Abstract
Introduction
Lenalidomide (LEN) is a relatively new and very effective therapy for multiple myeloma (MM). Prior LEN therapy is associated with an increased risk of peripheral blood stem cell collection (PBSC) failure, particularly with filgrastim (G-CSF) alone. We performed a retrospective chart review of 319 consecutive MM patients who underwent apheresis to collect PBSCs for the first autologous stem cell transplant (ASCT).
Results
The median number of PBSCs collected in the LEN (+) group was significantly less than the LEN (−) group (6.34 vs. 7.52×106 CD34+ cells/kg; p=0.0004). In addition, the median number of apheresis sessions required for adequate PBSCs collection were significantly more in the LEN (+) group as compared to LEN (−) group (2 vs. 1 sessions; p=0.002). In the LEN (+) group, there was a negative correlation between PBSCs collected and prior number of cycles of LEN (p=0.0001). Rate of PBSC collection failure was 9 % in the LEN (+) group and 5 % in the LEN (−) group (p=0.16). Only six patients who failed PBSC collection with G-CSF were able to collect adequate PBSCs with G-CSF + plerixafor. LEN exposure had no effect on neutrophil or platelet recovery post-ASCT.
Conclusions
Up to four cycles of LEN exposure have minimal negative impact on PBSC collection. Despite prolong exposure of LEN, PBSC collection was adequate for two ASCTs in the majority of patients and post-ASCT engraftment was not longer than expected; however, clinical relevance (complication rate, quality of life, cost) of prolonged LEN exposure on both PBSC and ASCT, should be evaluated in prospective clinical trials.
Keywords: Lenalidomide, Multiple myeloma, Peripheral stem cells collection
Background
Lenalidomide (LEN) is an immuno-modulatory agent approved for treatment of multiple myeloma (MM). In the upfront setting, the combination of LEN and dexamethasone (DEX) has shown an overall response rate (ORR) of 70–80 % and a greater than or equal to very good partial response achieved in roughly half of the patients [1, 2]. In relapsed patients, the ORR is approximately 60 % with LEN/DEX therapy [2]. The high response rates as well as ease of administration have contributed to the widespread off-label use of LEN as upfront induction therapy.
One of the common side effects of LEN is myelosuppression, with grades 3–4 neutropenia requiring dose reduction or discontinuation in about 20–30 % of patients [1–3]. Based on retrospective reviews, LEN has been reported to have an adverse effect on the peripheral blood stem cell (PBSC) yield prior to autologous stem cell transplant (ASCT). Clinical impact of this adverse effect on PBSC yield and patient’s ability to undergo ASCT is not known and is not studied in prospective trials. This has resulted in recommendations to limit LEN induction treatment regardless of achieving best treatment response. In addition, there are recommendations to consider cryopreserving PBSC without any prospective data and lack of universal insurance coverage for upfront PBSC collection with no intent to offer ASCT within 1 year and cryopreservation of back-up PBSCs. In these reports, LEN exposure was associated with a higher rate of PBSC collection failure, lower CD34+ cell-count yield and an increased number of apheresis sessions required for adequate PBSC collection [4, 5].
ASCT continues to retain importance in the treatment of MM and can improve progression-free survival as well as overall survival [6, 7]. Collection of an adequate number of PBSCs is a critical factor in performing ASCT. The dose of CD34+ cells infused affects the kinetics of hematopoietic recovery, with 2×106 infused CD34+ cells/kg considered a minimum requirement for ASCT [8, 9]. Filgrastim (G-CSF) is the most commonly used agent for PBSC mobilization, and is well tolerated by most patients. Alternatives or adjuncts to G-CSF include sargramostim (GMCSF) [10], cyclophosphamide [11], and the novel drug plerixafor, which is used upfront [12] or for poor PBSC mobilizers [13, 14]. All these other agents are commonly used in patients who fail to collect PBSC with G-CSF alone. To evaluate the potential influence of prior LEN exposure on subsequent PBSC mobilization and post-ASCT engraftment, we retrospectively analyzed MM patients undergoing PBSC mobilization for ASCT.
Methods
All MM patients eligible for ASCT and began attempts at PBSC mobilization between July 1, 2007 and June 30, 2011 were included in this retrospective analysis. Apheresis records of the Karmanos Cancer Institute were reconciled with the MM database to ensure a complete list of consecutive patients. Patients who had a prior ASCT were excluded. Patients who received at least one LEN cycle (21 consecutive days) at any time prior to PBSC collection were included in this analysis. Approval from institutional review board was obtained for this analysis.
Patients were divided into lenalidomide exposed LEN (+) and non-lenalidomide exposed LEN (−) groups. The primary outcome was failure to collect PBSCs using G-CSF alone. Per our institutional practice, failure of mobilization was defined as collection of minimum target of <2×106 CD34+ cells/kg (adequate target: ≥4×106 CD34+ cells/kg). Patients underwent through collection procedure if the peripheral blood CD34+ count on day 5 after starting G-CSF was >10/μl. They were considered collection failure if they were unable to collect ≥2×106 CD34+ cells/kg in 3 days of apheresis. The secondary endpoints included differences in the amount of PBSCs collected, number of apheresis sessions required to collect the adequate number of PBSCs, and days to neutrophils and platelet recovery following ASCT.
Statistical analysis
Due to skewed frequency distribution of CD34+ cells, Kruskal–Wallis tests were used to assess statistical significance of differences between treatment groups in the quantity of PBSCs collected, total number of apheresis days to collect at least the minimum desired number of PBSCs and days to platelet and neutrophil engraftment. A test for trend in proportions was performed to assess the statistical significance of the difference in the number of collection days between the LEN (+) and LEN (−) groups. Spearman’s rank correlation coefficient was used to evaluate the statistical significance of the association between the number of PBSCs collected per 100 ml and number of cycles of LEN.
Results
A total of 339 patients with MM underwent attempt at PBSC collection. Out of these, 15 patients had a prior ASCTand were excluded. Five patients were excluded because of missing data. Thus, a total of 319 patients were included in the final analysis. Out of these, 256 patients were initially mobilized with G-CSF alone, 4 patients were mobilized with combination of G-CSF and GMCSF. Twenty-eight patients were collected with the combination of cyclophosphamide and G-CSF, and 31 patients participating in a clinical trial were collected with G-CSF/plerixafor. Both the LEN (+) and LEN (−) groups were balanced according to the initial priming agent used (Table 1).
Table 1.
Demographic and clinical characteristics by induction therapy
| LEN (−) N=133 |
LEN (+) N=186 |
|
|---|---|---|
| Age at BMT [Median (IQR)] | 59 (52, 65) | 58 (52, 63) |
| Sex | ||
| Male | 69 (52 %) | 107 (58 %) |
| Female | 64 (48 %) | 79 (42 %) |
| Race | ||
| White | 99 (74 %) | 142 (76 %) |
| Black | 32 (24 %) | 39 (21 %) |
| Other | 2 (2 %) | 5 (3 %) |
| Durie–Salmon Stage at Diagnosis | ||
| I | 14/77 (18 %) | 16/104 (15.3 %) |
| II | 13/77 (17 %) | 15/104 (14.4 %) |
| III | 50/77 (65 %) | 73/104 (70.1 %) |
| Initial Priming Agent | ||
| G-CSF | 104 (78 %) | 139 (75 %) |
| G-CSF + GMCSF | 0 (0 %) | 4 (2 %) |
| Cyclophosphamide + G-CSF | 14 (11 %) | 14 (8 %) |
| Plerixafor + G-CSF | 15 (11 %) | 29 (16 %) |
| Induction cycles [median (IQR)] | 5 (4, 7) | 4 (4, 6) |
G-CSF granulocyte-colony stimulating factor (filgrastim); GMCSF granulocyte-macrophage colony-stimulating factor (sargramostim); Median IQR median interquartile range represents the middle value between 25th and 75th percentile values
Of the total 319 patients, 186 patients were LEN (+), and 133 patients were LEN (−) prior to PBSC collection. Both groups had similar demographic and clinical characteristics (Table 1). Median age was approximately 58 years; there was a slight predominance of males in the LEN (+) group and approximately 75 % of participants were Caucasians. About 70 % of patients presented with Durie–Salmon stage III disease. Dose of G-CSF administered for PBSC mobilization was 16 mcg/kg/day, calculated based on actual body weight.
In the LEN (+) arm, 123 patients were treated with only one induction regimen prior to PBSC collection which were LEN/DEX in 83 patients, and LEN/DEX/bortezomib (BTZ) in 40 patients. Sixty-three patients in the LEN (+) group received more than one induction therapy, most commonly LEN/DEX preceded or followed by BTZ-based therapy. Only one patient received melphalan-containing regimen. In the LEN (−) arm, 102 patients had received only one prior induction regimen: BTZ/DEX (n =54 patients), BTZ/DEX/pegylated doxorubicin (n=21), thalidomide (THAL)/DEX (n =12), BTZ/THAL/DEX (n=10), and BTZ/DEX/cyclophosphamide (n=5). Thirty-one patients in this group received more than one line of prior therapy and the regimens used were BTZ/DEX plus either THAL/DEX (23 patients) or melphalan/prednisone (8 patients).
The median number of cycles of induction therapy prior to attempting PBSC mobilization was five in LEN (−) and four in LEN (+) arm (Table 1). Rate of PBSC collection failure was 9 % in the LEN (+) group and 5 % in the LEN (−) group, which was not statistically different (p=0.16). Out of 16 patients in the LEN (+) arm who failed collection in the first attempt, 12 had received more than 4 cycles of LEN induction (ranging from 5–12 cycles) while 4 patients had received only 4 cycles of induction. None of these patients had received any alkylating agent or anthracycline-based chemotherapy. Six patients failed to collect in the LEN (−) arm and all of them had received melphalan- or doxil-based chemotherapy prior to collection. Out of the 22 patients who failed initial collection with G-CSF, 16 were successfully collected with second-line plerixafor + G-CSF priming, 3 patients failed to collect with both G-CSF and plerixafor + G-CSF priming and the remaining 3 patients did not attempt a second collection.
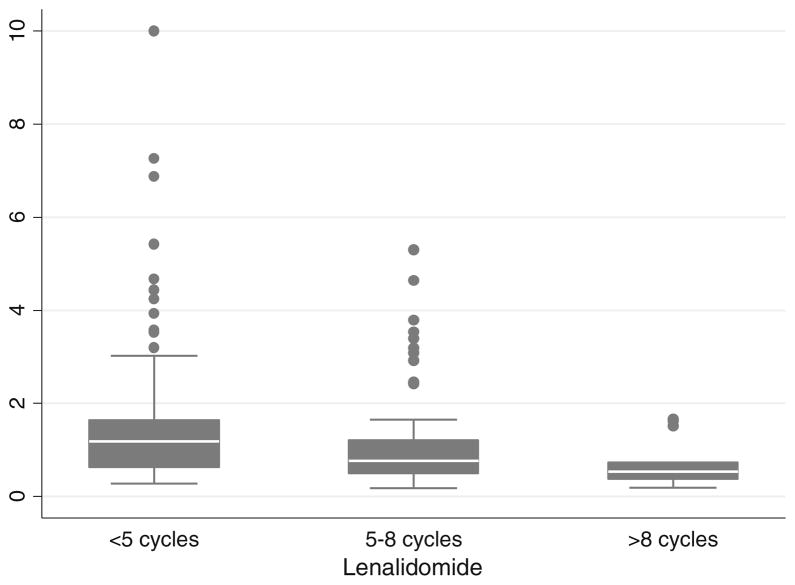
The median number of PBSCs collected in the LEN (+) group was significantly less than LEN (−) group (6.34 vs. 7.52 × 106 CD34+ cells/kg; p = 0.0003) (Table 2). The difference remained significant even after correction for the total volume collected (1.00 vs. 1.39×106 CD34+ cells/kg/100 ml; p=0.0001). As a result, the median number of apheresis sessions required for adequate PBSC collection were significantly more in the LEN (+) group as compared to LEN (−) group (2 vs.1 sessions; p=0.004). There was a statistically significant negative correlation between PBSC collected per 100 ml and prior cycles of LEN (Spearman’s rho=−0.30, p= 0.0001). To assess the effect of duration of LEN therapy on the PBSC collection we divided our LEN (+) patients into three groups, (<5 cycles, 5–8 cycles and >8 cycles) and compared the PBSC yield in these groups. There was a statistically significant difference in the PBSC yield among patients who received <5 cycles compared to those who received 5–8 or >8 cycles (p=0.004). However, the difference in yield between those who received 5–8 cycles of LEN and those who received >8 cycles was not statistically significant (p=0.14) (Table 3), (Fig. 1). The median number of PBSC collected in patients mobilized with G-CSF plus cyclophosphamide (n=28) as initial mobilizing agents were 12.4×106 CD34+ cells/kg and 7.34×106 CD34+ cells/kg in the G-CSF plus plerixafor group (n=31). Given the presence of these upfront mobilizing agents used in about 20 % of patients, we performed a separate analysis of patients (n=256) who were collected with upfront G- CSF and the differences in terms of total CD34+ cells collected, days to collection, CD34+ cells collected per 100 ml remained statistically significant in the LEN (−) and LEN (+) arms.
Table 2.
Outcomes by induction therapy
| Outcome | LEN (−) N=133 |
LEN (+) N=186 |
p value |
|---|---|---|---|
| CD34+ collected median (IQR) | 7.52 (5.71, 10.71) | 6.34 (5.12, 8.37) | 0.0006 |
| Volume collected (ml) median (IQR) | 500 (400, 800) | 600 (500, 900) | 0.02 |
| CD34+ per 100 units volume median (IQR) | 1.39 (0.75, 2.41) | 1 (0.59, 1.54) | 0.0002 |
| Day 5 peripheral CD34+ count median (IQR) | 60.7 (32.7, 107) | 37.6 (18.5, 64.1) | 0.00001 |
| CD34+ collected | 0.007 | ||
| 0 to 2a | 2 (2 %) | 4 (2 %) | |
| >2 to 4 | 10 (8 %) | 20 (11 %) | |
| >4 to 6 | 29 (22 %) | 57 (31 %) | |
| >6 to 8 | 29 (22 %) | 55 (30 %) | |
| >8 | 63 (47 %) | 50 (27 %) | |
| G-CSF failure (first attempt) | 0.16 | ||
| No | 127 (95 %) | 170 (91 %) | |
| Yes | 6 (5 %) | 16 (9 %) | |
| CD34+≤2a | 0.68 | ||
| No | 131 (98 %) | 182 (98 %) | |
| Yes | 2 (2 %) | 4 (2 %) | |
| CD34+≤4 | 0.28 | ||
| No | 121 (91 %) | 162 (87 %) | |
| Yes | 12 (9 %) | 24 (13 %) | |
| Days to collect target | 0.01 | ||
| 1 | 78 (60 %) | 72 (42 %) | |
| 2 | 34 (26 %) | 71 (41 %) | |
| 3 | 15 (12 %) | 24 (14 %) | |
| 4 to 8 | 2 (2 %) | 5 (3 %) | |
| Days to collect target median (IQR) | 1 (1, 2) | 2 (1, 2) | 0.004 |
| CD34+ infused dose × 106 kg median (IQR) | 4.09 (3.09, 5.8) | 3.51 (2.88, 4.39) | 0.001 |
| Date to neutrophil recovery median (IQR) | 12 (11, 13) | 12 (11, 12) | 0.87 |
| Days to platelet recovery median (IQR) | 20.5 (17, 28) | 21 (18, 28) | 0.17 |
This represents the number of patients unable to collect 2×106 CD34+ cells/kg after two attempts at collection
Table 3.
CD34+ yield per 100 ml by lenalidomide cycles
| Lenalidomide cycles | N | Median CD34+×106 cells/kg/100 ml (IQR) |
|---|---|---|
| <5 | 100 | 1.2 (0.7, 1.6) |
| 5–8 | 59 | 0.8 (0.5, 1.3) |
| >8 | 12 | 0.5(0.3, 1.1) |
Missing data in 15 patients
Fig. 1.
Effect of number of LEN cycles on yield of CD34 cells/100 ml
Analysis of day 5 peripheral CD34+ cells showed a statistically lower number of CD34+ cells in LEN (+) group as compared to the LEN (−) group (37.6 vs. 60.7) p=0.00001. Because of the overall lower median number of stem cells collected in LEN (+) group, a lower median number of stem cells were available for the first ASCT in the LEN (+) group (3.51 vs. 4.09×106 CD34+ cells/kg). This did not translate into difference in days to neutrophils or platelet recovery in these two groups (Table 2). There was no significant difference in two arms in terms of PBSC collection for two ASCTs, defined as collection of ≥4×106 CD34+ cells/kg (LEN (+) 87 % and LEN (−) 91 %, p=0.28) (Table 2). Out of the 319 patients, 10 patients did not proceed to ASCT because of various reasons, including being on a delayed ASCT study protocol, disease progression, inadequate PBSC collection and patient’s refusal.
Discussion
Our data confirms the results of the previous reports [4, 5] that LEN exposure negatively affects PBSC collection in patients undergoing ASCT for MM. There was a significant drop in the day 5 peripheral CD34+ count in the patients who were LEN exposed although the CD34+ counts still remained adequate for collection. It is unclear if this reduction in PBSC mobilization is clinically significant as in both groups adequate number of PBSCs were collected for at least two ASCTs in majority of patients. Recently published guidelines based on expert opinion from the International Myeloma Working Group recommends PBSC collection after 4 cycles of LEN induction therapy in order to minimize the risk of PBSC collection failure [15]. It is important to note that majority of health insurance carriers will not allow collection and cryopreservation of PBSCs if there is no intent to proceed with ASCT soon after PBSCs collection. In addition, there are no designated reimbursement codes for back-up PBSC storage raising concerns for increased cost and liability.
Our data defines the so called threshold for LEN exposure, as ≥5 prior cycles of LEN were associated with a drop in PBSC yield but without a negative impact on collecting the minimum PBSC collection target required to perform ASCT. Nonetheless, complete failure to collect PBSC was not statistically associated with prior LEN exposure questioning the utility of LEN exposure threshold and rationale for current guidelines for earlier PBSC collection in this group of MM patients. This practice requires limiting the number of cycles of LEN induction treatment with a disregard for disease response. Although our data is somewhat heterogeneous in terms of the induction therapy used, a small minority of patients were exposed to alkylating agents prior to PBSC collection. Majority of these patients received either proteasome inhibitor or immuno-modulator based front-line therapy which have become standards of care for MM therapy at this time. About 20 % of our patients were collected with upfront G-CSF + cyclophosphamide or plerixafor. These patients were distributed evenly in both groups and a separate analysis of patient receiving G-CSF only group showed no difference in the results of the study.
The mechanism of marrow toxicity of LEN is unclear. LEN induces expansion of the cytotoxic CD8+ T-cells and NK cells with resultant increases in levels of IL-2 and IFN-gamma [16, 17]. These cytokines are known to cause myelosuppression and could thus theoretically impair PBSC collection but this has not been confirmed. A report by Koh et al. looking at the effect of LEN on the bone marrow showed suppression of red cell lineage while demonstrating enhancement of myelopoiesis [18], but this does not explain observed leucopenia due to LEN. Myelosuppression is dose-dependent and seen more commonly in patients with renal dysfunction [19].
Some investigators have reported improvement in the PBSC collection in LEN (+) patients with the use of cyclophosphamide in addition to the G-CSF [11]. Although all patients in our series mobilized initially with cyclophosphamide + G-CSF, were successfully collected on the first attempt, the small number of such patients precludes us to draw any meaningful conclusions about the relative efficacy of this mobilization regimen compared to G-CSF alone. Of note, in a recent published analysis of 346 patients undergoing PBSC collection following four cycles of LEN/DEX induction and subsequent cyclophosphamide priming, 15 % of patients failed to mobilize ≥2×106 CD34+ cells/kg on the first collection attempt [20]. Given the unpredictability of PBSC recovery, risk of hospitalization, as well as the added cost and increased morbidity associated with cyclophosphamide priming, initial mobilization with G-CSF alone appears preferable, given the overall low failure rate.
In conclusion, our data suggests that G-CSF can be used to successfully mobilize PBSC in MM patients following LEN therapy. The rate of PBSC collection failure on the first attempt appears similar to that described in published reports of cyclophosphamide priming. Almost all patients who failed their first mobilization attempt were ultimately able to collect PBSCs after a second attempt using combination of other mobilizing agents.
We confirmed previous reports that more than four cycles of prior LEN therapy has a negative impact on PBSC collection in MM patients. However, we could not find sufficient evidence to demonstrate that this reduction in PBSC yields precludes either the feasibility of ASCT or a successful collection for storage and future use of PBSCs. Therefore, we suggest a reevaluation of current guidelines that erroneously limit the number of cycles of LEN induction therapy regardless of response and recommend to cryopreserve backup PBSC in patients treated with LEN. We also demonstrated that LEN (+) MM patients undergoing ASCT with an adequate PBSC dose had normal marrow recovery. These issues must be addressed in prospective clinical trials as more MM patients are exposed to prolong duration of LEN in the setting of delayed ASCT and maintenance therapy.
Footnotes
Conflict of interest: Muneer H. Abidi: Speaker for Millennium Pharmaceutical
Jeffrey Zonder: Advisory Board for Celgene Pharmaceutical
Contributor Information
Divaya Bhutani, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Jeffrey Zonder, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA.
Jason Valent, Cleveland Clinic, Cleveland, OH, USA.
Nishant Tageja, Medical Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Lois Ayash, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Abhinav Deol, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Zaid Al-Kadhimi, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Judith Abrams, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Lawrence Lum, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Voravit Ratanatharathorn, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Joseph Uberti, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA.
Muneer H. Abidi, Email: abidim@karmanos.org, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI 48201, USA, Wayne State University, Detroit, MI, USA
References
- 1.Zonder JA, Crowley J, Hussein MA, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232) Blood. 2010;116(26):5838–5841. doi: 10.1182/blood-2010-08-303487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 5.Popat U, Saliba R, Thandi R, et al. Impairment of filgrastim induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15:718–723. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 7.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 8.Bensinger W, Appelbaum F, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13(10):2547–2555. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 9.Tricot G, Jagannath S, Vesole D, et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85(2):588–596. [PubMed] [Google Scholar]
- 10.Gianni M, Alessandro S, Siena M, et al. Granulocyte-macrophage colony-stimulating factor to harvest circulating haematopoietic stem cells for autotransplantation. Lancet. 1989;334(8663):580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 11.Mark T, Stern J, Furst JR, et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 13.Malard F, Kröger N, Gabriel IH, et al. Plerixafor for autologous peripheral blood stem cell mobilization in patients previously treated with fludarabine or lenalidomide. Biol Blood Marrow Transplant. 2012;18(2):314–317. doi: 10.1016/j.bbmt.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz ME, Chute JP, Gasparetto C, et al. Preemptive dosing of plerixafor given to poor stem cell mobilizers on day 5 of G-CSF administration. Bone Marrow Transplant. 2012;47:1051–1055. doi: 10.1038/bmt.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Giralt S, Stadtmauer EA, et al. International Myeloma Working Group. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 17.Chang DH, Liu N, Klimek V, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh KR, Janz M, Mapara MY, et al. Immunomodulatory derivative of thalidomide (IMiD CC-4047) induces a shift in lineage commitment by suppressing erythropoiesis and promoting myelopoiesis. Blood. 2005;105(10):3833–3840. doi: 10.1182/blood-2004-03-0828. [DOI] [PubMed] [Google Scholar]
- 19.Niesvizky R, Naib T, Christos PJ, et al. Lenalidomide-induced myelosuppression is associated with renal dysfunction: adverse events evaluation of treatment-naïve patients undergoing front-line lenalidomide and dexamethasone therapy. Br J Haematol. 2007;138(5):640–643. doi: 10.1111/j.1365-2141.2007.06698.x. [DOI] [PubMed] [Google Scholar]
- 20.Cavallo F, Bringhen S, Milone G, et al. Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia. 2011;25(10):1627–1631. doi: 10.1038/leu.2011.131. [DOI] [PubMed] [Google Scholar]