tissues. It has been shown that certain sialylated Lewis antigens, membranous cell glycoproteins which end in nine carbon molecules named sialic acids, are involved in cellular adhesions with the ECM and with endothelial cell-related ligands, such as the selectins, during tumour progression. Typical sLe antigens associated with malignant diseases are sLex and sLea (Fig. 3). In the large bowel it has been discovered that both these antigens are expressed in tumours located throughout its epithelium, while sLeb and sLey are expressed only in neoplasms of the distal colon [79–81]. Glycosyltransferases constitute a wide category of enzymes that transfer monosaccharide units and include several families, such as sialyl- and fucosyltransferases. These two enzyme families are involved in neoplastic transformation and cancer development through their role in cell differentiation and adhesion. Therefore, any modification in their function may alter the status of cell connection [82–84].
Fig 3.
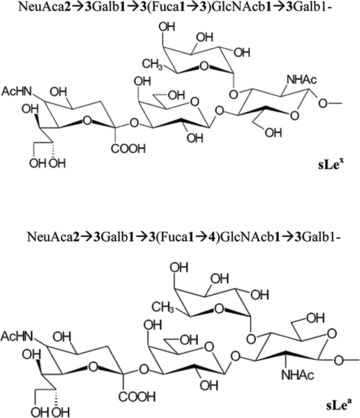
Chemical structure of sialyl Lewis x (sLex) and α (sLea) [80].
In the primary CRC with poor outcome, it was observed that both sialyl- and fucosyltransferases were up-regulated. The fucosyltransferases-3 and -4 (Fuc-TIII and Fuc-TVI) and sialyltransferase3galactosamine-4 (ST3Gal-IV) mediated sLe antigen synthesis in the colon, but were not responsible for the increased expression of these antigens in CRC. This increase should be attributed to a combinational up-regulation of multiple glycosyltransferases genes [85, 86]. Immunohistochemical studies revealed an augmented αFuc-TVI expression in human colorectal carcinomas, which was associated with lymph node metastasis and tumour stage. Notably, increased values of the enzyme correlated with the degree of tumour infiltration through the intestinal wall [87]. Murine experiments displayed that the expression of Fuc-TI induces lower levels of sLex in CRC cells, inhibiting their dissemination and thus liver metastases, due to decreased adhesion capacity to E-selectin [88]. Moreover, in colorectal liver metastases, there was a decrease in sialyltransferase levels and increase in fucosyltransferase expression [89].
The sLex antigen is a tetrasaccharide, usually attached to O-glycans on the cellular surface. It is an E-selectin ligand expressed on granulocytes and monocytes. It is well studied in CRC and appears to correlate closely with the bad prognosis of the disease. Its levels are reversely associated with survival of operated patients for primary CRC and analogous to CRC metastatic ability [90]. The same results were announced through an animal-based model of CRC liver metastases. High levels of sLex were associated with increased expression of E-selectin, cell adhesion and liver metastasis [91]. A multivariate analysis of patient records with CRC metastasis showed that sLex is an independent prognostic factor for the histologic type and the recurrence of the disease, as well as the invasion depth, in contrast with sLea that is not [92].
Sialyl Lewis α, also termed carbohydrate antigen 19–9 (CA 19–9), is most frequently linked with O-glycans on mucins of the cellular surface. On normal tissue it is restricted to ductal epithelium, but is widely expressed in multiple carcinomas including colon cancer [79, 93]. This tetrasaccharide binds to E- and P-selectin and controls the extravasation and the attachment of CRC cells to endothelium. It was also shown that down-regulation of this antigen on CRC cells via genetic modifications, substantially reduced their extravasation [94–96]. Several studies on murine models assessed the involvement of sLea in CRC liver metastases either in vitro or in vivo. It was reported that the antigen favoured the metastatic process mainly facilitating cellular adhesion. This action was also related to increased αFuc-TIII enzymatic activity [97–99]. Dabrowska et al. studied the association of Fuc-TIII with sLea synthesis in five different colon cancer cell lines and concluded in favour of a strong positive regulatory role for the enzyme [100]. Furthermore, in vitro studies demonstrated that hypoxic conditions enhanced the expression of E-selectin ligands, such as sLea and sLex, by colon cancer cells and promoted selectin-mediated cell adhesion during metastasis [101].
The clear role of sLea and sLex in CRC metastasis caused therapeutically oriented research, which targeted these molecules. Cimetidine, a histamine H2 receptor antagonist, was administered for 1 year to patients with CRC, who had undergone a curative resection and were under 5-fluorouracil treatment; significant beneficial effects were announced referring to 10-year survival. Importantly, patients highly expressing sLea and sLex presented the most favourable results. It was claimed that cimetidine could block the expression of E-selectin on vascular endothelium and thus inhibit the adhesion of CRC cell ligands [102]. Moreover, in vivo murine experiments, testing the effect of sLex analogue GSC-150 in CRC liver metastasis, reported that this agent reduced the number of metastatic nodules, inhibiting malignant cell adhesion; this was also displayed via in vitro studies on HUVECs [103]. Recently, sLea conjugates were synthesized and used as vaccines in mice, against CRC and lung cancer cells. The produced antibodies proved to be highly active and no reactivity with other sialyl Lewis antigens was detected. Further experiments will evaluate this new immunotherapy [104].
CD44 (H-CAM)
CD44 or homing-associated CAM (H-CAM) is a family of transmembrane glycoproteins, including several isoforms expressed on epithelial, endothelial and tumour cells. These isoforms differ in the extracellular domain, where combinations of 10 variant exons may occur. CD44 serves as a hyaluronan receptor, a glycosaminoglycan, responsible for cell motility and proliferation. Furthermore, through interactions with other molecules, such as osteopontin, collagen, matrix metalloproteinases and selectins, it regulates cell adhesion among endothelial and hematopoietic cells, fibroblasts and the ECM [25, 105, 106].
The wide expression of CD44 on neoplastic cells has been well studied and its predictive value has been proposed in tumour invasion, migration and angiogenesis [65]. Increased values of CD44 were associated with venous invasion in CRC and correlated, along with E-cadherin, to poor overall survival, especially in stage II [107]. Murine experiments demonstrated that it promotes tumour growth, anti-apoptosis and CRC cell motility, and its genetic suppression induces malignant cell apoptosis and migration via AKT kinase [108–110].
Various isoforms of CD44 are frequently present in advanced stages of colorectal carcinogenesis and its liver metastases. CD44 splice variant 6 (CD44v6) appears to be associated with CRC liver metastases, although there is no agreement if this glycoprotein is highly [111] or lowly [112, 113] expressed on tumour cells. Clinical and experimental studies associated CD44v6 with colon cancer stage (Dukes’ classification), hepatic metastasis and 5-year survival [114, 115]. Additional confirmation of CD44’s value in the prognosis of colonic malignancies was provided by Wang et al. who revealed that high levels of heat shock protein 72, a molecular chaperone which regulates cancer cell growth and apoptosis, were connected with high CD44v6 levels in human colonic cancer, compared to normal colon [116].
In contrast to the preceding conclusions, a clinical study on 56 patients with Dukes’ C or D CRC reported no difference of CD44v6 expression in the primary site and the metastatic lymph nodes [117]. A recent analysis of CRC metastatic lesions revealed that the complex of epithelial CAM (EpCAM), claudin-7, CD44v6 and a tetraspanin member was strongly related to poor prognosis and low disease free survival, although the solitary expression of each of these proteins showed no similar outcome [118]. Moreover, the expression of CD44v8 to -10 revealed no significant correlation with the histology, lymphatic and venous invasion of the primary CRC, but was significantly related with lymph node and haematogenous metastases. Also, patients with increased values of these ligands presented higher recurrence and lower 5- and 10-year survival rates [119]. Consequently, there is mounting evidence which connects CD44v isoforms with CRC prognosis and its migration to the liver, but this relationship is neither well defined, nor clearly demonstrated in the clinical field.
In vitro studies on multiple colon carcinoma cell lines concluded that CD44v isoforms mediate tumour cell adhesion to platelets, leucocytes and endothelial cells, through links with P-, L- and E- selectins, and fibrin. Additionally, heparin reduced cell adhesion to P- and L- selectins. As the selectin family and fibrin play a critical role in metastasis, these findings present CD44 as a potential target of future therapeutic applications [25, 120, 121].
Conclusions – future perspectives
Selectins and their ligands, such as sLex, sLea and CD44 isoforms, facilitate the metastatic process and promote CRC cell extravasation from the sinusoids. While selectins are expressed in platelets, leucocytes and SECs, their ligands are present on malignant cells and their connections mediate CRC cell apoptosis, proliferation, motility and adhesion to different cells and the ECM. Importantly, multiple cytokines released by Kupffer, stellate and other cells, are also involved in the expression of selectins and thus the cellular intercommunication within the sinusoids develops and appears more complicated (Fig. 4).
Fig 4.
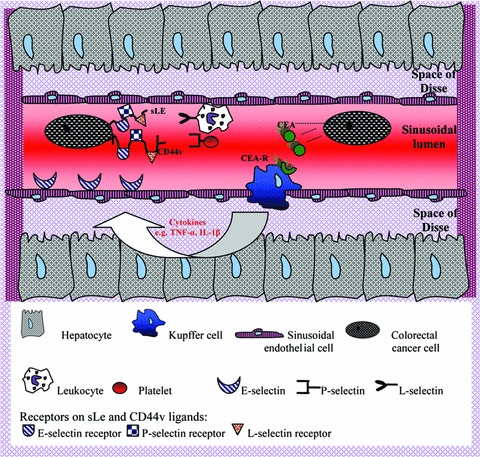
Schematic representation of CRC cell interactions with hepatic sinusoidal cells. KCs, presenting CEA receptors, may be activated through CEA molecules released by malignant cells, produce cytokines which stimulate endothelial cells to express E-selectin. Metastasizing colorectal cells also present sLe and CD44v ligands, which link with E-, P- and L-selectins. The last two molecules are expressed on platelets and leucocytes respectively. CD44v: CD44 variant, CEA: carcinoembryonic antigen, CEA-R: CEA receptor, CRC: colorectal cancer, IL-1β: Interleukin 1β, sLe: sialyl Lewis antigen, TNF-α: tumour necrosis factor α.
Effective anti-cancer treatment of the future will primarily rely on molecular analysis. In the field of CRC liver metastasis, current research has recognized the importance of selectins and their ligands. Several selectin inhibitors have been composed and tested via animal models and/or clinical trials, however progress was slow, because these CAMs are present in numerous physiological functions and their blockade could cause ample complications. Cimetidine, a well-established H2 receptor antagonist, showed significant anti-cancer action in preliminary studies with CRC patients and should be additionally studied, in conjunction with other medicines of this category. Further research on human tissue should consolidate successful therapeutic results from animal models and bigger clinical trials should evaluate the potential of old and new selectin inhibitors in the treatment of CRC liver metastases.
References
- 1.Wilkins T, Reynolds PL. Colorectal cancer: a summary of the evidence for screening and prevention. Am Fam Physician. 2008;78:1385–92. [PubMed] [Google Scholar]
- 2.Lin OS. Acquired risk factors for colorectal cancer. Methods Mol Biol. 2009;472:361–72. doi: 10.1007/978-1-60327-492-0_16. [DOI] [PubMed] [Google Scholar]
- 3.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Cho JY, Han HS, Yoon YS, et al. Outcomes of laparoscopic liver resection for lesions located in the right side of the liver. Arch Surg. 2009;144:25–9. doi: 10.1001/archsurg.2008.510. [DOI] [PubMed] [Google Scholar]
- 5.Yamane B, Weber S. Liver-directed treatment modalities for primary and secondary hepatic tumors. Surg Clin North Am. 2009;89:97–113. doi: 10.1016/j.suc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.McLoughlin JM, Jensen EH, Malafa M. Resection of colorectal liver metastases: current perspectives. Cancer Control. 2006;13:32–41. doi: 10.1177/107327480601300105. [DOI] [PubMed] [Google Scholar]
- 7.Braet F, Nagatsuma K, Saito M, et al. The hepatic sinusoidal endothelial lining and colorectal liver metastases. World J Gastroenterol. 2007;13:821–5. doi: 10.3748/wjg.v13.i6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vekemans K, Braet F. Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World J Gastroenterol. 2005;11:5095–102. doi: 10.3748/wjg.v11.i33.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudmik LR, Magliocco AM. Molecular mechanisms of hepatic metastasis in colorectal cancer. J Surg Oncol. 2005;92:347–59. doi: 10.1002/jso.20393. [DOI] [PubMed] [Google Scholar]
- 10.Haier J, Nicolson GL. The role of tumor cell adhesion as an important factor in formation of distant colorectal metastasis. Dis Colon Rectum. 2001;44:876–84. doi: 10.1007/BF02234713. [DOI] [PubMed] [Google Scholar]
- 11.Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: a review. J Surg Oncol. 2006;94:68–80. doi: 10.1002/jso.20558. [DOI] [PubMed] [Google Scholar]
- 12.Garcia J, Callewaert N, Borsig L. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology. 2007;17:185–96. doi: 10.1093/glycob/cwl059. [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. Structural basis for selectin mechanochemistry. Proc Natl Acad Sci USA. 2009;106:91–6. doi: 10.1073/pnas.0810784105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witz IP. The involvement of selectins and their ligands in tumor-progression. Immunol Lett. 2006;104:89–93. doi: 10.1016/j.imlet.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008;27:19–30. doi: 10.1007/s10555-007-9101-z. [DOI] [PubMed] [Google Scholar]
- 16.Huang MC, Zollner O, Moll T, et al. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–60. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- 17.Zipin A, Israeli-Amit M, Meshel T, et al. Tumor-microenvironment interactions: the fucose-generating FX enzyme controls adhesive properties of colorectal cancer cells. Cancer Res. 2004;64:6571–8. doi: 10.1158/0008-5472.CAN-03-4038. [DOI] [PubMed] [Google Scholar]
- 18.Borsig L. Selectins facilitate carcinoma metastasis and heparin can prevent them. News Physiol Sci. 2004;19:16–21. doi: 10.1152/nips.01450.2003. [DOI] [PubMed] [Google Scholar]
- 19.Doebis C, Siegmund K, Loddenkemper C, et al. Cellular players and role of selectin ligands in leukocyte recruitment in a T-cell-initiated delayed-type hypersensitivity reaction. Am J Pathol. 2008;173:1067–76. doi: 10.2353/ajpath.2008.080052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiese G, Barthel SR, Dimitroff CJ. Analysis of physiologic E-selectin-mediated leukocyte rolling on microvascular endothelium. J Vis Exp. 2009;pii:1009. doi: 10.3791/1009. . doi: 10.3791/1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laferriere J, Houle F, Taher MM, et al. Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. J Biol Chem. 2001;276:33762–72. doi: 10.1074/jbc.M008564200. [DOI] [PubMed] [Google Scholar]
- 23.Burdick MM, McCaffery JM, Kim YS, et al. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol. 2003;284:C977–87. doi: 10.1152/ajpcell.00423.2002. [DOI] [PubMed] [Google Scholar]
- 24.Hanley WD, Napier SL, Burdick MM, et al. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J. 2006;20:337–9. doi: 10.1096/fj.05-4574fje. [DOI] [PubMed] [Google Scholar]
- 25.Napier SL, Healy ZR, Schnaar RL, et al. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. J Biol Chem. 2007;282:3433–41. doi: 10.1074/jbc.M607219200. [DOI] [PubMed] [Google Scholar]
- 26.Voura EB, Sandig M, Siu CH. Cell-cell interactions during transendothelial migration of tumor cells. Microsc Res Tech. 1998;43:265–75. doi: 10.1002/(SICI)1097-0029(19981101)43:3<265::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Laferriere J, Houle F, Huot J. Adhesion of HT-29 colon carcinoma cells to endothelial cells requires sequential events involving E-selectin and integrin beta4. Clin Exp Metastasis. 2004;21:257–64. doi: 10.1023/b:clin.0000037708.09420.9a. [DOI] [PubMed] [Google Scholar]
- 28.Sawada T, Yoshida M, Yasukouchi Y, et al. Colon cancer cell adhesion to endothelial E-selectin inhibits detachment of endothelial cells through activation of beta(1)-integrin. Biochem Biophys Res Commun. 2001;286:20–7. doi: 10.1006/bbrc.2001.5298. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay PL, Auger FA, Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene. 2006;25:6563–73. doi: 10.1038/sj.onc.1209664. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Kiely JM, Szente BE, et al. E-selectin-dependent signaling via the mitogen-activated protein kinase pathway in vascular endothelial cells. J Immunol. 2000;165:2142–8. doi: 10.4049/jimmunol.165.4.2142. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay PL, Huot J, Auger FA. Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008;68:5167–76. doi: 10.1158/0008-5472.CAN-08-1229. [DOI] [PubMed] [Google Scholar]
- 32.Aychek T, Miller K, Sagi-Assif O, et al. E-selectin regulates gene expression in metastatic colorectal carcinoma cells and enhances HMGB1 release. Int J Cancer. 2008;123:1741–50. doi: 10.1002/ijc.23375. [DOI] [PubMed] [Google Scholar]
- 33.Ye C, Kiriyama K, Mistuoka C, et al. Expression of E-selectin on endothelial cells of small veins in human colorectal cancer. Int J Cancer. 1995;61:455–60. doi: 10.1002/ijc.2910610404. [DOI] [PubMed] [Google Scholar]
- 34.Ito K, Ye CL, Hibi K, et al. Paired tumor marker of soluble E-selectin and its ligand sialyl Lewis A in colorectal cancer. J Gastroenterol. 2001;36:823–9. doi: 10.1007/s005350170004. [DOI] [PubMed] [Google Scholar]
- 35.Uner A, Akcali Z, Unsal D. Serum levels of soluble E-selectin in colorectal cancer. Neoplasma. 2004;51:269–74. [PubMed] [Google Scholar]
- 36.Dymicka-Piekarska V, Kemona H. Does colorectal cancer clinical advancement affect adhesion molecules (sP- selectin, sE- selectin and ICAM-1) concentration? Thromb Res. 2009;124:80–3. doi: 10.1016/j.thromres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther. 2008;8:1247–55. doi: 10.1586/14737140.8.8.1247. [DOI] [PubMed] [Google Scholar]
- 38.Sierko E, Wojtukiewicz MZ. Inhibition of platelet function: does it offer a chance of better cancer progression control? Semin Thromb Hemost. 2007;33:712–21. doi: 10.1055/s-2007-991540. [DOI] [PubMed] [Google Scholar]
- 39.Peeters CF, Ruers TJ, Westphal JR, et al. Progressive loss of endothelial P-selectin expression with increasing malignancy in colorectal cancer. Lab Invest. 2005;85:248–56. doi: 10.1038/labinvest.3700217. [DOI] [PubMed] [Google Scholar]
- 40.McCarty OJ, Jadhav S, Burdick MM, et al. Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys J. 2002;83:836–48. doi: 10.1016/S0006-3495(02)75212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarty OJ, Mousa SA, Bray PF, et al. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96:1789–97. [PubMed] [Google Scholar]
- 42.Burdick MM, Konstantopoulos K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol. 2004;287:C539–47. doi: 10.1152/ajpcell.00450.2003. [DOI] [PubMed] [Google Scholar]
- 43.Wei M, Tai G, Gao Y, et al. Modified heparin inhibits P-selectin-mediated cell adhesion of human colon carcinoma cells to immobilized platelets under dynamic flow conditions. J Biol Chem. 2004;279:29202–10. doi: 10.1074/jbc.M312951200. [DOI] [PubMed] [Google Scholar]
- 44.Sousou T, Khorana AA. New insights into cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2009;29:316–20. doi: 10.1161/ATVBAHA.108.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousou T, Khorana A. Identifying cancer patients at risk for venous thromboembolism. Hamostaseologie. 2009;29:121–4. [PubMed] [Google Scholar]
- 46.Jadhav S, Konstantopoulos K. Fluid shear- and time-dependent modulation of molecular interactions between PMNs and colon carcinomas. Am J Physiol Cell Physiol. 2002;283:C1133–43. doi: 10.1152/ajpcell.00104.2002. [DOI] [PubMed] [Google Scholar]
- 47.Borsig L, Wong R, Hynes RO, et al. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA. 2002;99:2193–8. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sturm JW, Magdeburg R, Berger K, et al. Influence of TNFA on the formation of liver metastases in a syngenic mouse model. Int J Cancer. 2003;107:11–21. doi: 10.1002/ijc.11320. [DOI] [PubMed] [Google Scholar]
- 49.Schluter K, Gassmann P, Enns A, et al. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol. 2006;169:1064–73. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haier J, Korb T, Hotz B, et al. An intravital model to monitor steps of metastatic tumor cell adhesion within the hepatic microcirculation. J Gastrointest Surg. 2003;7:507–14. doi: 10.1016/S1091-255X(03)00023-4. ; discussion 14–5. [DOI] [PubMed] [Google Scholar]
- 51.Thomas SN, Zhu F, Schnaar RL, et al. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem. 2008;283:15647–55. doi: 10.1074/jbc.M800543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas P, Hayashi H, Zimmer R, et al. Regulation of cytokine production in carcinoembryonic antigen stimulated Kupffer cells by beta-2 adrenergic receptors: implications for hepatic metastasis. Cancer Lett. 2004;209:251–7. doi: 10.1016/j.canlet.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Gangopadhyay A, Lazure DA, Kelly TM, et al. Purification and analysis of an 80-kDa carcinoembryonic antigen-binding protein from Kupffer cells. Arch Biochem Biophys. 1996;328:151–7. doi: 10.1006/abbi.1996.0155. [DOI] [PubMed] [Google Scholar]
- 54.Minami S, Furui J, Kanematsu T. Role of carcinoembryonic antigen in the progression of colon cancer cells that express carbohydrate antigen. Cancer Res. 2001;61:2732–5. [PubMed] [Google Scholar]
- 55.Knittel T, Mehde M, Kobold D, et al. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 56.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–86. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 57.Wagnerova J, Cervenakova L, Balabanov R, et al. Cytokine regulation of E-selectin in rat CNS microvascular endothelial cells: differential response of CNS and non-CNS vessels. J Neurol Sci. 2002;195:51–62. doi: 10.1016/s0022-510x(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 58.Silverman MD, Babra B, Pan Y, et al. Differential E-selectin expression by iris versus retina microvascular endothelial cells cultured from the same individuals. Microvasc Res. 2005;70:32–42. doi: 10.1016/j.mvr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Petzelbauer P, Bender JR, Wilson J, et al. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993;151:5062–72. [PubMed] [Google Scholar]
- 60.Sepp NT, Gille J, Li LJ, et al. A factor in human plasma permits persistent expression of E-selectin by human endothelial cells. J Invest Dermatol. 1994;102:445–50. doi: 10.1111/1523-1747.ep12373008. [DOI] [PubMed] [Google Scholar]
- 61.Haraldsen G, Kvale D, Lien B, et al. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;156:2558–65. [PubMed] [Google Scholar]
- 62.Hauser IA, Johnson DR, Madri JA. Differential induction of VCAM-1 on human iliac venous and arterial endothelial cells and its role in adhesion. J Immunol. 1993;151:5172–85. [PubMed] [Google Scholar]
- 63.Mannori G, Crottet P, Cecconi O, et al. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995;55:4425–31. [PubMed] [Google Scholar]
- 64.Di Bella MA, Flugy AM, Russo D, et al. Different phenotypes of colon carcinoma cells interacting with endothelial cells: role of E-selectin and ultrastructural data. Cell Tissue Res. 2003;312:55–64. doi: 10.1007/s00441-003-0704-6. [DOI] [PubMed] [Google Scholar]
- 65.Gout S, Tremblay PL, Huot J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin Exp Metastasis. 2008;25:335–44. doi: 10.1007/s10585-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 66.Aoki M, Kanamori M, Yudoh K, et al. Effects of vascular endothelial growth factor and E-selectin on angiogenesis in the murine metastatic RCT sarcoma. Tumour Biol. 2001;22:239–46. doi: 10.1159/000050622. [DOI] [PubMed] [Google Scholar]
- 67.Antoine M, Tag CG, Gressner AM, et al. Expression of E-selectin ligand-1 (CFR/ESL-1) on hepatic stellate cells: implications for leukocyte extravasation and liver metastasis. Oncol Rep. 2009;21:357–62. [PubMed] [Google Scholar]
- 68.Taura K, De Minicis S, Seki E, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–38. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 69.Man K, Ng KT, Lo CM, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases–activation of cell invasion and migration pathways. Liver Transpl. 2007;13:1669–77. doi: 10.1002/lt.21193. [DOI] [PubMed] [Google Scholar]
- 70.Mayr FB, Firbas C, Leitner JM, et al. Effects of the pan-selectin antagonist bimosiamose (TBC1269) in experimental human endotoxemia. Shock. 2008;29:475–82. doi: 10.1097/shk.0b013e318142c4e8. [DOI] [PubMed] [Google Scholar]
- 71.Meyer M, Beyer D, Vollhardt K, et al. The pharmacokinetics of subcutaneously injected Bimosiamose disodium in healthy male volunteers. Biopharm Drug Dispos. 2007;28:475–84. doi: 10.1002/bdd.574. [DOI] [PubMed] [Google Scholar]
- 72.Romano SJ. Selectin antagonists: therapeutic potential in asthma and COPD. Treat Respir Med. 2005;4:85–94. doi: 10.2165/00151829-200504020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fritzsche J, Alban S, Ludwig RJ, et al. The influence of various structural parameters of semisynthetic sulfated polysaccharides on the P-selectin inhibitory capacity. Biochem Pharmacol. 2006;72:474–85. doi: 10.1016/j.bcp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 74.Stevenson JL, Varki A, Borsig L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb Res. 2007;120:S107–11. doi: 10.1016/S0049-3848(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 75.Niers TM, Klerk CP, DiNisio M, et al. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit Rev Oncol Hematol. 2007;61:195–207. doi: 10.1016/j.critrevonc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 76.He XY, Xu Z, Melrose J, et al. Humanization and pharmacokinetics of a monoclonal antibody with specificity for both E- and P-selectin. J Immunol. 1998;160:1029–35. [PubMed] [Google Scholar]
- 77.Kneuer C, Ehrhardt C, Radomski MW, et al. Selectins–potential pharmacological targets? Drug Discov Today. 2006;11:1034–40. doi: 10.1016/j.drudis.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Dernedde J, Enders S, Reissig HU, et al. Inhibition of selectin binding by colloidal gold with functionalized shells. Chem Commun. 2009;28:932–4. doi: 10.1039/b818263a. [DOI] [PubMed] [Google Scholar]
- 79.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–18. [PubMed] [Google Scholar]
- 80.Ugorski M, Laskowska A. Sialyl Lewis(a): a tumor-associated carbohydrate antigen involved in adhesion and metastatic potential of cancer cells. Acta Biochim Pol. 2002;49:303–11. [PubMed] [Google Scholar]
- 81.Asai S, Watanabe T, Sakamoto J, et al. Expression and prognostic indicators of type 1 and type 2 Lewis blood group antigens in colorectal cancers. Nippon Geka Gakkai Zasshi. 1994;95:753–62. [PubMed] [Google Scholar]
- 82.Blixt O, Allin K, Bohorov O, et al. Glycan microarrays for screening sialyltransferase specificities. Glycoconj J. 2008;25:59–68. doi: 10.1007/s10719-007-9062-z. [DOI] [PubMed] [Google Scholar]
- 83.Liu G, Marathe DD, Matta KL, et al. Systems-level modeling of cellular glycosylation reaction networks: O-linked glycan formation on natural selectin ligands. Bioinformatics. 2008;24:2740–7. doi: 10.1093/bioinformatics/btn515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mollicone R, Moore SE, Bovin N, et al. Activity, splice variants, conserved peptide motifs, and phylogeny of two new {alpha}1,3-fucosyltransferase families (FUT10 and FUT11) J Biol Chem. 2009;284:4723–38. doi: 10.1074/jbc.M809312200. [DOI] [PubMed] [Google Scholar]
- 85.Kudo T, Ikehara Y, Togayachi A, et al. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab Invest. 1998;78:797–811. [PubMed] [Google Scholar]
- 86.Malagolini N, Santini D, Chiricolo M, et al. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology. 2007;17:688–97. doi: 10.1093/glycob/cwm040. [DOI] [PubMed] [Google Scholar]
- 87.Muinelo-Romay L, Vazquez-Martin C, Villar-Portela S, et al. Expression and enzyme activity of alpha(1,6)fucosyltransferase in human colorectal cancer. Int J Cancer. 2008;123:641–6. doi: 10.1002/ijc.23521. [DOI] [PubMed] [Google Scholar]
- 88.Mejias-Luque R, Lopez-Ferrer A, Garrido M, et al. Changes in the invasive and metastatic capacities of HT-29/M3 cells induced by the expression of fucosyltransferase 1. Cancer Sci. 2007;98:1000–5. doi: 10.1111/j.1349-7006.2007.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petretti T, Kemmner W, Schulze B, et al. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–66. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Irimura T, Nakamori S, Matsushita Y, et al. Colorectal cancer metastasis determined by carbohydrate-mediated cell adhesion: role of sialyl-LeX antigens. Semin Cancer Biol. 1993;4:319–24. [PubMed] [Google Scholar]
- 91.Bresalier RS, Ho SB, Schoeppner HL, et al. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354–67. doi: 10.1053/gast.1996.v110.pm8613039. [DOI] [PubMed] [Google Scholar]
- 92.Nakamori S, Kameyama M, Imaoka S, et al. Involvement of carbohydrate antigen sialyl Lewis(x) in colorectal cancer metastasis. Dis Colon Rectum. 1997;40:420–31. doi: 10.1007/BF02258386. [DOI] [PubMed] [Google Scholar]
- 93.Zhang S, Zhang HS, Cordon-Cardo C, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–6. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 94.Takada A, Ohmori K, Yoneda T, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–61. [PubMed] [Google Scholar]
- 95.Klopocki AG, Laskowska A, Antoniewicz-Papis J, et al. Role of sialosyl Lewis(a) in adhesion of colon cancer cells–the antisense RNA approach. Eur J Biochem. 1998;253:309–18. doi: 10.1046/j.1432-1327.1998.2530309.x. [DOI] [PubMed] [Google Scholar]
- 96.Ben-David T, Sagi-Assif O, Meshel T, et al. The involvement of the sLe-a selectin ligand in the extravasation of human colorectal carcinoma cells. Immunol Lett. 2008;116:218–24. doi: 10.1016/j.imlet.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 97.Yamada N, Chung YS, Takatsuka S, et al. Increased sialyl Lewis A expression and fucosyltransferase activity with acquisition of a high metastatic capacity in a colon cancer cell line. Br J Cancer. 1997;76:582–7. doi: 10.1038/bjc.1997.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato M, Narita T, Kimura N, et al. The association of sialyl Lewis(a) antigen with the metastatic potential of human colon cancer cells. Anticancer Res. 1997;17:3505–11. [PubMed] [Google Scholar]
- 99.Opolski A, Laskowska A, Madej J, et al. Metastatic potential of human CX-1 colon adenocarcinoma cells is dependent on the expression of sialosyl Le(a) antigen. Clin Exp Metastasis. 1998;16:673–81. doi: 10.1023/a:1006502009682. [DOI] [PubMed] [Google Scholar]
- 100.Dabrowska A, Baczynska D, Widerak K, et al. Promoter analysis of the human alpha1,3/4-fucosyltransferase gene (FUT III) Biochim Biophys Acta. 2005;1731:66–73. doi: 10.1016/j.bbaexp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Koike T, Kimura N, Miyazaki K, et al. Hypoxia induces adhesion molecules on cancer cells: a missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci USA. 2004;101:8132–7. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsumoto S, Imaeda Y, Umemoto S, et al. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br J Cancer. 2002;86:161–7. doi: 10.1038/sj.bjc.6600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shirota K, Kato Y, Irimura T, et al. Anti-metastatic effect of the sialyl Lewis-X analog GSC-150 on the human colon carcinoma derived cell line KM12-HX in the mouse. Biol Pharm Bull. 2001;24:316–9. doi: 10.1248/bpb.24.316. [DOI] [PubMed] [Google Scholar]
- 104.Ragupathi G, Damani P, Srivastava G, et al. Synthesis of sialyl Lewis(a) (sLe (a), CA19-9) and construction of an immunogenic sLe(a) vaccine. Cancer Immunol Immunother. 2009;58:1397–405. doi: 10.1007/s00262-008-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–7. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 106.Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51:191–200. doi: 10.1136/mp.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ngan CY, Yamamoto H, Seshimo I, et al. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. J Surg Oncol. 2007;95:652–62. doi: 10.1002/jso.20638. [DOI] [PubMed] [Google Scholar]
- 108.Subramaniam V, Gardner H, Jothy S. Soluble CD44 secretion contributes to the acquisition of aggressive tumor phenotype in human colon cancer cells. Exp Mol Pathol. 2007;83:341–6. doi: 10.1016/j.yexmp.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Subramaniam V, Vincent IR, Gilakjan M, et al. Suppression of human colon cancer tumors in nude mice by siRNA CD44 gene therapy. Exp Mol Pathol. 2007;83:332–40. doi: 10.1016/j.yexmp.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 110.Subramaniam V, Vincent IR, Gardner H, et al. CD44 regulates cell migration in human colon cancer cells via Lyn kinase and AKT phosphorylation. Exp Mol Pathol. 2007;83:207–15. doi: 10.1016/j.yexmp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Streit M, Schmidt R, Hilgenfeld RU, et al. Adhesion receptors in malignant transformation and dissemination of gastrointestinal tumors. J Mol Med. 1996;74:253–68. doi: 10.1007/BF00196578. [DOI] [PubMed] [Google Scholar]
- 112.Coppola D, Hyacinthe M, Fu L, et al. CD44V6 expression in human colorectal carcinoma. Hum Pathol. 1998;29:627–35. doi: 10.1016/s0046-8177(98)80014-2. [DOI] [PubMed] [Google Scholar]
- 113.Nanashima A, Yamaguchi H, Sawai T, et al. Prognostic factors in hepatic metastases of colorectal carcinoma: immunohistochemical analysis of tumor biological factors. Dig Dis Sci. 2001;46:1623–8. doi: 10.1023/a:1010680815954. [DOI] [PubMed] [Google Scholar]
- 114.Nihei Z, Ichikawa W, Kojima K, et al. The positive relationship between the expression of CD44 variant 6 and prognosis in colorectal cancer. Surg Today. 1996;26:760–1. doi: 10.1007/BF00312104. [DOI] [PubMed] [Google Scholar]
- 115.Peng J, Lu JJ, Zhu J, et al. Prediction of treatment outcome by CD44v6 after total mesorectal excision in locally advanced rectal cancer. Cancer J. 2008;14:54–61. doi: 10.1097/PPO.0b013e3181629a67. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Chen W, Li X, et al. Heat shock protein 72 associated with CD44v6 in human colonic adenocarcinoma. Cell Biol Int. 2008;32:860–4. doi: 10.1016/j.cellbi.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Zhang JC, Wang ZR, Cheng YJ, et al. Expression of proliferating cell nuclear antigen and CD44 variant exon 6 in primary tumors and corresponding lymph node metastases of colorectal carcinoma with Dukes’ stage C or D. World J Gastroenterol. 2003;9:1482–6. doi: 10.3748/wjg.v9.i7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuhn S, Koch M, Nubel T, et al. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553–67. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- 119.Yamaguchi A, Urano T, Goi T, et al. Expression of a CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer patients. J Clin Oncol. 1996;14:1122–7. doi: 10.1200/JCO.1996.14.4.1122. [DOI] [PubMed] [Google Scholar]
- 120.Hanley WD, Burdick MM, Konstantopoulos K, et al. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 2005;65:5812–7. doi: 10.1158/0008-5472.CAN-04-4557. [DOI] [PubMed] [Google Scholar]
- 121.Alves CS, Burdick MM, Thomas SN, et al. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am J Physiol Cell Physiol. 2008;294:C907–16. doi: 10.1152/ajpcell.00463.2007. [DOI] [PubMed] [Google Scholar]
- 122.Brooks SA, Lomax-Browne HJ, Carter TM, et al. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112:3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]


