Abstract
Primary lateral sclerosis (PLS) has been regarded as a rare, extreme form of amyotrophic lateral sclerosis (ALS). Like ALS, it is a clinical diagnosis without established biomarkers. We sought to explore loss of cerebral myelin in relation to clinical features, including cognitive impairment, in cases of both ALS and PLS.
A novel MRI sequence (mcDESPOT) sensitive to water pools within myelin and intra- and extra-cellular spaces was applied to 23 ALS patients, seven PLS patients and 12 healthy controls, with interval follow-up in 15 ALS and four PLS patients.
Results demonstrated that PLS patients were distinguished by widespread cerebral myelin water fraction reductions, independent of disease duration and clinical upper motor neuron burden. ALS patients showed a significant increase in intra- and extra-cellular water, indirectly linked to neuroinflammatory activity. Limited measures of cognitive impairment in the ALS group were associated with myelin changes within the anterior corpus callosum and frontal lobe projections. Longitudinal changes were only significant in the PLS group. In conclusion, in this exploratory study, myelin imaging has potential to distinguish PLS from ALS, and may have value as a marker of extramotor involvement. PLS may be a more active cerebral pathological process than its rate of clinical deterioration suggests.
Keywords: Imaging, biomarker, pathology
Introduction
Charcot's pivotal observation of the pallor of the corticospinal tracts (CSTs) forms the diagnostic cornerstone of amyotrophic lateral sclerosis (ALS) as a neurodegenerative syndrome characterized by progressive loss of both upper motor neurons (UMNs) of the corticospinal tract (CST), and lower motor neurons (LMNs) arising from the brainstem and spinal anterior horns. ALS is clinically heterogeneous, and a significant proportion of cases clinically shows relative selectivity for LMN or UMN involvement. There is also clinical, pathological and genetic overlap between ALS and some forms of frontotemporal dementia (FTD) (1).
A small proportion who manifest solely UMN signs clinically, typically of lower limb-onset, are termed primary lateral sclerosis (PLS). This syndrome was first recognized by Erb (2) before clinicopathological papers defined the association with a consistently slower progression and survival typically 10–20 years from symptom onset (3,4). PLS is a clinical diagnosis, defined as UMN-only involvement for at least four years from symptom onset (5). Early in its course PLS is difficult to distinguish from the ALS clinical sub-type of ‘UMN-predominant’ ALS, which also has a consistently slower-than-average rate of progression (6). The prognostically more ‘benign’ implications of a diagnosis of PLS compared to ALS, means that biomarkers able to discriminate between the two conditions at an earlier stage might improve stratification of patients in therapeutic trials, reducing the potential for type II errors, as well as optimize care planning (7).
Advanced MRI applications have generated multiple candidate biomarkers in ALS (8). A consistent signature of white matter tract alteration is detectable, using diffusion tensor imaging (DTI), across clinically heterogeneous cases of ALS, involving the rostral corticospinal tract (CST) and corpus callosum (CC), assumed to reflect a secondary (Wallerian-type) loss of myelin (9).
Multi-component relaxation imaging separates MRI signal into contributions from sub-voxel anatomical water pools, broadly corresponding to intra- and extra-cellular water (IE-water), which relaxes slowly, and water trapped between the myelin bilayers, which relaxes more quickly. The myelin water fraction (MWF) correlates strongly with gold standard histopathological measurements of myelin content (10,11), thus providing a non-invasive surrogate. Shifts in the T2 of the IE-water have been linked to changes in water content, and specifically inflammation or oedema (12,13). Multi-component Driven Equilibrium Single Pulse Observation of T1 & T2 (mcDESPOT) (14), provides a whole-brain characterization of myelin- and IE-water changes. We explored mcDESPOT's sensitivity to differences in brain white matter microstructure to distinguish the pathological process in ALS and PLS.
Methods
Participants
Prevalent and incident cases of ALS and PLS seen in a large tertiary referral clinic, diagnosed by two experienced neurologists (KT, MRT) according to standard criteria, were offered participation as part of a larger multi-modal, six-monthly assessment, longitudinal Oxford Study for Biomarkers in Motor Neuron Disease (‘BioMOx’). All patients were apparently sporadic apart from one individual with ALS and a dominant family history associated with a hexanucleotide repeat expansion in C9orf72. Healthy volunteers without significant past medical history (spouses and friends of patients) were recruited for a single study only. All participants were capable of providing informed consent. The study was approved by the South Oxfordshire Research Ethics Committee.
Clinical assessment
All patients were examined at each visit (MRT). Disease duration was calculated in months from symptom onset to date of assessment. Disability was assessed using the revised ALS Functional Rating Score (ALSFRS-R, 0–48, lower score reflects greater disability). Progression rate was calculated as: (48 minus ALSFRS-R)/(disease duration). A sum score of pathological reflexes (0–15) was used to stratify the clinical UMN burden (9,15).
Participants were assessed on the same day as MRI using the revised Addenbrookes Cognitive Examination (lower score reflects poor cognitive performance (16)), except those few patients for whom significant communication impairment precluded study (n = 3). Verbal fluency sub-scores were analysed separately for the patients in addition to the total score. This included letter fluency scores (as many words beginning with p in one minute) and category fluency (as many animals beginning with any letter in one minute), with lower scores reflecting worse performance. Those participants without significant upper limb disability were also scored for the written Trail-Making Test (TMT) of executive function, in which the standard measure of Trail B minus Trail A time in seconds was used for scoring (higher score reflects greater impairment of executive function).
MRI acquisition
Scans were performed at the Oxford Centre for Clinical Magnetic Resonance Research using a 3T Siemens Trio scanner (Siemens AG, Erlangen, Germany) with a 12-channel head coil. The mcDESPOT protocol consisted of a 14-min series of sagittal spoiled gradient recalled echo (SPGR) and balanced steady state free procession (bSSFP) acquisitions across a range of flip angles (α), as well as an inversion–recovery-prepared SPGR (IR-SPGR) scan for correction of flip angle inhomogeneity (17). A common field of view of 22 = 22 = 18 cm and voxel size of 1.7 = 1.7 = 1.7 mm was used. Other scan parameters were:
SPGR: TE = 2.5 ms; TR = 5.6 ms; BW = ± 24 kHz; α = [3, 4, 5, 6, 7, 9, 12, 18]°.
bSSFP: TE = 2.2 ms; TR = 4.4 ms; BW = ± 36 kHz; α = [10, 13, 17, 20, 23, 30, 43, 60]°; all flip angles were acquired with phase-cycling patterns of ϕ = 0° and 180° for correction of off-resonance effects (17).
IR-SPGR: TE = 2.5 ms; TR = 5.6 ms; BW = ± 24 kHz, TI = 450 ms, α = 5°, slice thickness = 3.4 mm.
MRI analysis
mcDESPOT pre-processing. mcDESPOT datasets were linearly coregistered to account for subtle inter-scan motion using FLIRT, and non-brain parenchymal signal removed using an automated approach with BET (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl). Voxelwise MWF and IE-water T2 maps were derived using published mcDESPOT processing (17).
White matter tract-based analysis. To spatially compare MWF values across subjects and groups, tract-based spatial statistics (TBSS) were applied (18), reducing bias due to inaccurate spatial registration by performing analysis along a skeletonized WM mask representing the mid-line of each WM pathway. TBSS does not make use of DTI fibre orientation information, so may be directly applied to MWF maps with minor modification (19).
Individual MWF images were non-linearly aligned to every other using standard TBSS registration methods, and the most representative MWF map was assigned as the target template. The target was then affine-aligned into MNI152 standard space, and individual MWF maps were transformed to this space by combining the non-linear transform to the target template with the affine transform to MNI152 space. The cross-subject mean MWF map was computed and used to generate a WM tract ‘skeleton’, thresholded at MWF > 0.1. Individual MWF images were projected onto the skeleton for statistical comparison. The identical transformation was applied to the IE-water T2 maps to create skeletonized IE-water T2 maps.
Group differences in MWF or IE-water T2, and relationships between these metrics and clinical measures were tested using Randomise along the TBSS skeleton. Statistical significance was defined as p < 0.05, corrected for multiple comparisons by controlling family-wise error rate after Threshold-Free Cluster Enhancement (TFCE) (20). Global or ROI group differences were assessed using a Mann-Whitney test, and correlations using Spearman's rank order correlation coefficient.
Longitudinal analysis. Those patients with follow-up scans were included in a second TBSS analysis. Changes over time were assessed with a paired t-test using Randomise across the TBSS skeleton and also globally for the entire TBSS skeleton. Correlations with progression rate were also assessed using Spearman's rank order correlation coefficient.
Results
Participants
Thirty patients (23 ALS, seven PLS) and 12 control subjects underwent MRI. A total of 19 patients (15 ALS, four PLS) underwent a second scan (Table I).
Table I.
Clinical features and functional scores of the study participants.
| Diagnosis | Age at scan | Sex | Symptom onset | Duration (months) | MRI interval (months) | ALSFRS-R baseline, follow-up | UMN score baseline, follow-up | Baseline rate of progression | TMT B – A (seconds | ACE (max 100) | Verbal fluency (max 7) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Letter | Category | |||||||||||||
| ALS01 | 65 | M | BO | 30 | 7.4 | 43 | 43 | 13 | 11 | 0.17 | 41 | 98 | 6 | 7 |
| ALS02 | 67 | M | RUL | 70 | 5.7 | 35 | 35 | 3 | 3 | 0.19 | – | 96 | 4 | 7 |
| ALS03 | 70 | M | RUL | 46 | 7.8 | 27 | 27 | 6 | 5 | 0.46 | – | 90 | 4 | 7 |
| ALS04 | 61 | M | LLL | 34 | 8.0 | 28 | 21 | 7 | 3 | 0.59 | 50 | 91 | 6 | 6 |
| ALS06 | 67 | F | BO | 63 | 6.3 | 33 | – | 13 | – | 0.24 | 9 | – | – | – |
| ALS07 | 77 | M | BO | 57 | 6.5 | 27 | 26 | 10 | 10 | 0.37 | 73 | 73 | – | – |
| ALS08 | 64 | M | LLL | 88 | 6.7 | 28 | 28 | 1 | 1 | 0.23 | 68 | 87 | 6 | 4 |
| ALS09 | 55 | F | RLL | 50 | – | 23 | – | 9 | – | 0.49 | 31 | 95 | 5 | 7 |
| ALS11 | 49 | F | RUL | 80 | 6.0 | 29 | 29 | 5 | 5 | 0.24 | – | 97 | 5 | 7 |
| ALS12 | 41 | M | LUL | 41 | 6.9 | 26 | 23 | 15 | 15 | 0.54 | 27 | 99 | 6 | 7 |
| ALS13 | 69 | M | LUL | 33 | 5.3 | 39 | 34 | 4 | 4 | 0.27 | 29 | 93 | 5 | 6 |
| ALS14 | 48 | F | RLL | 135 | 9.7 | 30 | 30 | 10 | 10 | 0.13 | 41 | 92 | 5 | 6 |
| ALS15 | 61 | M | RUL | 141 | – | 23 | – | 1 | – | 0.18 | – | 93 | 6 | 7 |
| ALS16 | 56 | F | LLs | 65 | 7.4 | 27 | 24 | 3 | 3 | 0.32 | – | 99 | 7 | 7 |
| ALS18 | 46 | F | RLL | 21 | 7.3 | 32 | 18 | 12 | 12 | 0.77 | 10 | 97 | 6 | 7 |
| ALS19 | 56 | F | LLL | 98 | 7.9 | 31 | 30 | 3 | 4 | 0.17 | 21 | 100 | 7 | 7 |
| ALS20 | 49 | M | LLL | 28 | 6.2 | 35 | 31 | 15 | 15 | 0.46 | – | – | 5 | 5 |
| ALS21 | 76 | M | RLL | 14 | 6.0 | 34 | 28 | 7 | 11 | 1.00 | 63 | 85 | 5 | 6 |
| ALS22 | 76 | M | Trunk | 61 | – | 43 | – | 3 | – | 0.08 | 54 | 98 | 6 | 6 |
| ALS24 | 55 | M | RLL | 15 | – | 42 | – | 12 | – | 0.41 | 23 | 97 | 6 | 7 |
| ALS25 | 75 | M | LLL | 81 | – | 35 | – | 13 | – | 0.16 | 46 | 76 | 6 | 5 |
| ALS26 | 52 | F | RLL | 20 | – | 38 | – | 11 | – | 0.51 | 19 | 93 | 4 | 6 |
| ALS27 | 64 | F | RUL | 8 | – | 18 | – | 14 | – | 3.94 | – | – | – | – |
| ALS mean (SD) | 61 (11) | 9F:14M | 56 (36) | 7 (1) | 32 (7) | 28 (6) | 8 (5) | 8 (5) | 0.52 (0.78) | 38 (20) | 92 (7) | 5 (1) | 6 (1) | |
| PLS01 | 63 | F | LLL | 92 | 7.3 | 32 | 27 | 15 | 15 | 0.17 | 30 | 98 | 6 | 7 |
| PLS02 | 45 | F | LLs | 180 | – | 35 | – | 15 | – | 0.07 | 27 | 77 | 5 | 6 |
| PLS03 | 77 | F | LLs | 62 | 7.2 | 34 | 32 | 10 | 10 | 0.22 | 70 | 96 | 7 | 5 |
| PLS04 | 77 | F | BO | 155 | – | 30 | – | 15 | – | 0.12 | 18 | 92 | 5 | 5 |
| PLS05 | 64 | M | RLL | 184 | 6.3 | 20 | 18 | 15 | 15 | 0.15 | – | 98 | – | – |
| PLS06 | 70 | M | RLL | 366 | 7.4 | 29 | 27 | 13 | 15 | 0.05 | 45 | 94 | 5 | 5 |
| PLS07 | 65 | F | LLL | 260 | – | 28 | – | 15 | – | 0.08 | 42 | – | 4 | 3 |
| PLS mean (SD) | 66 (11) | 5F:2M | 186 (103) | 7 (1) | 30 (5) | 26 (14) | 14 (2) | 14 (8) | 0.12 (0.06) | 39 (18) | 93 (8) | 5 (1) | 5 (1) | |
| Control | 66 | M | – | – | – | – | – | – | – | – | 24 | 100 | 7 | 7 |
| Control | 38 | F | – | – | – | – | – | – | – | – | 23 | 93 | 7 | 7 |
| Control | 46 | M | – | – | – | – | – | – | – | – | 36 | 97 | 7 | 7 |
| Control | 68 | F | – | – | – | – | – | – | – | – | 14 | 99 | 7 | 7 |
| Control | 38 | F | – | – | – | – | – | – | – | – | 15 | 98 | 5 | 7 |
| Control | 56 | F | – | – | – | – | – | – | – | – | 12 | 100 | 7 | 7 |
| Control | 46 | M | – | – | – | – | – | – | – | – | 20 | 99 | 7 | 7 |
| Control | 65 | F | – | – | – | – | – | – | – | – | 17 | 98 | 5 | 7 |
| Control | 66 | M | – | – | – | – | – | – | – | – | 23 | 100 | 7 | 7 |
| Control | 72 | M | – | – | – | – | – | – | – | – | 16 | 99 | 7 | 7 |
| Control | 45 | F | – | – | – | – | – | – | – | – | 10 | 98 | 7 | 7 |
| Control | 64 | F | – | – | – | – | – | – | – | – | 19 | 99 | 7 | 7 |
| Control mean (SD) | 56 (12) | 7F:5M | 19 (7) | 98 (2) | 7 (1) | 7 (0) | ||||||||
ACE: Addenbrookes Cognitive Examination; BO: bulbar onset; LUL: left upper limb; LLL: left lower limb; LLs: bilateral lower limb onset; RUL: right upper limb; RLL: right lower limb; TMT: Trail-Making Test; UMN: upper motor neuron. Bold type: p < 0.05.
Cross-sectional MWF reductions
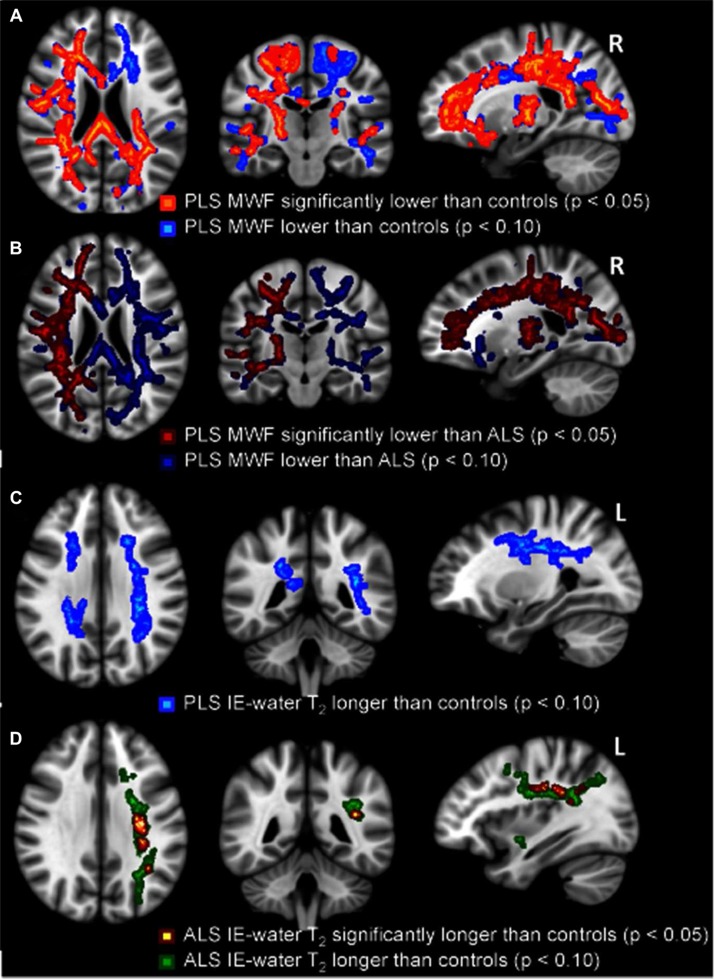
PLS compared to controls. Widespread, symmetrical regions of significantly decreased MWF were found in PLS (Figure 1A). The mean reduction was 2.0% across the entire WM skeleton (p < 0.05), and 4.4% for the area found to be significant (p < 0.0005).
Figure 1.
Reductions in MWF for PLS patients compared to healthy controls (A, significant reductions in orange with trends in blue), and PLS patients compared to ALS patients (B, significant reductions in red with trends in dark blue). Increases in IE-water T2 for PLS patients compared to healthy controls (C, non-significant trends in light blue) and ALS patients compared to healthy controls (D, significant increases in red-yellow with trends in green). All p-values corrected for multiple comparisons. Images displayed by radiological convention.
ALS compared to controls. MWF was not significantly decreased compared to controls, with a mean only 0.2% lower than controls across the entire WM skeleton (p = 0.3). In an analysis of a subgroup of ALS patients with the highest clinical UMN score (n = 7, mean UMN score = 14 (range 12–15)), thus matching for the UMN characteristics of the PLS group, there was still no significant decrease in MWF found anywhere in the WM skeleton between ALS and controls (p = 0.4). Similarly, no significant reduction (p = 0.3) in MWF was found within a subgroup of ALS patients with the longest disease durations (approaching the disease duration characteristics of the PLS group; n = 7, mean disease duration = 99 ± 28 months).
PLS compared to ALS. In direct comparison, MWF was significantly lower for PLS patients than for ALS patients (Figure 1B), with a right hemisphere predominance. The average MWF reduction in PLS compared to ALS was 1.8% across the entire WM skeleton (p < 0.05) and 4.3% for the area of most significant reduction (p < 0.0005).
Cross-sectional IE-water T2 increases
PLS compared to controls. There was a non-significant trend (0.05 < p < 0.1) to increased IE-water T2 for PLS patients compared to healthy controls, seen within the corona radiata bilaterally (Figure 1C). The average increase across the WM skeleton was 1.0% (p = 0.06). Both need guarded interpretation given the small group size.
ALS compared to controls. IE-water T2 was significantly increased in ALS patients compared to healthy controls (Figure 1D), with an average increase across the WM skeleton of 0.6% (p = 0.1), but 3.8% for the area of most significant increase (p < 0.0001). The non-significant trends of increase (0.05 < p < 0.1) lateralized to left corona radiata.
PLS compared to ALS. There were no significant differences in IE-water T2 found between the two patient groups.
Correlations with physical disability and disease duration
No significant correlations between MRI measures and UMN, ALSFRS-R, disease duration, or progression rate were found in either group of patients, nor for both groups combined (Table II).
Table II.
Spearman rank correlation coefficient and p-values for correlations between clinical measures and mcDESPOT MWF (top) and IE-water T2 (bottom) across the entire TBSS skeleton.
| ALSFRS-R |
Disease duration |
Progression rate |
ACE |
TMT |
Letter fluency |
Category fluency |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | |
| MWF | ||||||||||||||
| ALS | −0.39 | 0.06 | 0.06 | 0.73 | 0.15 | 0.50 | 0.54 | 0.01 | −0.49 | 0.05 | 0.22 | 0.36 | 0.40 | 0.09 |
| PLS | 0.00 | 1.00 | 0.21 | 0.64 | −0.32 | 0.48 | −0.40 | 0.50 | −0.49 | 0.33 | −0.62 | 0.27 | 0.30 | 0.62 |
| Combined | −0.26 | 0.17 | −0.12 | 0.55 | 0.24 | 0.20 | 0.42 | 0.04 | −0.49 | 0.02 | 0.13 | 0.56 | 0.48 | 0.02 |
| IE-water T2 | ||||||||||||||
| ALS | 0.17 | 0.44 | −0.04 | 0.85 | −0.14 | 0.51 | −0.65 | 0.002 | 0.66 | 0.005 | −0.21 | 0.38 | −0.65 | 0.003 |
| PLS | −0.11 | 0.82 | 0.00 | 1.00 | 0.11 | 0.82 | 0.40 | 0.50 | 0.43 | 0.40 | 0.41 | 0.49 | −0.10 | 0.87 |
| Combined | 0.09 | 0.64 | 0.05 | 0.80 | −0.15 | 0.43 | −0.52 | 0.008 | 0.57 | 0.005 | −0.12 | 0.57 | −0.65 | 0.0006 |
ACE: Addenbrookes Cognitive Examination; TMT: Trail-Making Test.
Correlations with cognitive dysfunction
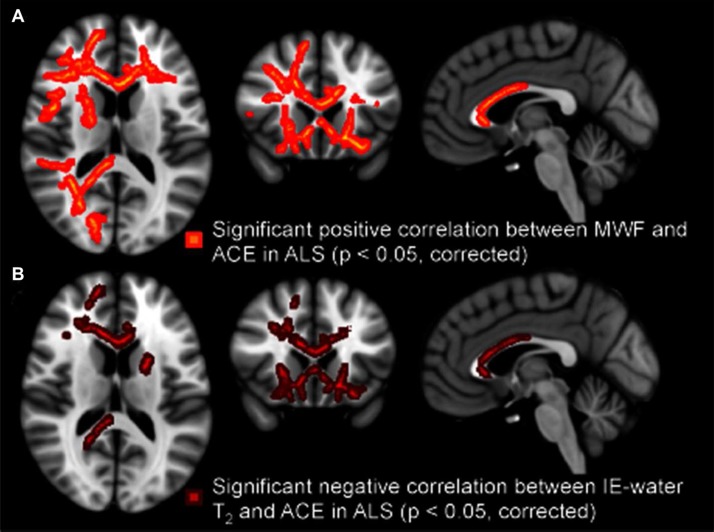
The total ACE score was found to correlate with MWF (positively) and IE-water T2 (negatively) in the ALS group, but not the PLS group (Table II). The correlations were found primarily in frontal regions (Figure 2). While the small PLS group size means these conclusions must be tentative, we noted that combining the patient groups reduced the significance of these differences compared to controls.
Figure 2.
Regional white matter tract correlations, notably including the anterior corpus callosum and frontal projections, between lower ACE scores and reduced MWF in ALS patients (A), and increased IE-water T2 in ALS patients (B). All p-values corrected for multiple comparisons. Images displayed by radiological convention.
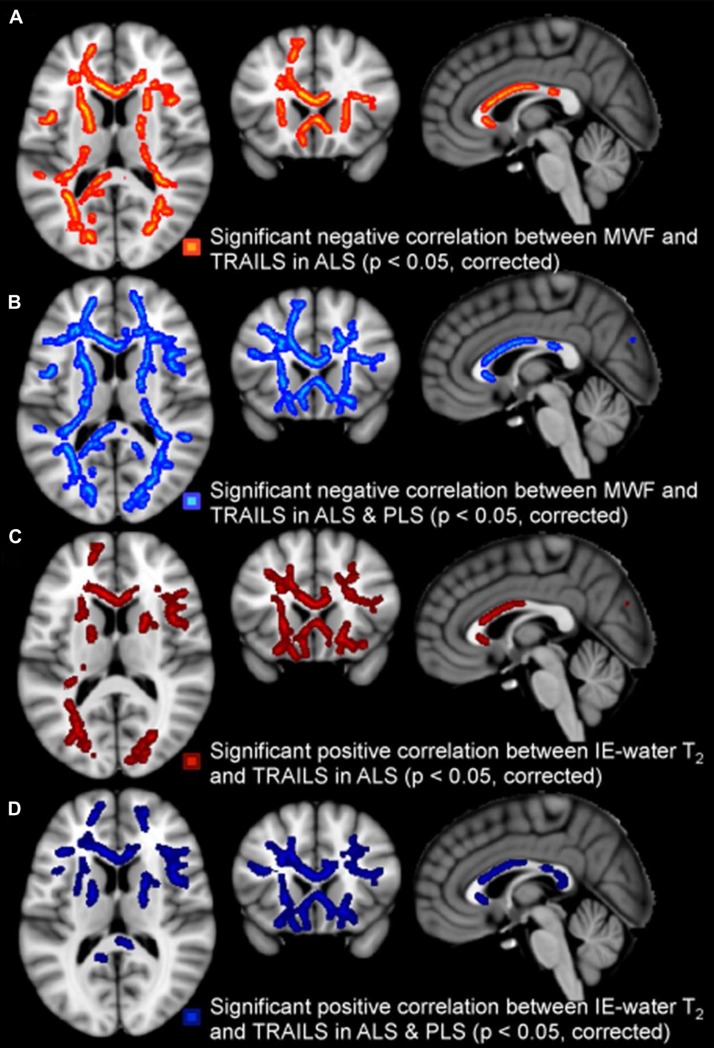
The TMT B-A score correlated with MWF (negatively) and IE-water T2 (positively) when both patient groups were combined, but the IE-water T2 increases also for the ALS patient group in isolation (Table II). The MWF correlations were spread throughout the white matter, but the IE-water T2 correlations were focused in frontal regions (Figure 3).
Figure 3.
Regional white matter tract correlations, notably including anterior corpus callosum and frontal projections, between higher TMT B-A scores (reflecting dysexecutive function) and reduced MWF in the ALS group (A), in the ALS and PLS patients combined (B); IE-water T2 increases in ALS patients (C) and IE-water T2 increases in the combined group (D). All p-values corrected for multiple comparisons. Images displayed by radiological convention.
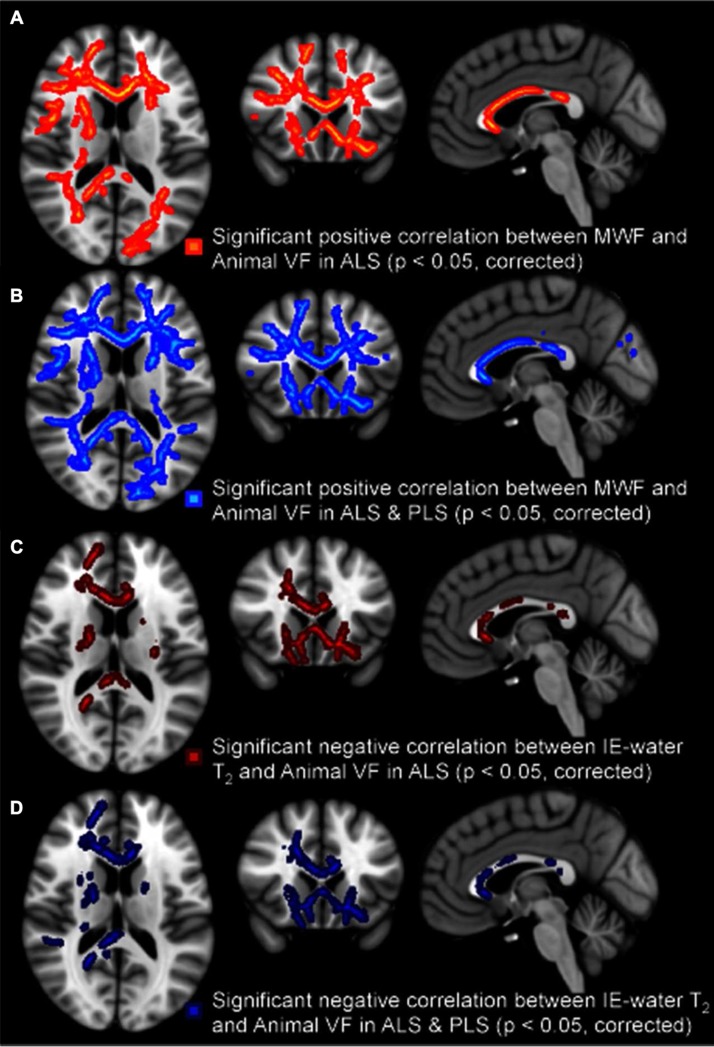
Letter fluency was not correlated with either MRI parameter. However, category fluency was correlated with MWF (positively) and IE-water T2 (negatively) for the ALS group, and more strongly with both patient groups combined. The MWF correlations were once again more widespread with the IE-water T2 correlations found primarily in frontal rather than temporal lobe projection tracts (Figure 4).
Figure 4.
Regional white matter tract correlations, with a notable involvement of frontal lobe projections, between reduced category fluency and decreased MWF in ALS patients (A), decreased MWF in all patients (B), increased IE-water T2 in ALS patients only (C), and increased IE-water T2 in all patients (D). All p-values corrected for multiple comparisons. Images displayed by radiological convention.
With the implementation of rigorous Bonferroni correction for multiple measures (in addition to those measures inherent to the image analysis software), only the correlations between IE-water T2 and the total ACE score in ALS patients, and category fluency in ALS patients and the combined patient group remained significant.
Longitudinal MWF reductions
The average MWF across the entire TBSS skeleton was significantly decreased at follow-up for the PLS patients (on average 1.4% lower, p = 0.02), but not for the ALS patients (0.06% higher, p = 0.9). However, no changes were significant on a voxelwise basis for either group (data not shown). Change in MWF was not significantly correlated with mean progression rate for PLS (r = − 0.80, p = 0.20) nor ALS (r = − 0.21, p = 0.5) patients, nor both groups combined (r = − 0.40, p = 0.09).
Longitudinal IE-water T2 increases
The average IE-water T2 across the entire TBSS skeleton was significantly increased at follow-up for the PLS patients (on average 1.6% higher, p = 0.03), but not significantly for the ALS patients (0.41% higher, p = 0.3) (data not shown). Voxelwise, neither group alone showed a significant increase, but combining both patient groups revealed significant increases, though only on the left side of the brain (data not shown). Change in IE-water T2 was not significantly correlated with mean progression rate for PLS (r = 0.80, p = 0.20) nor ALS (r = 0.15, p = 0.6) patients, nor both groups combined (r = 0.10, p = 0.7).
Discussion
This exploratory in vivo cerebral myelin imaging study involved only a small number of PLS patients, and so the conclusions are necessarily tentative:
PLS patients were distinguished by widespread cerebral MWF reductions that did not appear simply driven by longer disease duration, level of disability or the larger UMN burden.
ALS, not PLS, patients had ‘neuroinflammatory’ increased IE-water T2 in the cerebral deep white matter.
Reductions in global cognitive score and executive function (particularly category fluency) were linked to myelin changes with frontal lobe predominance in ALS.
Longitudinal changes in myelin imaging measures were observed in the PLS rather than ALS group.
PLS as a distinct disorder
Clinical, genetic and neuropathological features of PLS support it being a distinct disorder, rather than an extreme of the spectrum of ALS (Table III). The nosology of PLS emerged at the end of the 19th century (reviewed in (4)), followed in 1943 by a series of 21 cases contrasted clinically with ALS (21). The observation that humans have direct corticomotoneuronal connections to anterior horns has been postulated as the reason for UMN involvement more generally in ALS compared to LMN-only degenerations seen in lower-order animals (22). It is the striking atrophy of the primary motor cortex that has been most consistently observed as the distinguishing feature of PLS, visible on MRI (4,23), and matched by complete loss of Betz cells of layer V (4,24) to an extent rarely observed in ALS.
Table III.
Features that support PLS as a distinct pathological entity to ALS.
| Characteristic |
|---|
| Clinical features |
| Extreme length of survival from symptom onset |
| UMN-only involvement |
| Exclusively leg or bulbar-onset |
| No consistent FTD overlap |
| Genetics |
| Single familial pedigree (54), without ALS-like cases |
| Not described in most familial ALS pedigrees |
| Neuropathology |
| Profound atrophy of motor cortex with marked Betz cell loss |
Post mortem insights
Post mortem studies in ALS, including those with no UMN signs, reveal consistent brain and cord pyramidal tract demyelination (25,26). There are few dedicated studies in PLS (Table IV). Histology in three ALS patients with disease durations of 10, 17 and 20 years, respectively, revealed only mild loss of myelin staining in the cords of two (27), so that the more profound myelin changes seen in PLS might not simply be due to disease duration per se.
Table IV.
Neuropathological studies in PLS.
| n | Phenotype caveats | Main findings | Ref |
|---|---|---|---|
| 8 | Noted sub-clinical LMN involvement in 6/8 | (55) | |
| 1 | ‘ALS’ long-survivor (12 years) | Upper CST, CC, and extra-motor tract degeneration with myelin pallor in ALS; long-survivor, presumed PLS, case showed no spinal cord changes |
(56) |
| 1 | Profound PMC atrophy with marked Betz cell loss; loss of myelin throughout cerebral CST |
(24) | |
| 3 | Loss of myelin noted particularly in the PLIC | (32) | |
| 1 | Changes limited to PMC, with profound loss of Betz cells | (4) | |
| 1 | Less than 4 years from symptom onset | Myelin pallor throughout cerebral pyramidal tracts | (57) |
| 1 | Prominent cognitive involvement | Marked degeneration of frontal and temporal lobe tracts in addition to CST | (41–43) |
| 1 | Prominent cognitive involvement | Ubiquitin + ve skeins and Bunina bodies | (58) |
| 2 | TDP-43 + ve cytoplasmic inclusions in hypoglossal nuclei and glia of one of the cases | (59) |
CC: corpus callosum; CST: corticospinal tract; FTD: frontotemporal dementia; LMN: lower motor neuron; PMC: primary motor cortex; UMN: upper motor neuron; PLIC: posterior limb of internal capsule.
Previous MRI insights
Reductions in FA were noted in the CC and right subcortical CST in six PLS patients, in contrast to reduced FA in the superior frontal gyrus in the ALS group (28). Progression rate was linked to reduced FA in the sub-cortical white matter in the regions of the PMC in PLS, and to the CC and more caudal CST in ALS. In a larger study, the ‘core’ signature between both patient groups and healthy controls was similar, involving the CSTs and central motor fibres of the CC (29,30), and in a comparative study with hereditary spastic paraparesis (31). Degeneration of myelin was noted post mortem in the posterior limb of the internal capsule in a case of PLS (32), and reduced FA in this region has prognostic value in both ALS and PLS (33).
Insights into the neuroinflammatory signal
Shifts in IE-water T2, noted cross-sectionally in the ALS group, have been linked to inflammation or oedema (12,13). Neuroinflammatory mechanisms have been implicated in the pathogenesis of ALS (34), with activated microglia throughout motor and extramotor cerebral regions (15). In DTI-based studies, the coincidence of reduction in FA and increased RD (the diffusion direction perpendicular to the neuronal tract) has been postulated to reflect secondary Wallerian-type demyelination (35), rather than a primary immunological attack. The contribution of oligodendroglial cells to ALS pathogenesis is thought to involve a wider axonal support role (36).
Cognitive impairment in PLS versus ALS
Significant changes in myelin integrity were detected, predominantly frontal lobe regions, in relation to both global and executive measures of cognitive dysfunction in the ALS group, and in the patient groups combined, in keeping with the understanding of ALS and FTD as part of a clinicopathological spectrum (37). Myelin changes in the CC were strikingly anterior, probably reflecting the projection of such fibres to frontal regions (38), and raising the possibility of using regional CC involvement as a surrogate for extramotor pathology. Detailed cognitive studies in PLS are scarce, and though reported as similar dysfunction to ALS (39), it was to a lesser extent (40). In contrast to ALS, FTD is not frequently reported in the context of PLS beyond single case reports (41–43). Comparative flumazenil PET studies showed loss of GABAA receptor binding in frontal regions in the ALS compared to PLS group (44). PLS histopathology appears firmly based in the cerebral motor cortex, a region known to have strong connectivity with frontotemporal cortices (45). A blunted response of motor neurons to excitatory responses has been noted in PLS (46), which might relate to a wider theme of preserved inhibitory interneuronal function underlying differences in progression rate (47,48).
Longitudinal changes in cerebral white matter in PLS
Results of studies of longitudinal change in DTI measures in ALS have ranged from non-detectable (49) to very limited (50), to more widespread and including LMN-only cases (51). The present study did not find longitudinal changes in myelin in the ALS group but, across the whole white matter skeleton, longitudinal decreases in MWF and increases in IE-water T2 were noted in the PLS group. Given the slower progression of disease in PLS, this was unexpected but may provide evidence for PLS as a more active pathological process than is clinically obvious, or reflect the relative ‘reserve’ of unaffected white matter compared to a decimated pathological landscape in ALS.
Limitations of the technique
The mcDESPOT sequence is currently limited to examining two water pools. Studies using techniques that allow fitting of a T2 distribution can detect additional water pools, and long-T2 components have been demonstrated in multiple sclerosis (52). mcDESPOT also yields higher MWF values than are generally observed using multi-echo T2-relaxation, which could be the result of the two-pool model of mcDESPOT, magnetization transfer effects, or differences in modelling of exchange between pools (17,53).
Concluding remarks
Notwithstanding the need for a larger, prospective study using incident cases (ideally of shorter symptomatic duration), mcDESPOT offers novel potential to distinguish PLS from ALS by the degree and extent of myelin involvement. PLS may be a more active pathological process than its rate of clinical deterioration implies, and this study supports the view that it has a distinct pathogenesis.
Acknowledgements
We are indebted to our Research Assistant Melanie Lord and Clinic Coordinator Rachael Marsden, as well as to the patients and their carers for their great personal effort.
SK is supported by the Multiple Sclerosis Society of Canada and the Michael Smith Foundation for Health Research. MRT is funded by the Medical Research Council/Motor Neurone Disease Association Lady Edith Wolfson Fellowship. The Oxford Motor Neuron Disease Care & Research Centre receives funding from the Motor Neurone Disease Association UK Care Centre Program.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12:310–22. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erb WH. Ueber einen wenig bekannen spinalen symptomencomplex. Berliner Klinische Wochenschrift. 1875;12:357–9. [Google Scholar]
- 3.Stark FM, Moersch FP. Primary Lateral Sclerosis: A Distinct Clinical Entity. The Journal of Nervous and Mental Disease. 1945;102:332–7. [Google Scholar]
- 4.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis: clinical features, neuropathology and diagnostic criteria. Brain. 1992;115:495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- 5.Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, et al. The natural history of primary lateral sclerosis. Neurology. 2006;66:647–53. doi: 10.1212/01.wnl.0000200962.94777.71. [DOI] [PubMed] [Google Scholar]
- 6.Chio A, Calvo A, Moglia C, Mazzini L, Mora G. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82:740–6. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 7.Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- 8.Turner MR, Agosta F, Bede P, Govind V, Lule D, Verstraete E. Neuroimaging in amyotrophic lateral sclerosis. Biomark Med. 2012;6:319–37. doi: 10.2217/bmm.12.26. [DOI] [PubMed] [Google Scholar]
- 9.Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology. 2010;75:1645–52. doi: 10.1212/WNL.0b013e3181fb84d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb S, Munro CA, Midha R, Stanisz GJ. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magn Reson Med. 2003;49:638–45. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- 11.Laule C, Kozlowski P, Leung E, Li DK, Mackay AL, Moore GR. Myelin water imaging of multiple sclerosis at 7 T: correlations with histopathology. Neuroimage. 2008;40:1575–80. doi: 10.1016/j.neuroimage.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Stanisz GJ, Webb S, Munro CA, Pun T, Midha R. MR properties of excised neural tissue following experimentally induced inflammation. Magn Reson Med. 2004;51:473–9. doi: 10.1002/mrm.20008. [DOI] [PubMed] [Google Scholar]
- 13.Odrobina EE, Lam TY, Pun T, Midha R, Stanisz GJ. MR properties of excised neural tissue following experimentally induced demyelination. NMR Biomed. 2005;18:277–84. doi: 10.1002/nbm.951. [DOI] [PubMed] [Google Scholar]
- 14.Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60:1372–87. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- 15.Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [(11)C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15:601–9. doi: 10.1016/j.nbd.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrookes Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–85. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 17.Deoni SC. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magn Reson Med. 2011;65:1021–35. doi: 10.1002/mrm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Kolind S, Matthews L, Johansen-Berg H, Leite MI, Williams SC, Deoni S, et al. Myelin water imaging reflects clinical variability in multiple sclerosis. Neuroimage. 2012;60:263–70. doi: 10.1016/j.neuroimage.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Swank RL, Putnam TJ. Amyotrophic Lateral Sclerosis And Related Conditions: A Clinical Analysis. Arch Neurol Psychiat. 1943;49:151–77. [Google Scholar]
- 22.Eisen A. Amyotrophic lateral sclerosis: evolutionary and other perspectives. Muscle Nerve. 2009;40:297–304. doi: 10.1002/mus.21404. [DOI] [PubMed] [Google Scholar]
- 23.Kuipers-Upmeijer J, de Jager AE, Hew JM, Snoek JW, van Weerden TW. Primary lateral sclerosis: clinical, neurophysiological, and magnetic resonance findings. J Neurol Neurosurg Psychiatry. 2001;71:615–20. doi: 10.1136/jnnp.71.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beal MF, Richardson EP., Jr Primary lateral sclerosis: a case report. Arch Neurol. 1981;38:630–3. doi: 10.1001/archneur.1981.00510100058008. [DOI] [PubMed] [Google Scholar]
- 25.Lawyer T, Netsky MG. Amyotrophic lateral sclerosis: a clinico-anatomic study of 53 cases. ArchNeurol. 1953;69:171–92. doi: 10.1001/archneurpsyc.1953.02320260029002. [DOI] [PubMed] [Google Scholar]
- 26.Brownell B, Oppenheimer DR, Hughes JT. The central nervous system in motor neuron disease. J Neurol Neurosurg Psychiatry. 1970;33:338–57. doi: 10.1136/jnnp.33.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwanaga K, Hayashi S, Oyake M, Horikawa Y, Hayashi T, Wakabayashi M, et al. Neuropathology of sporadic amyotrophic lateral sclerosis of long duration. J Neurol Sci. 1997;146:139–43. doi: 10.1016/s0022-510x(96)00297-3. [DOI] [PubMed] [Google Scholar]
- 28.Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, et al. Investigation of white matter pathology in ALS and PLS using Tract-based spatial statistics. Hum Brain Mapp. 2009;30:615–4. doi: 10.1002/hbm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E, et al. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain. 2011;134:2642–55. doi: 10.1093/brain/awr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riad SM, Hathout H, Huang JC. High T2 signal in primary lateral sclerosis supports the topographic distribution of fibres in the corpus callosum: assessing disease in the primary motor segment. AJNR Am J Neuroradiol. 2011;32:61–4. doi: 10.3174/ajnr.A2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller HP, Unrath A, Huppertz HJ, Ludolph AC, Kassubek J. Neuroanatomical patterns of cerebral white matter involvement in different motor neuron diseases as studied by diffusion tensor imaging analysis. Amyotroph Lateral Scler. 2012;13:254–64. doi: 10.3109/17482968.2011.653571. [DOI] [PubMed] [Google Scholar]
- 32.Younger DS, Chou S, Hays AP, Lange DJ, Emerson R, Brin M, et al. Primary lateral sclerosis: a clinical diagnosis reemerges. Arch Neurol. 1988;45:1304–7. doi: 10.1001/archneur.1988.00520360022005. [DOI] [PubMed] [Google Scholar]
- 33.Menke RA, Abraham I, Thiel CS, Filippini N, Knight S, Talbot K, et al. Fractional anisotropy in the posterior limb of the internal capsule and prognosis in amyotrophic lateral sclerosis. Arch Neurol. 2012;69:1493–9. doi: 10.1001/archneurol.2012.1122. [DOI] [PubMed] [Google Scholar]
- 34.Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2012;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–9. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- 36.Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136:471–82. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2012;83:102–8. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- 38.Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172–87. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caselli RJ, Smith BE, Osborne D. Primary lateral sclerosis: a neuropsychological study. Neurology. 1995;45:2005–9. doi: 10.1212/wnl.45.11.2005. [DOI] [PubMed] [Google Scholar]
- 40.Grace GM, Orange JB, Rowe A, Findlater K, Freedman M, Strong MJ. Neuropsychological functioning in PLS: a comparison with ALS. The Canadian Journal of Neurological Sciences. 2011;38:88–97. [PubMed] [Google Scholar]
- 41.Konagaya M, Sakai M, Matsuoka Y, Konagaya Y, Hashizume Y. Upper motor neuron predominant degeneration with frontal and temporal lobe atrophy. Acta Neuropathol. 1998;96:532–6. doi: 10.1007/s004010050930. [DOI] [PubMed] [Google Scholar]
- 42.Mochizuki A, Komatsuzaki Y, Iwamoto H, Shoji S. Frontotemporal dementia with ubiquitinated neuronal inclusions presenting with primary lateral sclerosis and Parkinsonism: clinicopathological report of an autopsy case. Acta Neuropathol (Berl) 2004;8:348–55. doi: 10.1007/s00401-003-0818-7. [DOI] [PubMed] [Google Scholar]
- 43.Kosaka T, Fu YJ, Shiga A, Ishidaira H, Tan CF, Tani T, et al. Primary lateral sclerosis: upper motor-predominant amyotrophic lateral sclerosis with frontotemporal lobar degeneration: immunohistochemical and biochemical analyses of TDP-43. Neuropathology : Official Journal of the Japanese Society of Neuropathology. 2012;32:373–84. doi: 10.1111/j.1440-1789.2011.01271.x. [DOI] [PubMed] [Google Scholar]
- 44.Turner MR, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, et al. Distinct cerebral lesions in sporadic and ‘D90A’ SOD1 ALS: studies with [11C]flumazenil PET. Brain. 2005;128:1323–9. doi: 10.1093/brain/awh509. [DOI] [PubMed] [Google Scholar]
- 45.Verstraete E, Veldink JH, Mandl RC, van den Berg LH, van den Heuvel MP. Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS One. 2011;6:24239. doi: 10.1371/journal.pone.0024239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Floeter MK, Zhai P, Saigal R, Kim Y, Statland J. Motor neuron firing dysfunction in spastic patients with primary lateral sclerosis. J Neurophysiol. 2005;94:919–27. doi: 10.1152/jn.00185.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner MR, Kiernan MC. Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotroph Lateral Scler. 2012;13:245–50. doi: 10.3109/17482968.2011.636050. [DOI] [PubMed] [Google Scholar]
- 48.Turner MR, Douaud G. Faulty brakes: an inhibitory neuronal deficit in the pathogenesis of motor neuron disease. Advances in Clinical Neuroscience and Rehabilitation. 2012;12:10–1. [Google Scholar]
- 49.Blain CR, Williams VC, Johnston C, Stanton BR, Ganesalingam J, Jarosz JM, et al. A longitudinal study of diffusion tensor MRI in ALS. Amyotroph Lateral Scler. 2007;107:377–80. doi: 10.1080/17482960701548139. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Schuff N, Woolley SC, Chiang GC, Boreta L, Laxamana J, et al. Progression of white matter degeneration in amyotrophic lateral sclerosis: a diffusion tensor imaging study. Amyotroph Lateral Scler. 2011;12:421–9. doi: 10.3109/17482968.2011.593036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Graaff MM, Sage CA, Caan MW, Akkerman EM, Lavini C, Majoie CB, et al. Upper and extramotoneuron involvement in early motor neuron disease: a diffusion tensor imaging study. Brain. 2011;134:1211–28. doi: 10.1093/brain/awr016. [DOI] [PubMed] [Google Scholar]
- 52.Laule C, Vavasour IM, Madler B, Kolind SH, Sirrs SM, Brief EE, et al. MR evidence of long T2 water in pathological white matter. J Magn Reson Imaging. 2007;26:1117–21. doi: 10.1002/jmri.21132. [DOI] [PubMed] [Google Scholar]
- 53.Kolind SH, Deoni SC. Rapid three-dimensional multicomponent relaxation imaging of the cervical spinal cord. Magn Reson Med. 2011;65:551–6. doi: 10.1002/mrm.22634. [DOI] [PubMed] [Google Scholar]
- 54.Dupre N, Valdmanis PN, Bouchard JP, Rouleau GA. Autosomal dominant primary lateral sclerosis. Neurology. 2007;68:1156–7. doi: 10.1212/01.wnl.0000258678.58808.86. [DOI] [PubMed] [Google Scholar]
- 55.Spiller WG. Primary Degeneration of the Pyramidal Tracts: A Study of Eight Cases with Necropsy. Univ Pennsylvania M Bull. 1904;17:390–407. [Google Scholar]
- 56.Smith MC. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1960;23:269–82. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe R, Iino M, Honda M, Sano J, Hara M. Primary lateral sclerosis. Neuropathology: official journal of the Japanese Society of Neuropathology. 1997;17:220–4. [Google Scholar]
- 58.Tan CF, Kakita A, Piao YS, Kikugawa K, Endo K, Tanaka M, et al. Primary lateral sclerosis: a rare upper motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathol. 2003;105:615–20. doi: 10.1007/s00401-003-0687-0. [DOI] [PubMed] [Google Scholar]
- 59.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114:71–9. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]