Abstract
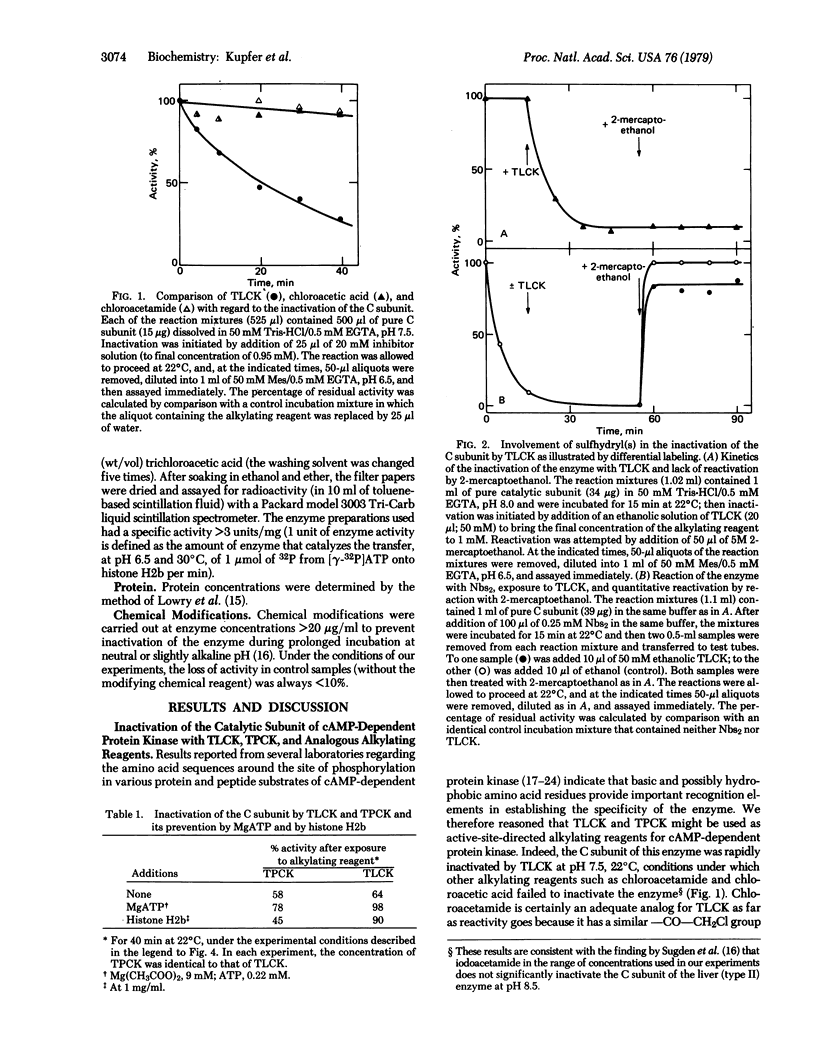
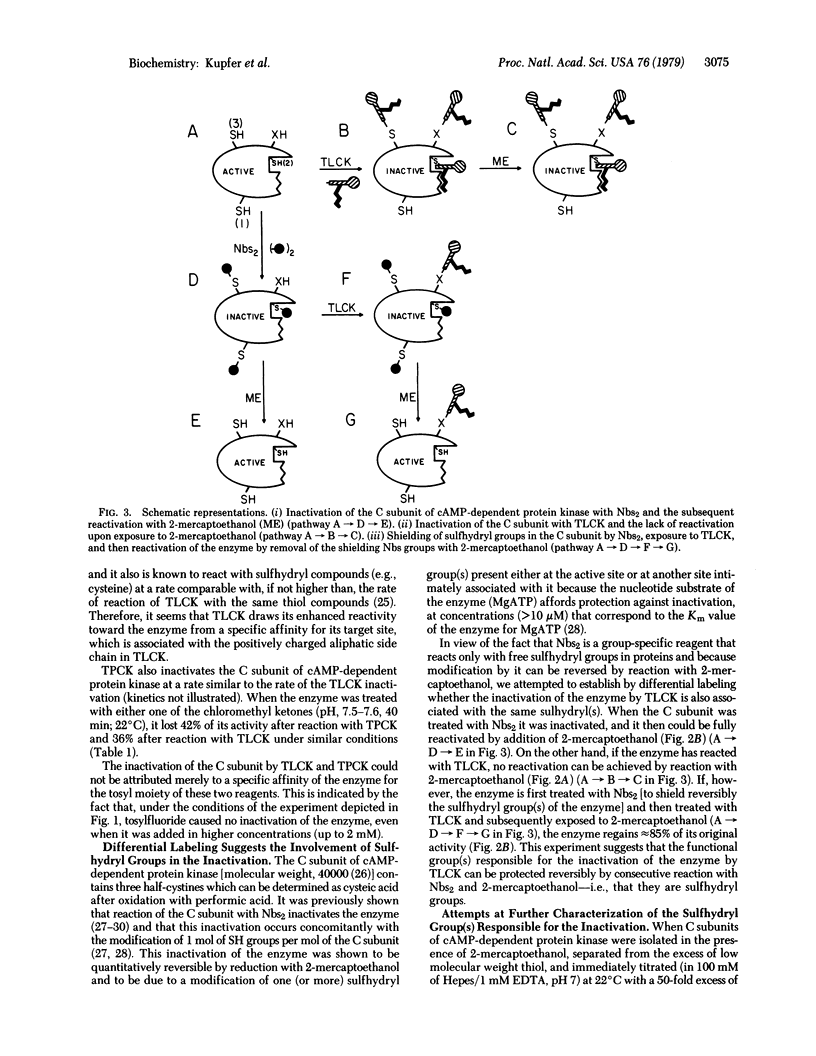
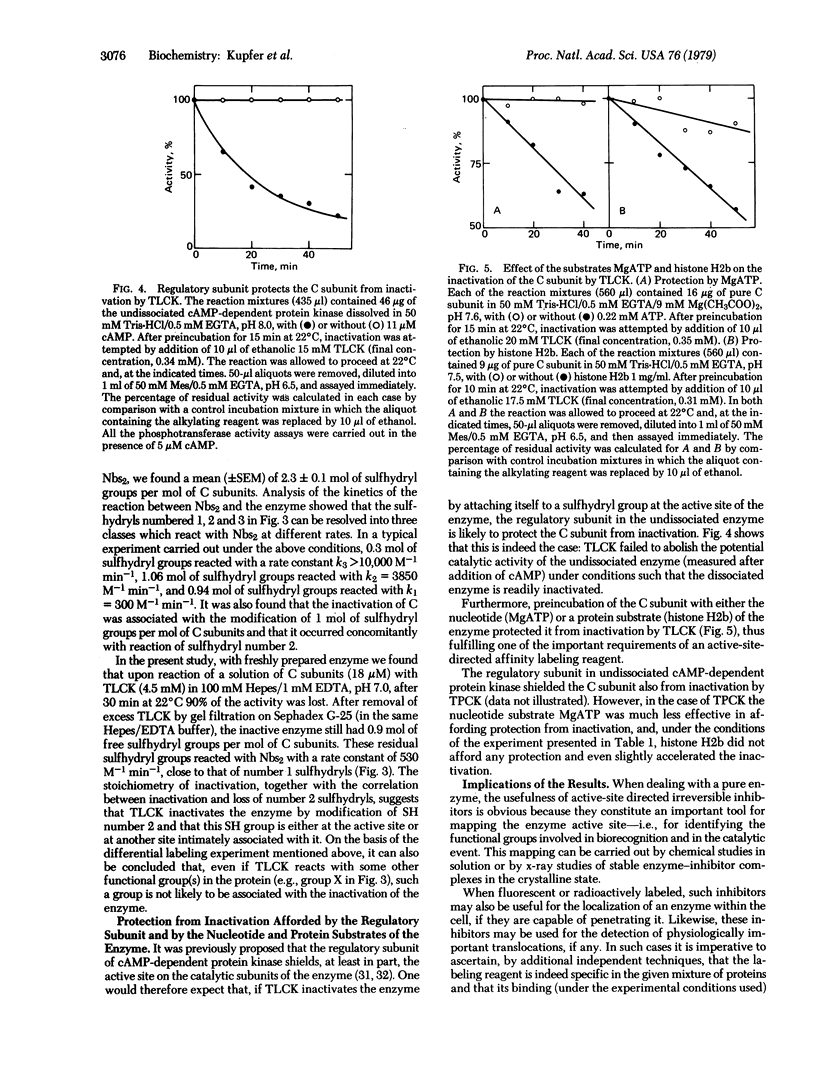
The catalytic subunit of cyclic AMP-dependent protein kinase (from rabbit skeletal muscle; ATP:protein phosphotransferase, EC 2.7.1.37) was found to be irreversibly inactivated by chloromethyl ketone derivatives of lysine and phenylalanine, chemical reagents originally designed for labeling the active sites of the proteolytic enzymes trypsin and chymotrypsin. This inactivation was shown to occur at pH 7.5 and 22 degrees C, conditions under which chemically related alkylating reagents such as chloroacetamide and chloroacetic acid (which do not possess the amino acid side chain) fail to inactivate the enzyme. In the case of the chloromethyl ketone derivative of N alpha-tosyl-L-lysine, the enzyme could be protected by its nucleotide substrate (MgATP), by one of its protein substrates (histone H2b), and by its regulatory subunit which, upon binding, shields the active site of the catalytic subunit. Differential labeling experiments, together with kinetic studies of the rates of modification of the sulfhydryl groups in the enzyme before and after inactivation with the chloromethyl ketone, suggest that the loss of activity is associated with one (kinetically characterized) sulfhydryl group present either at the active site of the enzyme or at a site intimately associated with it. The general implications of these results regarding the interpretation of affinity labeling experiments carried out in complex mixtures of proteins or under in vivo conditions are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. N., Kaiser E. T. Sulfhydryl group reactivity of adenosine 3',5'-monophosphate dependent protein kinase from bovine heart: a probe of holoenzyme structure. Biochemistry. 1978 Jul 11;17(14):2840–2845. doi: 10.1021/bi00607a022. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Mechanisms of control for cAMP-dependent protein kinase from skeletal muscle. Adv Cyclic Nucleotide Res. 1975;5:241–251. [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Brostrom M. A., Reimann E. M., Walsh D. A., Krebs E. G. A cyclic 3',5'-amp-stimulated protein kinase from cardiac muscle. Adv Enzyme Regul. 1970;8:191–203. doi: 10.1016/0065-2571(70)90017-8. [DOI] [PubMed] [Google Scholar]
- Böhm E. L., Strickland W. N., Strickland M., Thwaits B. H., van der Westhuizen D. R., von Holt C. Purification of the five main calf thymus histone fractions by gel exclusion chromatography. FEBS Lett. 1973 Aug 15;34(2):217–221. doi: 10.1016/0014-5793(73)80797-5. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R., Young J. D. Synthetic substrate for cyclic AMP-dependent protein kinase. Nature. 1975 Oct 2;257(5525):416–418. doi: 10.1038/257416a0. [DOI] [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Hirsch A. H., Rosen O. M. Interconversion of cyclic nucleotide-activated and cyclic nucleotide-independent forms of a protein kinase from beef heart. Proc Natl Acad Sci U S A. 1971 Apr;68(4):731–735. doi: 10.1073/pnas.68.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmquist G., Andersson J., Edlund B., Engstroöm L. Amino acid sequence of a (32P) phosphopeptide from pig liver pyruvate kinase phosphorylated by cyclic 3',5'-AMP-stimulated protein kinase and gamma-(32P)ATP. Biochem Biophys Res Commun. 1974 Nov 27;61(2):559–563. doi: 10.1016/0006-291x(74)90993-0. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Freist W., Marutzky R., Shaltiel S. Mapping the ATP-binding site in the catalytic subunit of adenosine-3':5'-monophosphate-dependent protein kinase. Spatial relationship with the ATP site of the undissociated enzyme. Eur J Biochem. 1978 Oct 16;90(3):427–432. doi: 10.1111/j.1432-1033.1978.tb12621.x. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Wagner K. G. An improved method for the purification of cAMP-dependent protein kinase from rabbit muscle using hydrophobic chromatography. FEBS Lett. 1977 Feb 15;74(1):95–98. doi: 10.1016/0014-5793(77)80761-8. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Bylund D. B., Stull J. T., Krebs E. G. The amino acid sequences of the phosphorylated sites in troponin-I from rabbit skeletal muscle. FEBS Lett. 1974 Jun 15;42(3):249–252. doi: 10.1016/0014-5793(74)80738-6. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Hadden J. W., Inoue A., Allfrey V. G. DNA binding by cyclic adenosine 3',5'-monophosphate dependent protein kinase from calf thymus nuclei. Biochemistry. 1975 Aug 26;14(17):3873–3884. doi: 10.1021/bi00688a022. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Cyclic AMP and histone phosphorylation. Ann N Y Acad Sci. 1971 Dec 30;185:166–180. doi: 10.1111/j.1749-6632.1971.tb45246.x. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Protein kinases and protein kinase substrates. Adv Cyclic Nucleotide Res. 1973;3:99–153. [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Powers J. C. Reaction of serine proteases with halomethyl ketones. Methods Enzymol. 1977;46:197–208. doi: 10.1016/s0076-6879(77)46020-8. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Separation of regulatory and catalytic subunits of the cyclic 3',5'-adenosine monophosphate-dependent protein kinase(s) of rabbit skeletal muscle. Biochem Biophys Res Commun. 1971 Jan 22;42(2):187–194. doi: 10.1016/0006-291x(71)90086-6. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Salas M. L., Lipmann F. Mechanism of activation by adenosine 3':5'-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Sep;67(1):408–414. doi: 10.1073/pnas.67.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P. The specificity of adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem Soc Trans. 1976;4(6):1027–1030. doi: 10.1042/bst0041027. [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U., Humble E., Berglund L., Engström L. The minimum substrate of cyclic AMP-stimulated protein kinase, as studied by synthetic peptides representing the phosphorylatable site of pyruvate kinase (type L) of rat liver. Biochem Biophys Res Commun. 1976 Jun 7;70(3):696–703. doi: 10.1016/0006-291x(76)90648-3. [DOI] [PubMed] [Google Scholar]



