Abstract
Mesenchymal stromal cells (MSC) have been suggested to provide a suitable cellular environment for in vitro expansion of haematopoietic stem and progenitor cells (HPC) from umbilical cord blood. In this study, we have simultaneously analysed the cell division history and immunophenotypic differentiation of HPC by using cell division tracking with carboxyfluorescein diacetate N-succinimidyl ester (CFSE). Co-culture with MSC greatly enhanced proliferation of human HPC, especially of the more primitive CD34+CD38− fraction. Without co-culture CD34 and CD133 expressions decreased after several cell divisions, whereas CD38 expression was up-regulated after some cell divisions and then diminished in fast proliferating cells. Co-culture with MSC maintained a primitive immunophenotype (CD34+, CD133+ and CD38−) for more population doublings, whereas up-regulation of differentiation markers (CD13, CD45 and CD56) in HPC was delayed to higher numbers of cell divisions. Especially MSC of early cell passages maintained CD34 expression in HPC over more cell divisions, whereas MSC of higher passages further enhanced their proliferation rate. Inhibition of mitogen-activated protein kinase 1 (MAPK1) impaired proliferation and differentiation of HPC, but not maintenance of long-term culture initiating cells. siRNA knockdown of N-cadherin and VCAM1 in feeder layer cells increased the fraction of slow dividing HPC, whereas knockdown of integrin beta 1 (ITGB1) and CD44 impaired their differentiation. In conclusion, MSC support proliferation as well as self-renewal of HPC with primitive immunophenotype. The use of early passages of MSC and genetic manipulation of proteins involved in HPC–MSC interaction might further enhance cord blood expansion on MSC.
Keywords: mesenchymal stromal cells, haematopoietic stem cells, stem cell niche, cord blood, co-culture, immunophenotype, proliferation, replicative senescence, adhesion proteins
Introduction
Umbilical cord blood (CB) has become a viable option for transplantation of haematopoietic stem cells (HSC) [1]. However, in adults their use is hampered by the insufficient numbers of stem cells per graft. In vitro expansion prior to transplantation might provide a strategy to overcome this limitation and to reduce time for haematopoietic recovery after transplantation [2–4]. Despite intensive research over the last decades, there are still no reliable methods for expansion of primitive and self-renewing haematopoietic stem and progenitor cells (HPC). Recent studies indicated that this might be facilitated by novel growth factor combinations [5] or ectopic expression of HOXB4 [6]. It is however unclear whether cytokines or genetic modifications could lead to real expansion of HPC with self-renewing capacity, as direct interaction between HPC and cellular components in the stem cell niche is crucial for regulation of haematopoiesis [7–9]. Mimicking this cellular microenvironment by stromal cells therefore provides a more promising alternative for in vitro expansion of CB-HSC [10–15].
Mesenchymal stromal cells (MSC; alternatively named ‘mesenchymal stem cells’) are precursors of mesodermal cell types such as osteocytes, adipocytes and chondrocytes. They are defined by plastic adherent growth, a panel of surface markers (e.g. CD105, CD73 and CD90) and their in vitro differentiation potential under specific culture conditions [16–18]. Le Blanc and coworkers have reported that co-transplantation of allogeneic MSC enhanced engraftment in seven patients [19] and recently, a clinical trial has been activated to analyse CB expansion on MSC (NCT00498316; M.D. Anderson Cancer Center, Houston, TX, USA). Preliminary experiments from our group indicated that the haematopoiesis supportive potential varies between MSC from different tissues. This underlines the need for molecular and functional characterization of MSC preparations in relationship to their HPC supportive function [20–22].
Real expansion of HPC requires maintenance of ‘stemness’ despite proliferation [23]. This appeared to be an oxymoron, as proliferation in vitro is usually associated with differentiation. In this study, we have used the carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-dilution method to simultaneously analyse the number of population doublings within 7 days of culture and of immunophenotypic differentiation [24–26]. Thereby, we have investigated maintenance of CD34+CD38− immunophenotype in relation to the cell division history after co-culture with MSC and the mechanisms involved in this process.
Materials and methods
Isolation of haematopoietic progenitor cells
HPC were collected from fresh umbilical CB after written consent using guidelines approved by the Ethic Committee on the Use of Human Subjects at the University of Heidelberg. Mononuclear cells were isolated after centrifugation on Ficoll-hypaque (Biochrom KG, Berlin, Germany). CD34+ cells were enriched with a monoclonal anti-CD34 antibody labelled using magnetic beads on an affinity column (Miltenyi Biotec, Bergisch-Gladbach, Germany). After additional staining with anti-CD34-allophycocyanin or anti-CD34-fluorescein-isothiocyanate (FITC) (both clone 8G12, Becton Dickinson Biosciences, San Jose, CA, USA [BD]) and CD38-phycoerythrin (PE, BD, clone HB-7), further purification of CD34+ cells or of CD34+CD38− cells was achieved using a FACS-Vantage-SE flow cytometry cell sorting system. Staining with propidium iodide (PI) was performed to allow exclusion of non-viable cells. Re-analysis always revealed a purity of more than 95%.
Isolation and culture of mesenchymal stromal cells
MSC were isolated from human bone marrow and characterized as described in our previous work [27, 28]. All samples were taken after written consent using guidelines approved by the Ethic Committee on the Use of Human Subjects at the University of Heidelberg. In brief, MNC were seeded in tissue culture flasks that have been coated with 10 ng/ml fibronectin (Sigma). The medium consists of 58% Dulbecco’s Modified Eagles Medium-Low Glucose (DMEM-LG, Cambrex, Apen, Germany) and 40% MCDB201 (Sigma, Deisenhofen, Germany), 2% FCS (HyClone, Bonn, Germany), supplemented with 2 mM L-Glutamine, 100 U/ml Pen/Strep (Cambrex), 1% insulin transferrin selenium, 1% linoleic acid bovine serum albumin, 10 nM dexamethasone, 0.1 mM L-ascorbic-acid-2-phosphate (all from Sigma), PDGF-bb and EGF (10 ng/ml each, PeproTech, Hamburg, Germany) [29]. If not indicated otherwise, we have used sub-confluent MSC feeder layer (70–80%) of the third to sixth passage in these studies.
Culture conditions and expansion of HPC
HPC were expanded in long-term culture medium (LTBMC medium) that consists of IMDM (Gibco, Carlsbad, CA, USA) with 12.5% FCS, 12.5% horse serum (Terry Fox Laboratories, Vancouver, Canada), 2 mmol/l L-glutamine (Gibco), penicillin 1000 U/ml, streptomycin 100 U/ml (Gibco) and 10−6 mol/l hydrocortisone. Culture was either performed without stromal support, or HPC were directly seeded on a confluent layer of MSC that was irradiated (20 Gy) in 24-well plates 24 hrs before use.
Analysis of the number of cell divisions
HPC were labelled with carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Sigma-Aldrich, Steinheim, Germany) to monitor cell divisions. In brief, cells were washed in PBS with 0.1% FCS and then stained with CFSE at a final concentration of 2.5 μM for 10 min. at 37°C. Staining reaction was stopped with ice cold RPMI with 10% FCS for 5 min. with three subsequent washes. Alternatively, we have used the fluorescent membrane-dye PKH26 (SIGMA, Saint Louis, MO, USA) according to the manufacture’s instructions. The number of gated events was determined as a further parameter for cell proliferation.
Immunophenotypic analysis
To investigate the immunophenotype of HPC after co-culture with MSC, the cells were harvested by vigorous pipetting, washed in PBS and stained with CD34-allophycocyanin (APC; Becton Dickinson, San Jose, CA, USA [BD], clone 8G12), CD38-phycoerythrin (PE, BD, clone HB-7), CD133-PE (Miltenyi Biotec, clone AC141), CD13-APC (BD, clone WM15), CD45-APC (BD, clone HI30), CD56-PE (BD, clone MY31), CD3-PE (BD, clone SK3), CD19-PE (BD, clone 4G7) or CD20-APC (BD, clone L27). Labelled cells were acquired and expression of surface antigens was then analysed using a FACScan flow cytometry system with five colour up-grade (BD) running CellQuest 3.3 software (BD). Further analysis was performed using WinMDI software (WinMDI 2.8; The Scripps Institute, San Diego, CA, USA). Reliable discrimination of HPC and MSC was possible by higher forward-scatter, side-scatter and higher autofluorescence in the PI channel of MSC [30].
siRNA treatment and MAPK-inhibition
The role of specific adhesion proteins for the stromal function of MSC was further analysed by siRNA knockdown. We have used the RNAi starter kit (301799; Qiagen, Hilden, Germany) and validated siRNA constructs for integrin-beta-1 (ITGB1; SI00034370), N-cadherin (N-CDH; SI00028441), cadherin-11 (CDH11; SI00028511), Jagged-1 (SI02780134), vascular cell adhesion molecule (VCAM-1; SI00021630) and mitogen-activated protein kinase 1 (MAPK1; 1022564). Upon passaging, MSC were transfected with 5 nM of siRNA and 3 μl HiPerFect transfection reagent according to the manufacturer’s instructions. Knockdown efficiency was verified by immunoblot analysis or RT-PCR analysis. Alternatively, activation of MAPK1 was inhibited by blocking MAP kinase kinase-1 by adding 25 μM PD098059 (2′;-Amino-3′-methoxyflavone; Promega, Madison, WI, USA).
Immunoblot
MSC were harvested and treated for 15 min. in 50-μl lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton, 1% protease inhibitor cocktail [Sigma-Aldrich]). Protein concentration was measured using a Nano Drop ND-1000 spectrophotometer (Peqlab Biotechnology, Erlangen, Germany). Equal amounts were resolved on each lane of 4–12% TRIS-glycine gradient gels (Anamed, Darmstadt, Germany). As a protein molecular weight marker, the Page Ruler pre-stained protein ladder (SM0671, Fermentas GmbH, St. Leon-Rot, Germany) was used. Proteins were transferred on a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA), labelled with monoclonal antibodies for either cadherin-11 (clone 5B2H5, Zymed Laboratories, San Francisco, CA, USA), N-cadherin (clone 32, BD), ERK2 (BD; 610030), integrin beta 1 (ITGB1; clone 4B4LDC9LDH8, Beckman Coulter, Fullerton, CA, USA) or CD44 (clone DF1485, 03–16080, Gentaur, Brussels, Belgium) and detected with a secondary goat antimouse antibody (sc-2005, Santa Cruz Biotechnology, Santa Cruz, CA, USA) by chemiluminescence (ECL, Amersham Biosciences, UK). As a reference for the protein concentration, we have used the ERK2 antibody (BD; 610030) or the β-actin antibody (sc-47778, Santa Cruz Biotechnology).
Quantitative real-time PCR analysis
siRNA effects for ITGB1, VCAM1 and Jagged1 were validated on mRNA level by RT-PCR. Total RNA was isolated using TRIzol reagent (Invitrogen, Paisley, Scotland), controlled using the RNA 6000 Pico LabChip kit (Agilent, Waldbronn, Germany) and the Nano Drop ND-1000 spectrophotometer (Peqlab Biotechnology, Erlangen, Germany) and reverse transcribed by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Applera Deutschland GmbH, Darmstadt, Germany). Quantification of mRNA expression was performed with SYBR green using the ABI PRISM® 7700HT Sequence Detection System Instrument (Applied Biosystems). Primers were obtained from Biospring (Frankfurt, Germany): ITGB1-forward primer (F): 5′-CGTAGCAAAG GAACAGCAGA-3′, ITGB1-reverse primer (R): 5′-GCTTAGCTGT TGTGCTA-3′, VCAM1-F: 5′-TACCCATTTG ACAGGCTGGA-3′, VCAM1-R: 5′-TGGAACAGGT ATGGTCACA-3′, Jagged1-F: 5′-CTTCCAACGA ACACCTGAA-3′, GAPDH-F: 5′-TTCGTCATGG TGTGAACCA-3′, GAPDH-R: 5′-CTGTGGTCAT AGTCCTTCCA-3′. Differential gene expression was normalized to GAPDH.
LTC-IC Assay
Long-term culture-initiating cell (LTC-IC) frequency was performed as described before [31]. In some experiments, MSC were treated with siRNA for ITGB1 or CD44 to investigate the impact of these adhesion proteins on LTC-IC maintenance. Confluent layer of MSC were then irradiated (20 Gy) in 96-well plates. CD34+ cells were plated in limiting dilutions (22 replicates per concentration: 150, 50, 15, 5 cells/well) on these feeder layers and cultured in LTBMC medium. After 5 weeks, cells were overlaid with clonogenic methylcellulose medium (HSC-CFU lite with EPO, Miltenyi Biotec, Bergisch-Gladbach, Germany). Cultures were scored for secondary colony-forming cells (CFC) after additional 2 weeks of growth. LTC-IC frequency was determined using the L-Calc™ Software for Limiting Dilution Analysis (Stem Cell Technologies). Three independent experiments were performed in duplicate. To estimate the probability of differences in LTC-IC frequency, we used the paired Student’s t-test.
Results
MSC support proliferation of primitive HPC
HPC from CB were stained with CFSE and cultured either with or without MSC. The fluorescence dye is precisely halved at each successive cell generation. To determine the fluorescence intensity of non-dividing cells, an aliquot was analysed after 24 hrs. After 7 days, each CFSE fluorescence peak could be attributed to a certain number of cell doublings. The number of cell divisions significantly increased upon co-culture with MSC (Fig. 1). Without co-culture, hardly any proliferation of the more primitive fraction (CD34+CD38− cells) could be observed, whereas they underwent between 3 and 11 population doublings in co-culture with MSC (Fig. 2A).
Fig 1.
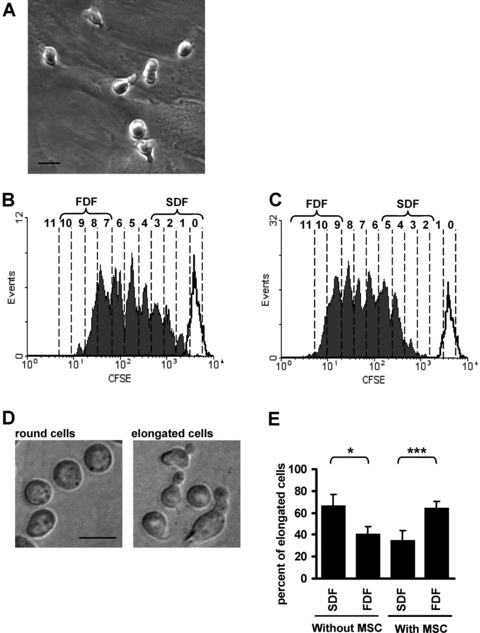
Co-culture with MSC supports proliferation of HPC. Most HPC are elongated upon co-culture with MSC (A). CD34+ cells were stained with CFSE and cultured for 7 days without stromal support (B) or in co-culture with MSC (C, black lines represent fluorescence intensity of non-dividing cells after 24 hrs). The number of cell divisions is indicated for each peak and cells were discerned in a slow dividing fraction (SDF, CFSE+) and a fast dividing fraction (FDF, CFSE−). Without stromal support, the fraction of elongated cells was significantly higher in the SDF and this was inversed by co-culture with MSC (D, E; magnification 400×, scale bar 10 μm; *=P < 0.05; ***=P < 0.001; n= 7).
Fig 2.
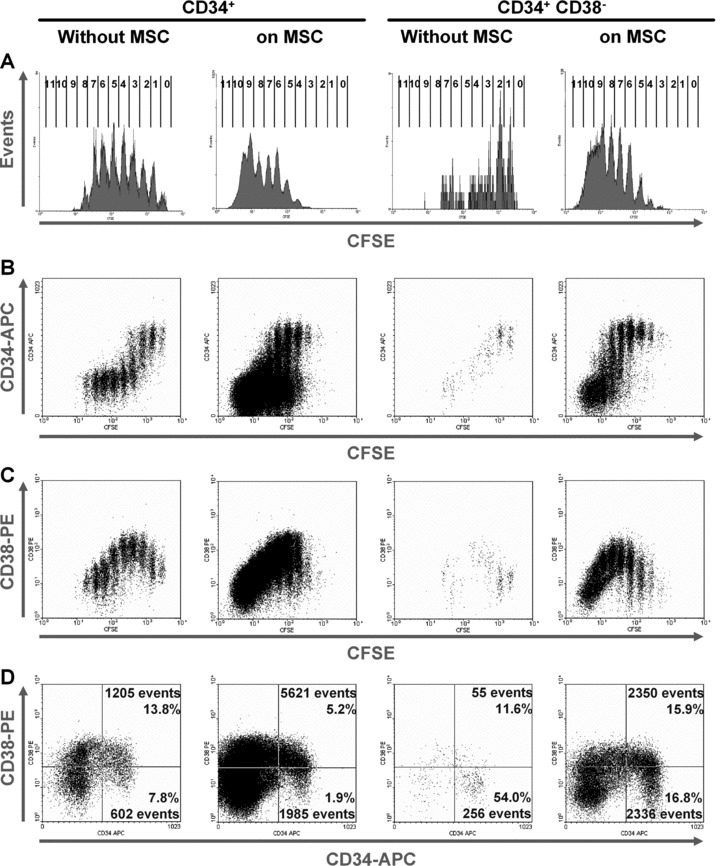
CD34/CD38 expression in relation to the number of HPC divisions. CD34+ cells (left panels) or CD34+CD38− cells (right panels) were stained with CFSE and cultured without or with MSC for 7 days. The number of cell divisions is indicated for each peak (A). CD34 expression is maintained in the CFSE+ cells and decreases after several cell divisions (B). CD38 expression is transiently up-regulated after several cell divisions and decreases thereafter in the FDF (C). Upon co-culture with MSC, the percentage of primitive CD34+CD38− cells was lower, whereas the number of gated events was higher (D).
Co-culture with MSC results in fast dividing cells with more elongated morphology
The cell morphology of HPC is heterogeneous. They display either a round or an elongated phenotype with various types of podia and membrane protrusions [32, 33]. After 7 days of culture without or with MSC, the CFSE-labelled HPC were separated into a slow dividing fraction (SDF) and a fast dividing fraction (FDF) [34]. SDF and FDF were defined as the 10% of cells with the slowest or fastest proliferation rate, respectively. Subsequently, cells were cultured for 12 hrs in culture medium without stromal support to allow settlement of HPC and formation of cytoplasmatic protrusions. Cell morphology was then analysed by phase contrast microscopy. If HPC were cultured without cellular support, morphologic analysis revealed that significantly more elongated cells were found in the SDF than in the FDF. Notably, this relation was inversed upon co-culture with MSC (Fig. 1).
Simultaneous analysis of cell divisions and immunophenotype
The immunophenotype of HPC was analysed in relation to the cell division history after 7 days. This was performed by simultaneous analysis of surface marker expression and remaining CFSE dye. CD34 expression was retained at high levels in the slow-dividing fraction, whereas the faster proliferating cells became more and more CD34 negative. Interestingly, after the same number of cell divisions (4 to 7 doublings) CD34 expression remained higher upon co-culture with MSC than without MSC (Fig. 2B). In analogy, expression of CD133 decreased in the fast proliferating fraction and this was delayed to higher numbers of cell division by co-culture with MSC (Fig. 3B). Analysis of initially CD34+CD38− cells revealed that expression of CD38 was up-regulated after some cell divisions and decreased again in the FDF. This gain and loss of CD38 expression was also delayed by co-culture with MSC (Figs 2C and 3B). Overall, the percentage of primitive CD34+CD38− cells decreased, whereas their absolute number was significantly increased by stromal support (Fig. 2D). Expression of differentiation markers CD45 (common lymphocyte antigen), CD13 (myeloid marker) and CD56 (NCAM, expressed on NK cells) was delayed to a higher number of cell divisions if cultured with MSC (Fig. 3B). Lymphatic markers (CD3, CD19 and CD20) were not detected on HPC after 7 days or after 14 days of culture either without or with MSC (results not shown). Taken together, these results clearly demonstrate that MSC not only support proliferation of HPC, but also maintain their primitive immunophenotype over a higher number of population doublings.
Fig 3.
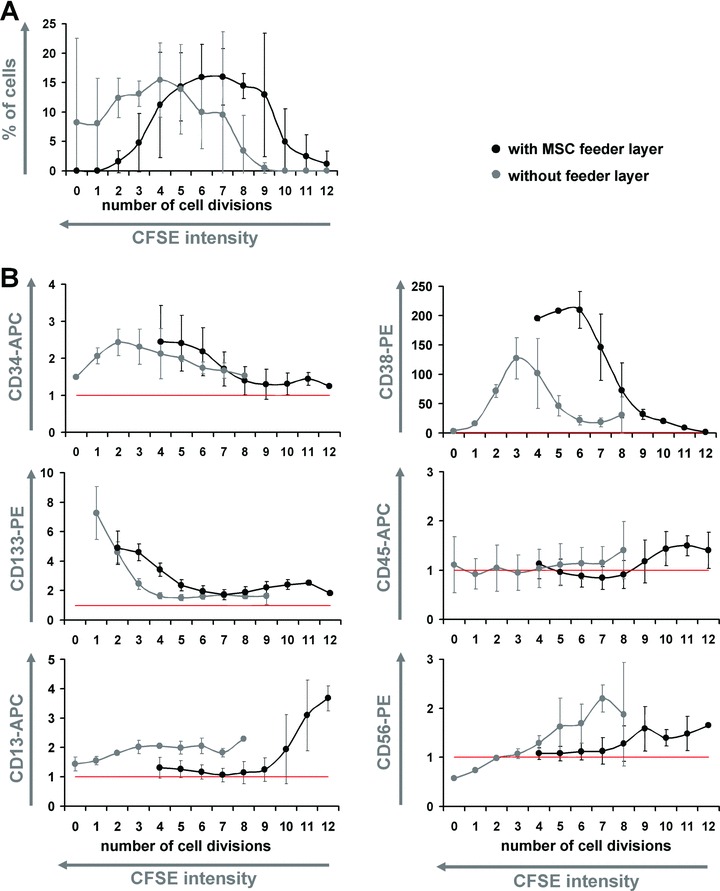
Co-culture shifts differentiation to higher numbers of cell division. CD34+ cells were stained with CFSE and simultaneously cultured either without (grey dots) or with MSC (black dots) for 7 days. Each CFSE-peak (representing 0 to 12 cell divisions) was individually gated and analysed. Co-culture with MSC enhanced the number of cell divisions (A). Decrease of CD34 expression of CD133 and of CD38 expression was delayed to a higher number of cell divisions by co-culture with MSC. On the other hand, up-regulation of differentiation associated markers CD45, CD13 and CD56 shifted to higher numbers of cell division (B). Data of three independent experiments were normalized to the corresponding auto-fluorescence (red lines) and mean ± S.D. are demonstrated.
Replicative senescence of MSC affects their stromal function
Long-term culture and replicative senescence of MSC impair their differentiation potential and influence their global gene expression profile [28]. In this study, we have therefore analysed, if replicative senescence of MSC might have an impact on their haematopoiesis supportive function. MSC of different passages of the same donor samples were cryopreserved and simultaneously taken into culture for comparative studies. CD34+ cells were stained with CFSE and co-cultured with these MSC feeder layers of different cell passages for 7 days. MSC of higher passages supported proliferation of HPC more than those of earlier passages (Fig. 4A). On the other hand, CD34 expression remained high for more population doublings if HPC were co-cultured with MSC of earlier passages (Fig. 4B). Replicative senescence of MSC did not have a clear impact on CD38 expression of HPC (results not shown). Similar results have been observed in independent experiments with passages of five different MSC donor samples. Thus, MSC of higher passages support cell proliferation, whereas MSC of earlier passages maintain a CD34+ phenotype for a higher number of cell divisions. This indicates that MSC of earlier passage enhance the ratio of self-renewal versus differentiation and are therefore more suitable for in vitro expansion of CD34+ HPC.
Fig 4.
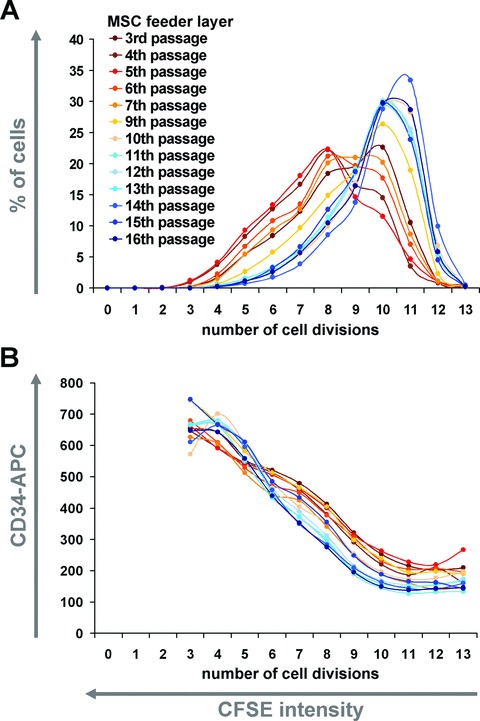
Replicative senescence affects the haematopoiesis supportive function of MSC. MSC of higher passages enhanced proliferation of HPC (blue lines, 9–12 population doublings) in comparison to MSC of early passages (red lines, 5–10 population doublings) (A). Cells remained CD34+ for more cell divisions if cultured on MSC of earlier passage (B).
MAPK1 is involved in proliferation and differentiation of HPC
MAPK1 has been demonstrated to play an important role for proliferation as well as for differentiation towards granulocyte/macrophage lineages [35]. We have addressed the role of MAPK1 in our co-culture system using either the inhibitor PD098059 or knockdown with small interfering RNA (Fig. 5). Treatment with the inhibitor reduced proliferation of HPC. A similar effect was observed with siRNA treatment although knockdown of MAPK1 was aimed for MSC (Fig. 6A and B). This was attenuated if MSC were repeatedly washed with culture medium after transfection indicating that siRNA was also reverse transfected in the HPC (data not shown). Slower proliferation consequently resulted in a higher fraction of CD34+ and CD34+CD38− cells. On the other hand, simultaneous analysis of cell proliferation and immunophenotype demonstrated that inhibition of MAPK1 impaired up-regulation of CD38 (Fig. 6F). This indicates that MAPK1 plays a role for proliferation as well as for differentiation of HPC. Inhibition with PD098059 during LTC-IC assays did not impair maintenance of colony forming cells (Fig. 6G). Thus, MAPK1 seems to play a role for proliferation and differentiation, rather than maintenance of primitive function in quiescent and slow-dividing cells.
Fig 5.
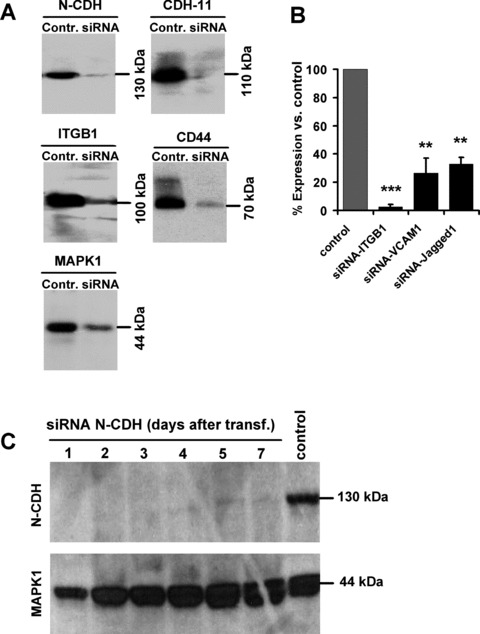
Specific knockdown of adhesion proteins by siRNA. Knockdown of N-cadherin (N-CDH), cadherin-11 (CDH11), integrin beta 1 (ITGB1), CD44 and MAPK1 in MSC was verified after 2 days by Western blot analysis (A). Knockdown of ITGB1, VCAM1 and Jagged1 was validated after 2 days by quantitative RT-PCR (B; **=P < 0.01; ***=P < 0.001). The transient siRNA effect lasted for more than 7 days (C).
Fig 6.
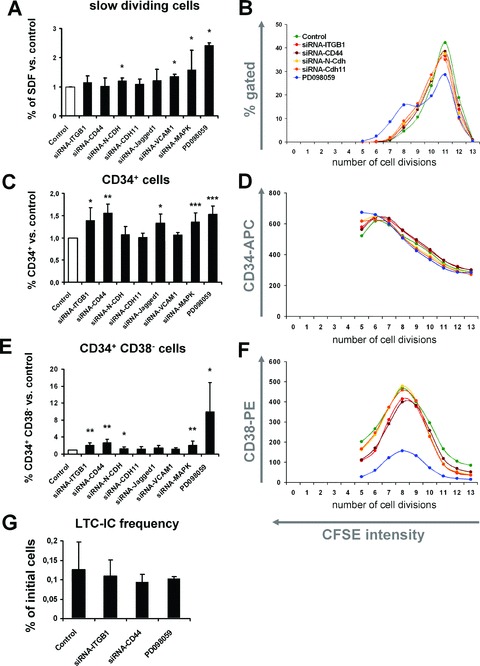
The role of various adhesion proteins for stromal function. CD34+ HPC were co-cultured on MSC upon siRNA knockdown of specific proteins. Alternatively, MAPK1 was inhibited by PD098059. The percentage of slow dividing cells (A), CD34+ cells (C) and CD34+CD38− cells (E) was determined after 7 days of co-culture. Means ± S.D. of eight independent experiments are presented in relation to untreated MSC feeder layer. Furthermore, representative results for proliferation (B), CD34 expression (D) and CD38 expression (F) in relation to the number of population doublings (residual CFSE stain) are demonstrated. Despite the increase of CD34+ and CD34+CD38− cells, there was no significant effect on the maintenance of long-term culture-initiating cells (LTC-IC) upon knockdown/inhibition of ITGB1, CD44 or MAPK1 (G; *=P < 0.05; **=P < 0.01; ***=P < 0.001).
The role of adhesion proteins for HPC–MSC interaction
Various adhesion proteins including N-cadherin (N-CDH), cadherin-11 (CDH11), integrin beta 1 (ITGB1), CD44, VCAM1 and Jagged1 have been suggested to play a crucial role for interaction of HPC with their niche. The role of these adhesion proteins was analysed in our co-culture system upon treatment of MSC with specific siRNA constructs. Knockdown was highly efficient as demonstrated after 3 days either by Western blot (N-CDH, CDH11, ITGB1 and CD44) or by quantitative RT-PCR (ITGB1, VCAM1 and Jagged1) and this effect sustained for more than 7 days (Fig. 5). CD34+ HPC were labelled either with CFSE or PKH26 and co-cultured simultaneously on untreated MSC and on siRNA-treated MSC feeder layer for 7 days. This was performed in eight independent experiments. Knockdown of N-cadherin and VCAM1 slightly enhanced the fraction of slow dividing cells. This indicates that N-cadherin and VCAM1 promote proliferation of HPC. Knockdown of cadherin-11, ITGB1, CD44 and Jagged1 did not affect proliferation of HPC. However, down-regulation of ITGB1 and CD44 resulted in a higher proportion of HPC with a primitive immunophenotype (CD34+ and CD34+CD38− cells). Furthermore, the maximal up-regulation of CD38 expression was reduced by knockdown of ITGB1 or CD44 (Fig. 6). In contrast, maintenance of long-term culture-initiating cell (LTC-IC) frequency was not affected by siRNA treatment for ITGB1 and CD44 (Fig. 6G). This suggests that ITGB1 and CD44 are involved in differentiation of HPC rather than maintenance of primitive HPC.
Discussion
Homeostasis of the haematopoietic system is regulated by the stem cell niche in the bone marrow. Understanding of the molecular mechanisms of cell–cell interaction might facilitate reliable CB expansion for therapeutic application. Our data demonstrate that co-culture with MSC activates proliferation of HPC and maintains a primitive immunophenotype over a higher number of cell divisions. Proliferation of HPC is enhanced by MSC of higher cell passages and it involves MAPK1, N-cadherin and VCAM1. In contrast, maintenance of a primitive immunophenotype is favoured by MSC of early cell passages, whereas MAPK1, ITGB1 and CD44 are involved in immunophenotypic differentiation (Table 1).
Table 1.
Effects of MSC on culture expansion of HPC
| Proliferation | Percentage of primitive cells (CD34+/CD38−) | Differentiation delayed to higher number of cell divisions | |
|---|---|---|---|
| MSC of early passage | − | + | + |
| MSC of late passage | + | − | − |
| MAPK1 inhibitor PD098059 | − | + | − |
| MAPK1-siRNA | − | + | NA |
| ITGB1-siRNA | No effect | + | + |
| CD44-siRNA | No effect | + | + |
| N-CDH-siRNA | − | No effect | No effect |
| CDH11-siRNA | No effect | No effect | No effect |
| Jagged1-siRNA | No effect | + | NA |
| VCAM1-siRNA | − | No effect | NA |
Effects of MSC passage, treatment with PD098059 or knockdown of specific genes by siRNA in MSC were analysed with regard to their haematopoiesis supportive potential. Proliferation, the percentage of CD34+ and CD34+CD38− cells and maintenance of this primitive immunophenotype for more cell divisions were determined (+, positive effect; −, negative effect; NA, not analysed).
The relation of self-renewal versus differentiation is regulated by asymmetric cell divisions [7]. This has been proposed for HPC already 30 years ago [36, 37]. In these experiments, individual daughter cells were physically separated and cultured under different culture conditions and the results indicated (i) that lineage commitment was not influenced by cytokines and (ii) that asymmetric cell divisions of HPC might occur in a stochastic manner [38, 39]. Using time lapse microscopy, we have demonstrated that asymmetric cell division of HPC correlates with asymmetric cell division kinetics: one daughter cell remained quiescent or divided very slowly while the other multiplied exponentially to yield committed progenitors and lineage specific colonies [34, 40]. LTC-IC and global gene expression profiling indicated that the slow-dividing fraction of HPC is highly enriched in more primitive HPC [41]. Interestingly, the symmetry of the initial cell divisions was only altered by a cellular environment [42].
The slow-dividing fraction of HPC is enriched in elongated cells with uropod formation and this has been shown in our previous work using the PKH26-dilution method [41]. These results were now verified with the CFSE-dilution method. It was however unexpected that the ratio of elongated cells in the SDF and FDF was inversed upon co-culture with MSC. The majority of HPC is polarized upon co-culture and the uropod at the trailing edge is involved in cell–cell adhesion [33, 43, 44]. Either these morphologic changes are induced by co-culture with MSC or they are determined cell intrinsically. Induction of an elongated phenotype by co-culture with MSC should involve both SDF and FDF. Under the assumption that polarized cell morphology is inherited upon cell division and discerns a subset of primitive HPC, our results indicate that elongated cells are preferentially recruited into the fast-proliferating fraction by MSC.
Slow dividing HPC maintained a more primitive immunophenotype and this is in line with the perception that proliferation in vitro is associated with differentiation. Decrease of CD34 expression has been described by other authors before [26, 45]. Our results demonstrate that CD38 is transiently up-regulated upon proliferation. Co-culture with MSC maintained CD34+ and CD133+ cells for a higher number of population doublings and it delayed up-regulation of differentiation markers (CD13, CD38, CD45 and CD56). Myeloid differentiation of CD34+ cells by co-culture with stromal cells has been demonstrated before [2]. Our results provide evidence that this differentiation is only acquired after several cell divisions and this is in line with the notion that it is linked to asymmetric cell divisions.
Preparation methods of MSC influence the stromal function [20, 46]. MSC need to be cultured for several weeks to generate sufficient numbers of feeder layer cells. Long-term culture and replicative senescence have far-reaching functional implications [47]. After 30 to 50 population doublings, MSC enter a senescent state and unequivocally stop proliferation. Recently, we have demonstrated that the global gene expression profile and microRNA expression changes continuously in the course of replicative senescence [28]. Here, we have demonstrated that later passages of MSC enhance proliferation of HPC, whereas earlier passages assist maintenance of a primitive phenotype for a higher number of population doublings. Thus, MSC of earlier passage increase the absolute number and percentage of the self-renewing population and are more suitable for expansion of primitive HPC.
The MAPK pathway plays a central role in cellular physiology and mediates numerous responses including cell cycle progression and differentiation [48]. In the haematopoietic system, it has been demonstrated that MAPK1 plays an important role for myeloid lineage commitment [35], megakaryocyte [49] and erythrocyte differentiation [50]. MAPK1 functions in proliferation and at the onset of lineage commitment. Furthermore, activation of MAPK1 mediates the effects of various cytokines and growth factors such as erythropoietin, stem cell factor, interleukin-3 and stromal derived factor 1 alpha [51, 52]. Therefore, we have tested the role of MAPK1 in our co-culture system. MAPK1 inhibition resulted in a lower proliferation of HPC. It also played a role for differentiation as CD34 expression was reduced after a lower number of cell divisions and up-regulation of CD38 was impaired. A similar effect on CD38 expression has been described before in smooth muscle cells [53]. The simultaneous analysis of this study provides further evidence that MAPK1 is essential for both proliferation and differentiation of HPC.
The natural haematopoietic stem cell niche attracts and anchors HSC. N-cadherin, CD44, VCAM1, Jagged1 and integrins have been suggested to be involved in this process [54–58]. We have previously compared gene expression profiles of MSC preparations from various tissues. Genes up-regulated in MSC preparations that have been associated with maintenance of stemness included N-cadherin, cadherin-11, integrin beta-1 (ITGB1, CD29) and VCAM1 [20]. Therefore, we reasoned that these adhesion proteins might play a crucial role for HPC–MSC interaction. It has been suggested that N-cadherin mediates anchorage of HPC in their niche and thereby keeps them in a quiescent state [58, 59]. Our results indicate that N-cadherin and VCAM1 rather promote proliferation of HPC. Furthermore, we have recently observed that N-cadherin is also involved in adhesion of HPC towards MSC and that N-cadherin mediated contact supports maintenance of LTC-IC (manuscript submitted). Unexpectedly, knockdown of ITGB1 and CD44 resulted in a significantly higher fraction of CD34+CD38− cells, whereas it did not impair proliferation or enhance maintenance of LTC-IC. Numerous studies indicated that ITGB1 and CD44 are involved in the homing process of HPC [57, 60–62]. Furthermore, CD44 is critical for formation of lymphoid and myeloid cells within the bone marrow [63–65]. In analogy, a critical role of ITGB1, especially in association with integrin alpha 4 (VLA4), has been demonstrated for haematopoietic differentiation in vitro[66, 67] and there is evidence that this effect is mediated via MAPK1 activation [68]. In our previous work, we have shown that ITGB1-mediated contact with MSC increases self-renewal and LTC-IC maintenance by using a blocking-function antibody [62]. Here, we demonstrate that expression of ITGB1 and CD44 in stromal cells also plays a role for differentiation of HPC.
In conclusion, simultaneous analysis of cell divisions and immunophenotypic differentiation has provided information that is of significance for expansion of HPC. Our data have demonstrated that MSC stimulate expansion especially of the more primitive and elongated HPC. Co-culture with MSC maintains HPC with a primitive immunophenotype (CD34+CD38− or CD133+CD38−) for a higher number of cell divisions. It needs to be demonstrated if co-culture also expands HSC with long-term repopulating capacity. This could be addressed in appropriate in vivo models such as serial transplantation in immune-deficient mice [69]. However, the immunophenotypic results of this study support the notion that expansion of HPC can be facilitated by co-culture with MSC, preferentially with MSC of early passages. Proliferation and differentiation can be independently influenced by replicative senescence of stromal cells or specific adhesion proteins. Thus, our data support the use of MSC in early passages for expansion of HPC and to increase the fraction of the self-renewing population.
Acknowledgments
We thank Kerstin Wörner and Katrin Miesala for excellent technical assistance in cell culture and cell sorting. This work was supported by the German Ministry of Education and Research (BMBF) within the supporting program ‘cell based regenerative medicine’ (START-MSC and CB-HERMES); the German Research Foundation DFG (HO 914/7–1); the Joachim Siebeneicher-Stiftung; the KIND-Stiftung; the Heidelberg Academy of Sciences (WIN-Kolleg), the Stem Cell Network North Rhine Westphalia and the Network for Aging Research (NAR, Heidelberg).
References
- 1.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–32. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Da Silva CL, Goncalves R, Crapnell KB, et al. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2005;33:828–35. doi: 10.1016/j.exphem.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeister CC, Zhang J, Knight KL, et al. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39:11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]
- 4.Kohler T, Plettig R, Wetzstein W, et al. Defining optimum conditions for the ex vivo expansion of human umbilical cord blood cells. Influences of progenitor enrichment, interference with feeder layers, early-acting cytokines and agitation of culture vessels. Stem Cells. 1999;17:19–24. doi: 10.1002/stem.170019. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CC, Kaba M, Iizuka S, et al. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–23. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiedlmeier B, Santos AC, Ribeiro A, et al. HOXB4’s road map to stem cell expansion. Proc Natl Acad Sci USA. 2007;104:16952–7. doi: 10.1073/pnas.0703082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho AD, Wagner W. The beauty of asymmetry – asymmetric divisions and self-renewal in the hematopoietic system. Curr Opin Hematol. 2007;14:330–6. doi: 10.1097/MOH.0b013e3281900f12. [DOI] [PubMed] [Google Scholar]
- 8.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 9.Harder F, Henschler R, Junghahn I, et al. Human hematopoiesis in murine embryos after injecting human cord blood-derived hematopoietic stem cells into murine blastocysts. Blood. 2002;99:719–21. doi: 10.1182/blood.v99.2.719. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chai C, Jiang XS, et al. Co-culture of umbilical cord blood CD34(+) cells with human mesenchymal stem cells. Tissue Eng. 2006;12:2161–70. doi: 10.1089/ten.2006.12.2161. [DOI] [PubMed] [Google Scholar]
- 11.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–66. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Feugier P, Serrurrier B, et al. Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol. 2007;35:507–15. doi: 10.1016/j.exphem.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Kadereit S, Deeds LS, Haynesworth SE, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(-) early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20:573–82. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 14.Magin AS, Koerfer NR, Partenheimer H, et al. Primary cells as feeder cells for coculture expansion of human hematopoietic stem cells from umbilical cord blood – a comparative study. Stem Cells Dev. 2008;18:173–86. doi: 10.1089/scd.2007.0273. [DOI] [PubMed] [Google Scholar]
- 15.Breems DA, Blokland EA, Siebel KE, et al. Stroma-contact prevents loss of hematopoietic stem cell quality during ex vivo expansion of CD34+ mobilized peripheral blood stem cells. Blood. 1998;91:111–7. [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Wagner W, Ho AD. Mesenchymal stem cell preparations-comparing apples and oranges. Stem Cell Rev. 2007;3:239–48. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 18.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–8. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 20.Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;10:2638–57. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 21.Ruster B, Gottig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–44. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 22.Schallmoser K, Rohde E, Reinisch A, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185–96. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- 23.Marciniak-Czochra A, Stiehl T, Ho AD, et al. Modeling of asymmetric cell division in hematopoietic stem cells – regulation of self-renewal is essential for efficient repopulation. Stem Cells Dev. 2008;18:377–85. doi: 10.1089/scd.2008.0143. [DOI] [PubMed] [Google Scholar]
- 24.Oostendorp RA, Audet J, Eaves CJ. High-resolution tracking of cell division suggests similar cell cycle kinetics of hematopoietic stem cells stimulated in vitro and in vivo. Blood. 2000;95:855–62. [PubMed] [Google Scholar]
- 25.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 26.Gotze KS, Schiemann M, Marz S, et al. CD133-enriched CD34(-) (CD33/ CD38/CD71)(-) cord blood cells acquire CD34 prior to cell division and hematopoietic activity is exclusively associated with CD34 expression. Exp Hematol. 2007;35:1408–14. doi: 10.1016/j.exphem.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–16. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells – a continuous and organized process. PLoS ONE. 2008;5:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 30.Thiemann FT, Moore KA, Smogorzewska EM, et al. The murine stromal cell line AFT024 acts specifically on human CD34+CD38- progenitors to maintain primitive function and immunophenotype in vitro. Exp Hematol. 1998;26:612–9. [PubMed] [Google Scholar]
- 31.Sutherland HJ, Eaves CJ, Eaves AC, et al. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989;74:1563–70. [PubMed] [Google Scholar]
- 32.Giebel B, Corbeil D, Beckmann J, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104:2332–8. doi: 10.1182/blood-2004-02-0511. [DOI] [PubMed] [Google Scholar]
- 33.Freund D, Bauer N, Boxberger S, et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity. Stem Cells Dev. 2006;15:815–29. doi: 10.1089/scd.2006.15.815. [DOI] [PubMed] [Google Scholar]
- 34.Huang S, Law P, Francis K, et al. Symmetry of initial cell divisions among primitive hematopoietic progenitors is independent of ontogenic age and regulatory molecules. Blood. 1999;94:2595–604. [PubMed] [Google Scholar]
- 35.Hsu CL, Kikuchi K, Kondo M. Activation of mitogen-activated protein kinase kinase (MEK)/extracellular signal regulated kinase (ERK) signaling pathway is involved in myeloid lineage commitment. Blood. 2007;110:1420–8. doi: 10.1182/blood-2007-02-071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suda J, Suda T, Ogawa M. Analysis of differentiation of mouse hemopoietic stem cells in culture by sequential replating of paired progenitors. Blood. 1984;64:393–9. [PubMed] [Google Scholar]
- 37.Leary AG, Strauss LC, Civin CI, et al. Disparate differentiation in hemopoietic colonies derived from human paired progenitors. Blood. 1985;66:327–32. [PubMed] [Google Scholar]
- 38.Brummendorf TH, Dragowska W, Zijlmans JMJM, et al. Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J Exp Med. 1998;188:1117–24. doi: 10.1084/jem.188.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayani H, Dragowska W, Lansdorp PM. Lineage commitment in human hemopoiesis involves asymmetric cell division of multipotent progenitors and does not appear to be influenced by cytokines. J Cell Physiol. 1993;157:579–86. doi: 10.1002/jcp.1041570318. [DOI] [PubMed] [Google Scholar]
- 40.Giebel B, Zhang T, Beckmann J, et al. Primitive human hematopoietic cells give rise to differentially specified daughter cells upon their initial cell division. Blood. 2006;107:2146–52. doi: 10.1182/blood-2005-08-3139. [DOI] [PubMed] [Google Scholar]
- 41.Wagner W, Ansorge A, Wirkner U, et al. Molecular evidence for stem cell function of the slow-dividing fraction among human hematopoietic progenitor cells by genome-wide analysis. Blood. 2004;104:675–86. doi: 10.1182/blood-2003-10-3423. [DOI] [PubMed] [Google Scholar]
- 42.Punzel M, Liu D, Zhang T, et al. The symmetry of initial divisions of human hematopoietic progenitors is altered only by the cellular microenvironment. Exp Hematol. 2003;31:339–47. doi: 10.1016/s0301-472x(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 43.Wagner W, Saffrich R, Wirkner U, et al. Hematopoietic progenitor cells and cellular microenvironment: behavioral and molecular changes upon interaction. Stem Cells. 2005;23:1180–91. doi: 10.1634/stemcells.2004-0361. [DOI] [PubMed] [Google Scholar]
- 44.Wagner W, Wein F, Roderburg C, et al. Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell-cell interaction. Exp Hematol. 2007;35:314–25. doi: 10.1016/j.exphem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Ko KH, Odell R, Nordon RE. Analysis of cell differentiation by division tracking cytometry. Cytometry A. 2007;71:773–82. doi: 10.1002/cyto.a.20437. [DOI] [PubMed] [Google Scholar]
- 46.Wagner W, Saffrich R, Ho AD. The stromal function of mesenchymal stromal cells. Transfus Med Hemother. 2008;35:185–93. doi: 10.1159/000128956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–30. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Wagner W, Bork S, Horn P, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–79. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 50.Racke FK, Lewandowska K, Goueli S, et al. Sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J Biol Chem. 1997;272:23366–70. doi: 10.1074/jbc.272.37.23366. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzaki T, Aisaki K, Yamamura Y, et al. Induction of erythroid differentiation by inhibition of Ras/ERK pathway in a friend murine leukemia cell line. Oncogene. 2000;19:1500–8. doi: 10.1038/sj.onc.1203461. [DOI] [PubMed] [Google Scholar]
- 52.Fuhler GM, Drayer AL, Olthof SG, et al. Reduced activation of protein kinase B, Rac, and F-actin polymerization contributes to an impairment of stromal cell derived factor-1 induced migration of CD34+ cells from patients with myelodysplasia. Blood. 2008;111:359–68. doi: 10.1182/blood-2006-11-060632. [DOI] [PubMed] [Google Scholar]
- 53.Sui X, Krantz SB, You M, et al. Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and stem cell factor is essential for expanded erythropoiesis. Blood. 1998;92:1142–9. [PubMed] [Google Scholar]
- 54.Tirumurugaan KG, Jude JA, Kang BN, et al. TNF-alpha induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1385–95. doi: 10.1152/ajplung.00472.2006. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 56.Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107:79–86. doi: 10.1182/blood-2005-05-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ alpha4(f/f) mice are hematopoietic cell autonomous. Blood. 2007;109:109–11. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 59.Haug JS, He XC, Grindley JC, et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell. 2008;2:367–79. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- 61.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci USA. 1993;90:9374–8. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner W, Wein F, Roderburg C, et al. Adhesion of human hematopoietic progenitor cells to mesenchymal stromal cells involves CD44. Cells Tissues Organs. 2007;188:160–9. doi: 10.1159/000112821. [DOI] [PubMed] [Google Scholar]
- 63.Gottschling S, Saffrich R, Seckinger A, et al. Human mesenchymal stroma cells regulate initial self-renewing divisions of hematopoietic progenitor cells by a beta1-integrin-dependent mechanism. Stem Cells. 2007;25:798–806. doi: 10.1634/stemcells.2006-0513. [DOI] [PubMed] [Google Scholar]
- 64.Khaldoyanidi S, Denzel A, Zoller M. Requirement for CD44 in proliferation and homing of hematopoietic precursor cells. J Leukoc Biol. 1996;60:579–92. doi: 10.1002/jlb.60.5.579. [DOI] [PubMed] [Google Scholar]
- 65.Miyake K, Medina KL, Hayashi S, et al. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990;171:477–88. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosel M, Khaldoyanidi S, Zawadzki V, et al. Involvement of CD44 variant isoform v10 in progenitor cell adhesion and maturation. Exp Hematol. 1999;27:698–711. doi: 10.1016/s0301-472x(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 67.Eshghi S, Vogelezang MG, Hynes RO, et al. Alpha4beta1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: integrins in red cell development. J Cell Biol. 2007;177:871–80. doi: 10.1083/jcb.200702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyake K, Weissman IL, Greenberger JS, et al. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapur R, Cooper R, Zhang L, et al. Cross-talk between alpha(4)beta(1)/alpha(5)beta(1) and c-Kit results in opposing effect on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood. 2001;97:1975–81. doi: 10.1182/blood.v97.7.1975. [DOI] [PubMed] [Google Scholar]
- 70.Gan OI, Murdoch B, Larochelle A, et al. Differential maintenance of primitive human SCID-repopulating cells, clonogenic progenitors, and long-term culture-initiating cells after incubation on human bone marrow stromal cells. Blood. 1997;90:641–50. [PubMed] [Google Scholar]


