Abstract
To compare the severity of Plasmodium vivax malaria with that of P. falciparum malaria, we conducted a retrospective cross-sectional study of 356 adults hospitalized with malaria (2009–2011) in Pakistan. P. vivax and P. falciparum accounted for 83% and 13% of cases, respectively; 79.9% of patients with severe malaria were infected with P. vivax.
Keywords: malaria, severe malaria, complications, Plasmodium vivax, Pakistan; parasites
Malaria is endemic to Pakistan and 64% and 36% of malaria cases are attributed to Plasmodium vivax and P. falciparum, respectively (1). The purpose of this study was to identify the complications of P. vivax among hospitalized malaria patients and compare the prevalence of these complications with those of P. falciparum malaria.
The Study
We conducted a retrospective cross-sectional study using convenience sampling at the Aga Khan University Hospital in Karachi, Pakistan. Participants were all adult patients (>16 years of age) who were hospitalized with malaria during January 2009–December 2011. Reasons for hospitalization included intravenous antimalarial therapy, management of associated diagnoses, and complications. The following data on patients were retrieved through the hospital’s electronic and file records: age, sex, infecting Plasmodium species, malaria diagnosis methods, co-existing conditions, results of biochemical and microbiological investigations, radiographic findings, complications, hospital course, and outcome.
Records showed that Giemsa-stained peripheral blood smears, the malaria rapid diagnostic test (RDT), or both, were used for malaria diagnosis. The RDT used antibodies against P. falciparum histidine-rich protein 2 and P. vivax lactate dehydrogenase. For 45 case-patients for which results from peripheral blood smears and RDTs were discordant or unreliable, surface protein-specific PCR was performed by using stored patient blood samples to identify the Plasmodium species (2,3). Clinical syndromes were classified as severe on the basis of the World Health Organization’s 2010 severe falciparum malaria criteria (4).
Statistical analysis was performed by using SPSS version 20 (http://www-01.ibm.com/software/analytics/spss/). Averages, χ2 test of independence, odds ratios with 95% CIs, and analysis of variance were computed when applicable.
Case-patients with prior co-morbid conditions were excluded from relevant subanalyses, for example, diabetes mellitus patients were excluded from hypoglycemia analysis. All analysis was also repeated after excluding all case-patients with associated infections and comorbid illnesses. The classification “comorbidity” included all conditions in the Charlson comorbidity index for mortality (5). The study was approved by the Aga Khan University’s Ethics Review Committee.
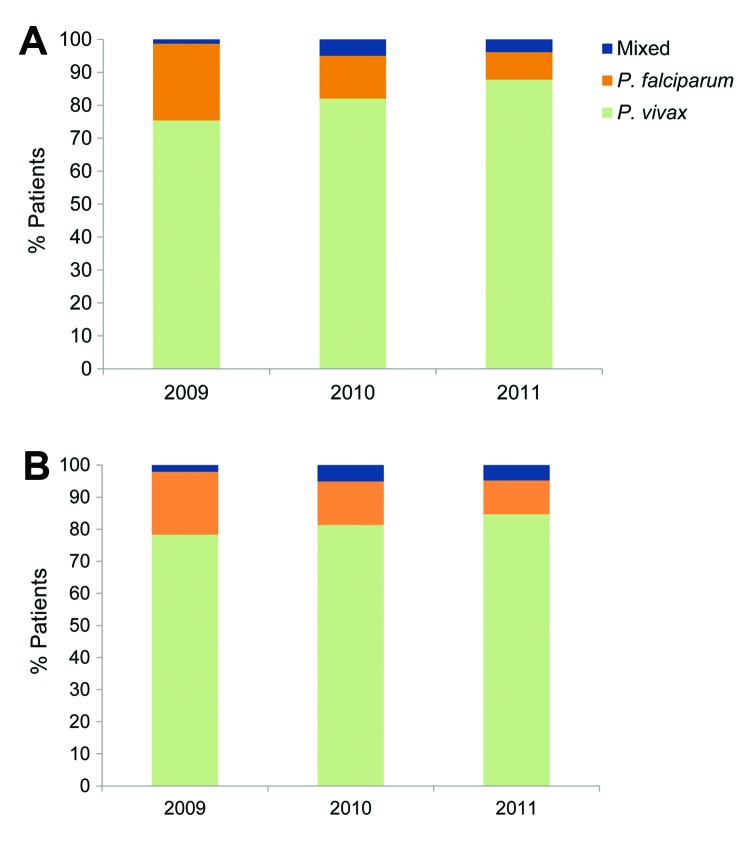
A total of 356 patients with malaria (mean ± SD age 42 ± 18 years) were hospitalized in the Aga Khan Hospital during 2009–2011. Among these, 296 (83.1%), 47 (13.2%), and 13 (3.7%) were found to have P. vivax infection, P. falciparum infection, and mixed infections ( P. vivax and P. falciparum), respectively. Baseline patient demographics are given in Table 1. The proportion of P. vivax infection among hospitalized malaria patients increased from 75.0% in 2009 to 87.7% in 2011 (p<0.02) (Figure 1, panel A).
Table 1. Demographic profile of study participants with Plasmodium vivax and P. falciparum malaria, Karachi, Pakistan, 2009–2011* .
| Characteristic |
Frequency (%) |
||
|---|---|---|---|
|
P. vivax
|
P. falciparum
|
Mixed |
|
| Sex | |||
| F | 98 (33) | 12 (25) | 6 (46) |
| M | 198 (67) | 35 (75) | 7 (54) |
| Previously healthy adults | 189 (64) | 30 (64) | 10 (77) |
| Concurrent illness | |||
| Diabetes | 49 (17) | 4 (9) | 0 |
| Ischemic heart disease | 37 (12) | 2 (4) | 3 (23) |
| Chronic kidney disease | 10 (3) | 3 (6) | 0 |
| Co-existing infection† | 34 (12) | 5 (11) | 0 |
| Others‡ | 10 (3) | 5 (11) | 0 |
| Total§ | 107 (36) | 17 (36) | 3 (23) |
*n = 356. †Co-existing infections included dengue fever, urinary tract infection, enteric fever, and hepatitis C, diagnosed by appropriate serologic testing/culture. ‡Other conditions included chronic obstructive pulmonary disease, chronic liver disease, malignancy, and other conditions from the Charlson Comorbidity Index (5). §Many patients had multiple comorbidities; therefore, the total does not sum the above.
Figure 1.
A) Proportion of hospitalized cases of Plasmodium vivax (n = 296), P. falciparum (n = 47), and mixed (n = 13) infections, Karachi, Pakistan, 2009–2011. B) Number of hospitalized cases of P. vivax (n = 189), P. falciparum (n = 30), and mixed (n = 10) infections, after excluding patients with concurrent illnesses, 2009–2011.
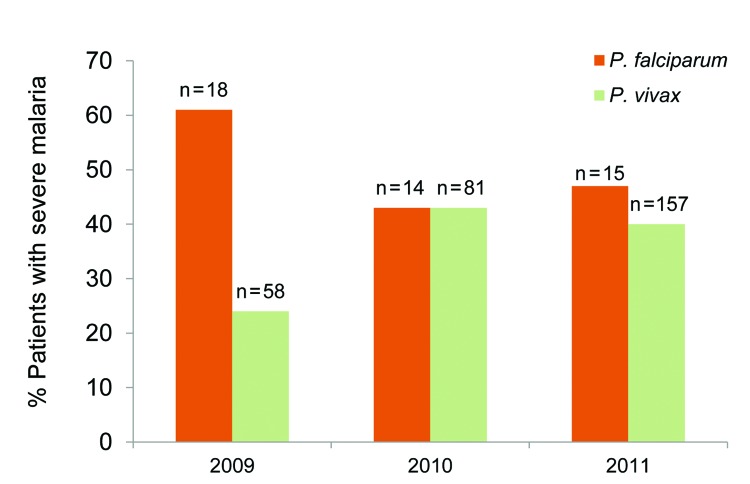
One hundred thirty-nine (39.0%) patients had at least 1 complication by World Health Organization criteria (4), among which 111 (79.9%) patients had P. vivax infection. In 24 (51.0%) cases of P. falciparum infections and in 111 cases (37.5%) of P. vivax infections, respectively, severe malaria developed (p = 0.077). As shown in Figure 2, the proportion of severe malaria among P. vivax patients increased from 24.1% in 2009 to 43.2% in 2010 and 39.5% in 2011 (p = 0.02).
Figure 2.
Percentage of Plasmodium falciparum and P. vivax patients with severe malaria, Karachi, Pakistan, 2009–2011. The number of mixed infections (n = 13) over 3 years was too small for comparison.
The most common complications in the patients are shown in Table 2. P. vivax and P. falciparum were responsible for comparable rates of pulmonary edema, the need for mechanical ventilation, coagulopathy, hypoglycemia, hemoglobinuria, metabolic acidosis, renal impairment, liver dysfunction, bleeding, and multi-organ dysfunction. Altered consciousness, anemia, and jaundice were associated with P. falciparum malaria. The mean platelet count for P. vivax patients was 55, significantly lower than that of P. falciparum patients (67.5; p = 0.001) and those with mixed infections (61; p = 0.024).
Table 2. Comparison of complication rates in P. falciparum versus P. vivax infections, Karachi, Pakistan, 2009–20011*.
| Complications |
Case definition |
No. (%) P. falciparum cases; n = 47 |
No. (%) P. vivax cases; n = 296 |
Odds ratio (CI) |
p value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO criteria† | |||||||||||
| Altered consciousness | Disorientation or confusion | 5 (10.6) | 6 (2.0) | 5.7 (1.7–19.7) | 0.002 | ||||||
| Metabolic acidosis | Plasma bicarbonate <15 mmol/L | 5 (10.6) | 17 (5.7) | 1.9 (0.7–5.6) | 0.203 | ||||||
| Pulmonary edema | Respiratory distress and bilateral diffuse infiltrates on chest radiograph | 6 (12.8) | 23 (7.8) | 1.7 (0.7–4.5) | 0.253 | ||||||
| Abnormal spontaneous bleeding | Bleeding from gastrointestinal, genitourinary or respiratory tracts | 1 (2.1) | 16 (5.4) | 0.4 (0.049–2.9) | 0.336 | ||||||
| Jaundice | Serum bilirubin >3.0 mg/dL | 12 (25.5) | 28(9.5) | 3.3(1.5–7.0) | 0.001 | ||||||
| Hemoglobinuria | Hemoglobin in urine | 15 (31.9) | 62 (20.9) | 1.8 (0.9–3.4) | 0.094 | ||||||
| Shock | Systolic blood pressure <80 mm Hg | 4 (8.5) | 5 (1.7) | 5.4 (1.4–20.9) | 0.007 | ||||||
| Hypoglycemia‡ | Blood glucose <40 mg/dL | 1 (2.1) | 3 (1.0) | 2.1 (0.2–20.9) | 0.509 | ||||||
| Renal impairment§ | Serum creatinine >3 mg/dL | 2 (4.3) | 10 (3.4) | 1.3 (0.3–6.0) | 0.761 | ||||||
| Other | |||||||||||
| Hyperpyrexia | Core body temperature >40°C | 4 (8.5) | 32 (10.8) | 0.8 (0.4–1.9) | 0.416 | ||||||
| Thrombocytopenia | Platelets <150,000/mm3 | 39 (83.0) | 272 (91.9) | 0.4 (0.2–1.0) | 0.051 | ||||||
| Profound | <20,000/mm3 | 5 (10.6) | 58 (19.6) | 0.5 (0.2–1.0) | 0.141 | ||||||
| Anemia | Hemoglobin <7 mg/dL | 10 (21.3) | 15 (5.1) | 5.0 (2.1–12.1) | 0.000 | ||||||
| Multiorgan dysfunction | Biochemical and /or radiographic evidence of ≥2 organs involved | 5 (10.6) | 21 (7.1) | 1.6 (0.6–4.4) | 0.394 | ||||||
| Secondary infection | Radiographic/microbiological evidence of infection | 9 (19.1) | 2 (7.4) | 2.9 (1.3–6.9) | 0.009 | ||||||
| Coagulopathy | Deranged PT/APTT | 5 (10.6) | 17 (5.7) | 2.0 (0.7–5.6) | 0.203 | ||||||
| Liver dysfunction | ALT level >normal | 16 (44.4) | 97 (40.9) | 1.1 (0.5–1.9) | 0.690 | ||||||
*WHO, World Health Organization; PT, prothrombin time; APTT, activated partial thromboplastin time. ALT, alanine aminotransferase. †Source: (4). ‡Patients with preexisting diabetes were excluded from this count; n = 303. §Patients with preexisting chronic kidney disease were excluded from this count; n = 343.
The mean hospital stay was 4.1 days for P. falciparum patients, 3.6 days for P. vivax patients, and 2.9 days for patients with mixed infections. Three P. vivax malaria patients experienced fatal acute myocardial infarctions. One patient, who had metastatic myeloma and P. falciparum malaria, died. The mortality rate was 2.1% for P. falciparum patients and 1.0% for P. vivax patients (p = 0.50).
Analysis was repeated after all patients with comorbid conditions were excluded (Table 1), which left 229 case-patients who had no illness other than malaria. Among these, 30 (13%) patients had P. falciparum infection, 189 (83%) had P. vivax infection, and 10 (4%) had mixed infection (Figure 1, panel B). In these patients, severe malaria appeared significantly more common in falciparum versus vivax malaria (53% and 33%, respectively, p = 0.029); however, 79.5% of the severe cases were caused by P. vivax. Hemoglobinuria and a higher mean creatinine level were more likely to occur with falciparum malaria than with vivax malaria (p<0.02). Shock and secondary bacterial infections were no longer associated with P. falciparum infection. All other statistical associations held, although the strength of association varied.
Conclusions
A study of hospitalized malaria patients at the Aga Khan University Hospital during 1997–2001 showed that 51.8% of cases were caused by P. vivax and 46.5% by P. falciparum, with mortality rates of 1.5% and 2.0%, respectively (6). Recent studies from elsewhere in Asia reported that 20%– 40% of patients hospitalized with malaria had P. vivax malaria (7), with mortality rates of 0.8%–1.6% (7). In our study, a much greater proportion of malaria cases were caused by P. vivax (83%), which was not unexpected because of the decreasing number of P. falciparum cases during the study period. Despite this high incidence of P. vivax malaria, the mortality rate found in our study is reassuring and stable at 1.0%.
The higher prevalence of jaundice, anemia, and hemoglobinuria seen with falciparum malaria in our study reflect the greater degree of hemolysis caused by P. falciparum. P. vivax has been reported elsewhere to cause a similar degree of anemia as P. falciparum (8). Differences in the level of endemic anemia between these study populations and may explain this discrepancy. Similar to our findings, another study reported the incidence of thrombocytopenia in hospitalized patients with vivax malaria as high as 96.3% (9). Pulmonary involvement has often been reported in complicated vivax malaria (7), as we found in our study. Hepatic dysfunction with jaundice has been reported in up to 57% of hospitalized P. vivax patients (10); our findings were similar.
To estimate the true effects of severe disease with vivax malaria, researchers have recommended excluding comorbid conditions (7) and other infections (11). In this study, excluding concurrent illness enabled a stronger association between P. falciparum and severe malaria to emerge. Thus, P. falciparum caused a higher likelihood of specific complications such as central nervous system disturbance and hemolysis than did P. vivax. Yet, ≈80% of severe malaria still occurred in patients with P. vivax malaria.
Limitations of the study include its retrospective design, low power, and lack of PCR diagnostics for all the samples. Furthermore, the study findings reflect the malaria situation at a single urban tertiary care hospital, which cannot be generalized without knowing the denominator of all hospitalized malaria cases in the study area.
P. vivax is a major contributor to the disease effects of malaria, including severe malaria, in a tertiary care setting in Karachi, Pakistan. Furthermore, P. falciparum and P. vivax have similar rates for several complications (pulmonary edema, metabolic acidosis, abnormal bleeding, renal impairment) and death.
Acknowledgments
We acknowledge collaboration with Raymond A. Smego from the University of the Free State, Bloemfontein, South Africa, whose intellectual contribution to this study continued until his untimely death.
PCR studies for this project were funded by an Aga Khan University Research Council grant.
Biography
Dr Zubairi is an Associate Professor and Section Head in Pulmonary and Critical Care Medicine in the Department of Medicine, Aga Khan University Hospital, Karachi. His research interests are asthma, interstitial lung disease, and respiratory tract infections.
Footnotes
Suggested citation for this article: Zubairi ABS, Nizami S, Raza A, Mehraj V, Rasheed AF, Ghanchi NK, et al. Severe Plamodium vivax malaria in Pakistan. Emerg Infect Dis [Internet]. 2013 Oct [date cited]. http://dx.doi.org/10.3201/eid1911.130495
References
- 1.World Health Organization. World malaria report: 2011. Geneva: The Organization; 2011. [Google Scholar]
- 2.Imwong M, Pukrittayakamee S, Grüner AC, Rénia L, Letourneur F, Looareesuwan S, et al. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar J. 2005;4:20 . 10.1186/1475-2875-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zakeri S, Bereczky S, Naimi P, Pedro Gil J, Djadid ND, Färnert A, et al. Multiple genotypes of the merozoite surface proteins 1 and 2 in Plasmodium falciparum infections in a hypoendemic area in Iran. Trop Med Int Health. 2005;10:1060–4. 10.1111/j.1365-3156.2005.01477.x [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines for the treatment of malaria, 2nd ed. Geneva: The Organization; 2010. [Google Scholar]
- 5.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–94. 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 6.Beg MA, Sani N, Mehraj V, Jafri W, Khan MA, Malik A, et al. Comparative features and outcomes of malaria at a tertiary care hospital in Karachi, Pakistan. Int J Infect Dis. 2008;12:37–42. 10.1016/j.ijid.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–5. 10.1097/QCO.0b013e32832f14c1 [DOI] [PubMed] [Google Scholar]
- 8.Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, et al. The anaemia of Plasmodium vivax malaria. Malar J. 2012;11:135. 10.1186/1475-2875-11-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Khanduri U. How benign is benign tertian malaria? J Vector Borne Dis. 2009;46:141–4 . [PubMed] [Google Scholar]
- 10.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8 . [PubMed] [Google Scholar]
- 11.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66 . 10.1016/S1473-3099(09)70177-X [DOI] [PubMed] [Google Scholar]