Abstract
Daily scheduled feeding is a potent time cue that elicits anticipatory activity in rodents. This food-anticipatory activity (FAA) is controlled by a food-entrainable oscillator (FEO) that is distinct from light-entrained oscillators of the suprachiasmatic nucleus (SCN). Circadian rhythms within the SCN depend on transcription-translation feedback loops in which CLOCK protein is a key positive regulator. The Clock gene is expressed in rhythmic tissues throughout the brain and periphery, implicating its widespread involvement in the functioning of circadian oscillators. To examine whether CLOCK protein is also necessary for the FEO, the effect of daily food restriction was studied in homozygous Clock mutant (Clk/Clk) mice. The results show that Clk/Clk mutant mice exhibit FAA, even when their circadian wheel-running behavior is arrhythmic. As in wild-type controls, FAA in Clk/Clk mutants persists after temporal feeding cues are removed for several cycles, indicating that the FEO is a circadian timer. This is the first demonstration that the Clock gene is not necessary for the expression of a circadian, food-entrained behavior and suggests that the FEO is mediated by a molecular mechanism distinct from that of the SCN.
Keywords: food-anticipatory activity, feeding, entrainment, clock genes, suprachiasmatic nucleus
The Ability of Animals to temporally organize their behavior and physiology in anticipation of periodically occurring environmental stimuli is key to survival. Although the cycle of day and night is the most predictable time cue, animals can also respond to other synchronizing signals such as the daily availability of food. Animals with restricted access to food exhibit a marked increase in activity in anticipation of feeding time [i.e., food-anticipatory activity (FAA)] during the hours preceding mealtime (9, 30). FAA is accompanied by increases in plasma corticosterone (24), body temperature, ketone bodies, and free fatty acids (10) and a phase shift in plasma melatonin (2). Light- and food-entrainable circadian rhythms share many properties, including limits of entrainment in the circadian range (23–31 h), free running under constant conditions, transients following phase shifts, and sustained rhythmicity in the absence of feedback (20).
Although they have similar properties, light- and food-entrainable circadian rhythms do not share a common neural basis. The suprachiasmatic nucleus (SCN) of the anterior hypothalamus is necessary for the expression of endogenous circadian rhythms and entrainment by light of many behavioral and physiological responses (38). Lesions of the SCN disrupt these rhythms (22, 36), but food-entrained rhythms survive SCN ablation (16, 35). In constant light or darkness, FAA is synchronized to the food availability schedule, whereas a separate component of locomotor activity free runs, suggesting separate phase control mechanisms (3, 13, 20). Furthermore, the free-running period is lengthened by deuterium oxide treatment in intact or SCN-transplanted animals (18), whereas food-entrained rhythms are insensitive (21). Because the SCN is entrainable by light, it has been termed the light-entrainable oscillator (LEO) to distinguish it from a second circadian control system, termed the food-entrainable oscillator (FEO) (20, 35). The neural locus, as well as the molecular mechanism, of the FEO remains elusive (34).
Substantial progress has been made in understanding the cellular and molecular basis of circadian rhythmicity in SCN oscillators. Circadian rhythms in mammals are based on transcription-translation autoregulatory feedback loops in which the Clock gene acts as a positive element (11, 40). CLOCK protein forms a heterodimer with BMAL1 and activates the transcription of circadian oscillator genes, such as Per and Cry. In turn, the protein products of these genes accumulate and eventually feed back to inhibit CLOCK:BMAL1-regulated transcription of their own genes. Expression of Clock and other circadian genes is widespread and occurs in rhythmic brain and peripheral tissues (1, 15, 33). The discovery that central and peripheral oscillators share the same circadian genes implicates CLOCK protein in the functioning of all oscillators (41).
It has been proposed that the SCN directly entrains feeding behavior to the light-dark (LD) cycle by setting the phase of activity, and then food-related cues generate the entraining signals for the liver and other organs (7). Daytime feeding does not change the phase of cyclic Per gene expression in the SCN but does shift the timing of Per expression in the liver in both intact and SCN-lesioned mice (7, 12). Changes in feeding time, but not in LD, seem to be the most important cue for phase-shifting the liver and other organs (37). Daytime-restricted feeding forces the FEO and SCN, which are normally in synchrony, to be out of phase with each other. Greater understanding of the resetting mechanism of the FEO during food restriction may reveal how food can serve as an entraining signal under normal feeding conditions.
The goal of the present study was to examine the effect of restricted feeding on food-entrained circadian rhythms in animals with a mutation in the transcription-translation feedback loop underlying the molecular rhythms of the SCN. When fed ad libitum in an LD cycle, homozygous Clock mutant (Clk/Clk) mice entrain such that their locomotor activity begins at lights off; in constant darkness (DD), their activity becomes arrhythmic, indicating that CLOCK protein is a necessary component of the LEO (39). If CLOCK is also a necessary component of the FEO, then FAA should be disrupted in Clk/Clk mice. If FAA is sustained in Clk/Clk mice under both entrained and constant conditions, this would suggest that the FEO is not a CLOCK-based oscillator and therefore utilizes a different circadian molecular mechanism than that of the SCN.
Materials and Methods
Subjects
Subjects were adult Clk/Clk mice from a C57BL/6j X BALB/cj intercross background from breeding pairs originally obtained from Northwestern University, Evanston, IL (39). Wild-type (WT) mice were derived from C57BL/6j × BALB/cj F1 mice (Jackson Laboratories, Bar Harbor, ME) and were interbred and reared in our colony. Mice weighed 30–38 g at the start of the experiment, with no significant differences in weight, age, or health between the two genotypes. All animal maintenance and experimental procedures were in accordance with the Institutional Animal Care and Use Committee Animal Welfare regulations at Columbia University.
Genotyping
Genotypes were determined using a polymerase chain reaction (PCR) mutagenesis method that introduces a HincII restriction site, which cleaves the WT allele but not the amplified product of the Clock mutant allele (14). Briefly, mouse genomic DNA was extracted from toe clippings of pups by proteinase K digestion and phenol-chloro-form-isoamyl alcohol purification. Genomic DNA was subjected to PCR amplification (for primers and cycling parameters, see Ref. 14) by use of Ready-To-Go PCR beads (Amersham Pharmacia Biotech, Piscataway, NJ). PCR products were digested with HincII, and gel electrophoresis was used to separate and visualize the alleles. Genotypes were also confirmed by phenotyping the free-running period of wheel-running activity in DD.
Housing
Experimental animals were housed individually in cages (13.0 × 7.5 × 8.0 in.; Ancare, Bellmore, NY), which were placed in light-tight, ventilated chambers in a room equipped with a white-noise generator (91dB SPL; Lafayette Instruments, Lafayette, IN) to mask environmental sounds. The room was maintained at 23 ± 2°C and constant humidity. The light portion of the LD cycle was 800 lux at the level of the cages. A dim red light (<1 lux; Delta 1, Dallas, TX) was used to aid in maintenance and provisioning of chambers kept in DD. The food hopper and water bottle holder were built into the cage top. Adding or removing food from the cage top controlled access to food.
Breeder Chow (Purina, St. Louis, MO) was provided during food access times. The mice were weighed every 3–4 days and compared with ad libitum-fed controls to monitor the health of the experimental animals. Mice were maintained at ∼90% of control weight. Food intake was checked periodically to make certain that the animals had adjusted to the restricted schedule. Water was freely available throughout the experiment.
Monitoring activity
Cages were equipped with running wheels (5 in. diameter; Learning Curve, Mansfield, MA). Wheel-running activity was monitored using DataQuest (Data Sciences International, St. Paul, MN) on a PC computer with a sampling interval of 10 min. The activity of the animals was checked daily, and data were analyzed using ClockLab (Actimetrics, Evanston, IL). Quantification of activity between designated time intervals and determination of bout start times was assessed by custom-written software in MatLab (The Mathworks, Natick, MA).
Experimental design
Food restriction was carried out under both LD and DD conditions (Fig. 1). For the LD portion of the experiment, mice (WT n = 16 and Clk/Clk n = 18) were acclimated to the running wheels and entrained to a 12:12-h LD cycle (lights on at 0400, lights off at 1600) for 7–25 days with ad libitum (AL) access to food. On the last day of AL feeding, food was removed at zeitgeber time (ZT) 10 (ZT 12 defined as time of lights off). Food restriction (FR) was started by a stepwise reduction in food access from 8 h (ZT 2–10) to 6 h (ZT 4–10) to 4 h/day (ZT 6–10, or 1000 to 1400) over 3 days and then persisted at 4 h/day for a total of 15 days of restricted food access. The food availability times were determined by a pilot study to maximize the time between lights on and the start of daytime restricted feeding (unpublished data).
Fig. 1.
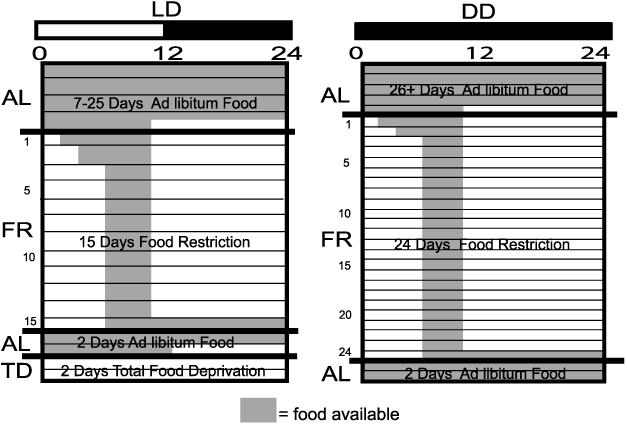
Schematic depicting lighting and feeding schedule. The lighting cycle is shown by the horizontal bar on top of the figures; white denotes light (L) and black denotes darkness (D). DD, constant darkness. Shaded areas represent time of food availability. Each horizontal line represents 1 day. Feeding condition is shown on the y-axis as follows: AL, ad libitum feeding; FR, food restricted; TD, total deprivation of food. Number of days spent in each stage is shown within each diagram.
To determine whether FAA was the result of an endogenous clock or a driven, metabolic signal, we examined the persistence of FAA behavior during 2 days of total deprivation of food (TD). In a subset of the LD group (WT n = 3 and Clk/Clk n = 3), AL resumed for 2 days starting at ZT 6 on day 15 of FR. Then at ZT 12 on the 2nd day of AL, food was removed, and mice underwent 2 days of TD.
For the DD portion of the experiment, mice (WT n = 4 and Clk/Clk n = 8) were monitored in DD for ≥26 days with AL access to food. Access to food was then gradually restricted as described above. Food was available for the same 4-h period as in LD food restriction (1000 to 1400), for a total of 24 days. To examine the persistence of FAA after a return to AL feeding, without the masking effects of light, the animals were then given 2 days of free access to food in DD.
Data analysis
The three measures of FAA used in the present study were magnitude, bout frequency, and bout start time. The magnitude of FAA to scheduled daily feedings was measured by the percentage of total daily activity occurring during the 3 h before the availability of food (0700 to 1000). Three-hour FAA and total daily activity were averaged over 4- to 5-day stages for each genotype. The LD food restriction protocol was divided into stages as follows. The last 5 days of AL access, and then the 15 days of FR, were averaged in 5-day blocks: FR (1–5), (6–10), and (11–15). Under DD, the stages analyzed were the last 5 days of AL, and the 24 days of FR divided into 4- to 5-day blocks: FR (1–5), (6–10), (11–15), (16–20), and (21–24).
The second measure of FAA, the bout frequency, was the percentage of FR days that an individual met following the FAA bout criterion. The third measure, FAA bout start time, was defined as the earliest time bin before food delivery (1000), when the rate of activity was ≥50 counts/10-min bin with no more than a 20-min gap at this activity level. This objective criterion matched closely with FAA bout start times determined by visual inspection. For each FR day, if an individual did not meet FAA bout criterion, then that day was coded as having no FAA bout for frequency and start time analysis. FAA bout frequency was averaged by genotype under each lighting condition. Mean FAA bout start time was an average of all FAA bout start times by genotype.
To determine whether start times for FAA or nocturnal activity changed after removal of food-related temporal cues (i.e., return to free feeding and then total food deprivation), average FAA start times by genotype for the 2 days of total deprivation of food (TD1,2) were subtracted from the average start times for the last 2 days of FR (FR14,15) (see Fig. 8). Difference scores for nocturnal bout start times were similarly calculated. The start time of the nocturnal bout was determined by the earliest time point when activity was ≥50 counts/10 min and contiguous with the onset of darkness (1600). The phase of FAA during TD was determined by visual inspection of the daily average waveforms (6).
FAA magnitude and total activity means were subjected to two-way repeated-measures analyses of variance (ANOVA) with genotype and experimental stage as factors, within a lighting condition or lighting condition and experimental stage as factors, calculated by genotype. Where significant main or interaction effects were found, as reported in Results, post hoc Tukey's tests were performed on individual comparisons. FAA bout frequencies were subjected to t-tests between both the two genotypes and the two lighting conditions. FAA start time means were compared by two-way repeated-measures ANOVA with genotype and FR day as factors within a lighting condition and by one-way repeated-measures ANOVA with days as a factor within a genotype. Other analyses performed are reported in Results.
Results
FAA: actograms
Representative WT and Clk/Clk actograms under LD and DD conditions are depicted in Figs. 2 and 3, top, respectively. Average daily waveforms of counts per minute for each change in feeding condition are shown below each actogram. Shaded areas denote the food-available periods in both the actograms and the average waveforms.
Fig. 2.
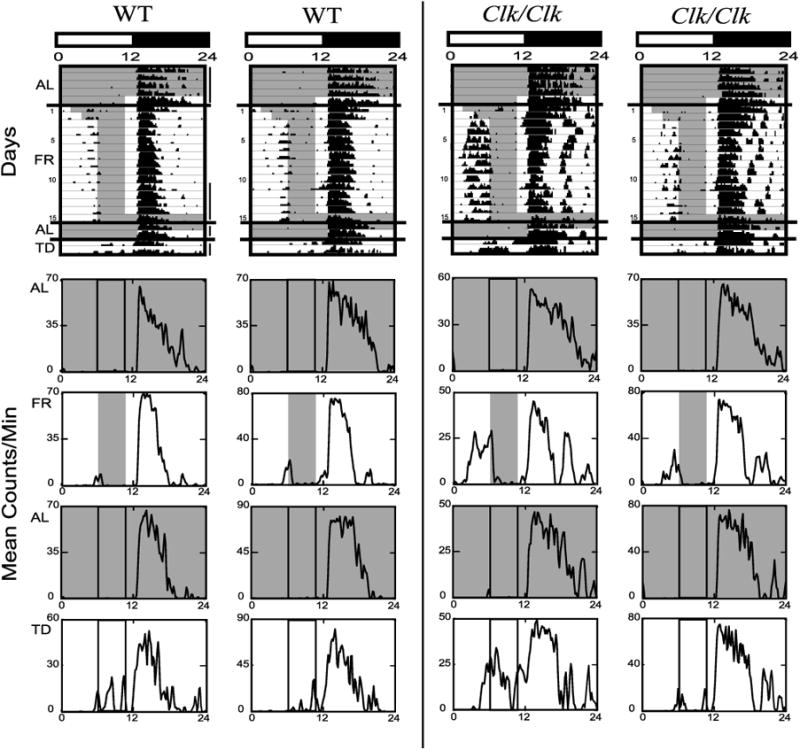
Wheel-running activity of 2 representative wild-type (WT, left) and 2 homozygous Clock mutant (Clk/Clk, right) mice housed in light-dark (LD) is shown with lighting and feeding schedule as represented in Fig. 1, left. The last 5 days of AL, the last 5 days of FR, the 2 days of return to AL, and the 2 days of TD, as denoted by vertical lines to the right of the 1st actogram, are also shown as average waveforms below each actogram. Waveforms are the mean counts/min over 24 h for the days averaged. Note that the y-axis scale for each waveform varies by the maximal running intensity for that period. Shaded areas represent time of food availability. Vertical lines within average waveforms denote time of food availability during the FR portion of the experiment.
Fig. 3.
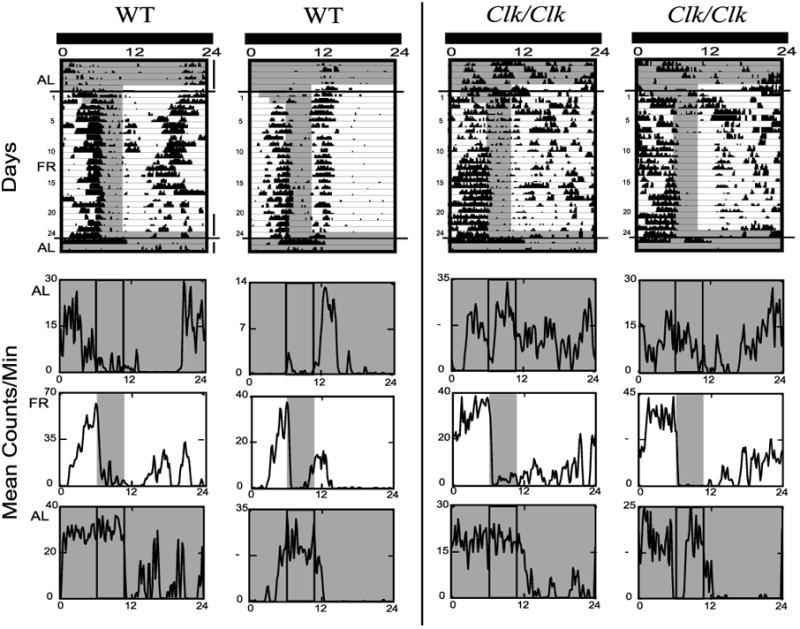
Wheel-running activity of 2 representative WT (left) and 2 Clk/Clk(right) mice housed in DD is shown with lighting and feeding schedule as represented in Fig. 1, right. The last 5 days of AL, the last 5 days of FR, and the 2 days of return to AL are also shown as average waveforms below each actogram. Details are as in Fig. 2.
During the daytime hours in LD, there was little activity throughout the initial AL period. In contrast, when food was restricted to 4 h/day, daytime activity increased just before food availability (Fig. 2 actograms). Both WT and Clk/Clk mice demonstrated FAA under LD. This change in the distribution of activity was also seen in the average waveforms as an increase in activity before food access and the corresponding reduction of the nocturnal bout (compare Fig. 2 AL and FR waveforms). The frequency of FAA bouts also increased with FR days. WT and Clk/Clk mice both initially demonstrated only nocturnal bouts, which started at lights off, but over the course of FR, more and more animals demonstrated FAA bouts that preceded the delivery of food. Linear regression analysis of variance indicates both WT [F(1,14) = 6.9, P < 0.05] and Clk/Clk [F(1,14) = 16.1, P < 0.001] mice significantly increased the number of FAA bouts over days. Return to AL feeding extinguished daytime activity, but TD triggered activity during the former mealtime in all mice (both WT and Clk/Clk).
During the initial AL period in DD, WT mice showed free-running activity rhythms, and Clk/Clk mice were mostly arrhythmic, with activity distributed relatively equally throughout the day (Fig. 3). The free-running period of WT animals was 23.5 ± 1.3 h [mean amplitude value (a) = 670, P < 0.01, as determined by χ2 analysis (ClockLab)], and four of eight Clk/Clk mice were arrhythmic after extended DD. The other four Clk/Clk mice expressed very low amplitude periods averaging 25.7 ± 4.3 h (a = 444, P < 0.01). During DD FR, just as in LD FR, activity increased just before food availability, resulting in an increasing number of FAA bouts over days in WT [F(1,23) = 28.2, P < 0.001] and Clk/Clk mice [F(1,23) = 16.4, P < 0.001] mice. In DD FR compared with DD AL, all animals, even the formerly arrhythmic Clk/Clk mice, expressed a higher amplitude activity rhythm with a 24-h interval, reflecting the periodicity of food availability (WT: 24 ± 0.0 h, a = 1250, P < 0.01; Clk/Clk: 24.0 ± 0.2 h, a = 544, P < 0.01). The phase of FAA persisted after return to AL feeding in DD from DD FR in all mice (WT and Clk/Clk) but did not continue after return to AL in LD after LD FR.
Magnitude of FAA
To determine whether the percentage of activity during the FAA period increased during FR and whether this was dependent on genotype, FAA magnitude was calculated under both LD and DD conditions. In LD (Fig. 4A), both Clk/Clk and WT mice significantly increased activity during the 3-h period before food availability over the course of the experimental stages [F(3,94) = 38.0, P < 0.001]. WT mice showed significantly more activity before feeding time during the first FR stages [FR (1–5), (6–10)] compared with activity during the same 3-h period under AL. Clk/Clk mice showed significantly greater FAA during the last two stages of food restriction [FR (6–10), (11–15)] compared with activity during the same time period under AL. Clk/Clk mice expressed significantly more FAA overall than WT mice [F(1,32) = 10.1, P < 0.01], with significantly higher FAA scores than WT animals during the last two stages of food restriction [FR (6–10): q = 5.9, P < 0.001; FR (11–15): q = 6.3, P < 0.001].
Fig. 4.
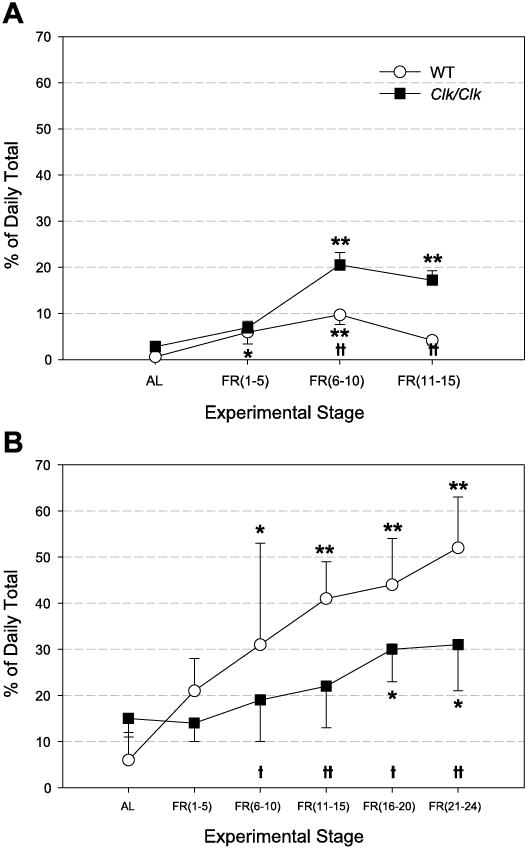
Food-anticipatory activity (FAA) magnitude (means ± SE), displayed as % daily total activity, in animals housed in LD (A) and DD (B). Percent activity corresponding to the 3-h FAA period is averaged over the last 5 days of AL feeding and over the number of days during FR as shown on the x-axis. *Differences between AL and other stages within a genotype; †differences between genotypes within a stage. * and †P < 0.05; ** and ††P < 0.001.
Under DD (Fig. 4B), as in LD, there were significant stage differences in amount of FAA [F(5,50) = 20.67, P < 0.001]. FAA in the last four FR stages in WT mice and in the last two FR stages in Clk/Clk mice was significantly greater than activity during the corresponding period during AL. In contrast to LD conditions, under DD, WT mice showed a greater percentage of total activity during the 3-h period before food availability than Clk/Clk mice [F(1,10) = 14.03, P < 0.01]. This trend was significantly greater during the last four stages of food restriction [FR (6–10): q = 3.1, P < 0.05; FR (11–15): q = 4.9, P < 0.001; FR (16–20): q = 3.5, P < 0.05; FR (21–24): q = 5.4, P < 0.001]. The lower FAA percentage seen in Clk/Clk mice was due to their increased activity starting well before the 3-h FAA period, whereas WT mice tended to show increased activity closer to the time of food availability (Fig. 3). FAA magnitude was also dependent on the lighting cycle. It was greater under the first 15 days of DD food restriction than under LD conditions in both genotypes [WT: F(1,18) = 38.27, P < 0.001; Clk/Clk: F(1,24) = 4.7, P < 0.05]. Specifically, the maximum average FAA magnitude in Clk/Clk mice was 20% in LD FR compared with 30% during the first 15 days of DD FR. Similarly, mean WT FAA magnitude was at most 10% in LD vs. 40% in DD at FR (11–15) stage. The significant increase in FAA magnitude over stages was replicated when LD FR and DD FR data were combined by genotype. In addition, there was a significant interaction between type of light cycle and stage of FR [WT: F(3,53) = 11.28, P < 0.001; Clk/Clk: F(3,71) = 5.96, P < 0.001].
Total activity
To determine whether genotype, FR, or lighting affected the total amount of activity, we calculated the average total daily activity under LD (Fig. 5A) and DD (Fig. 5B) conditions. In contrast to the genotypic differences in FAA, total activity did not differ between WT and Clk/Clk mice in either LD, [ F(1,32) = 0.03, P = nonsignificant (NS)] or DD, [ F(1,10) = 0.02, P = NS]. Overall, there were no systematic FR stage differences in total activity, with animals averaging 15,000 to 20,000 wheel rotations daily. There were statistically significant differences in LD FR (1–5) vs. LD FR (11–15) [q = 4.4, P < 0.05] and in DD AL stage vs. DD FR (1–5) [q = 6.05, P < 0.001]. Neither WT nor Clk/Clk mice showed significant differences in total activity between LD and DD conditions [WT: F(1,18) = 0.30, P = NS; Clk/Clk: F(1,24) = 1.5, P = NS]. Total activity output was relatively impervious to FR and to lighting condition, and even to genotype.
Fig. 5.
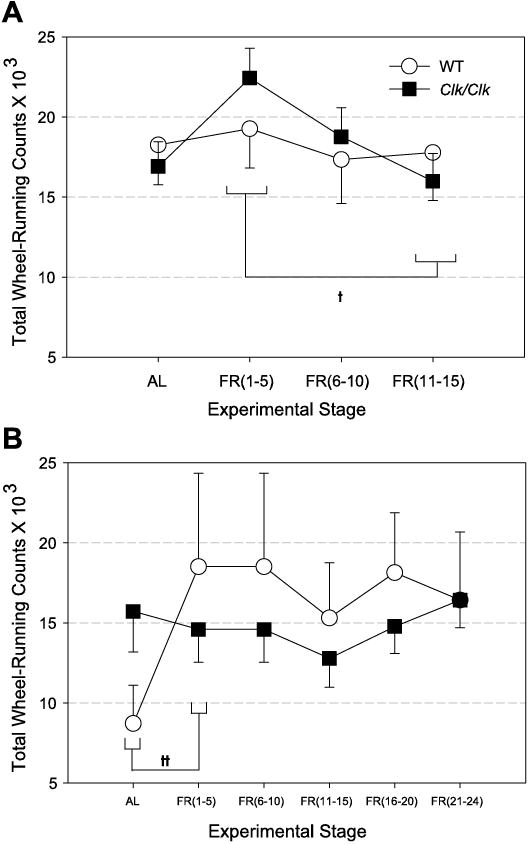
Total daily wheel-running counts (means ± SE) are shown in animals housed in LD (A) and DD (B). Total activity is averaged over a period of days as shown on the x-axis, details as in Fig. 4. The LD FR (1–5) stage is significantly different from LD FR (11–15), and the DD AL stage is different from DD FR (1–5) when the data are collapsed across genotypes ††P < 0.001. No other genotypic or stage differences are significant.
FAA bouts: frequency and start times
To determine whether there were genotypic differences in either FAA bout frequency or start time, the occurrence of FAA bouts and their start times were assessed for each individual for both genotypes. Under LD FR, Clk/Clk mice had an FAA bout frequency of 75%, whereas WT mice demonstrated FAA only 50% of the time (Fig. 6A). A t-test confirmed that the Clk/Clk group had a greater occurrence of activity meeting FAA criterion over days compared with WT controls [t(32) = 7.2, P < 0.001].
Figure 6.
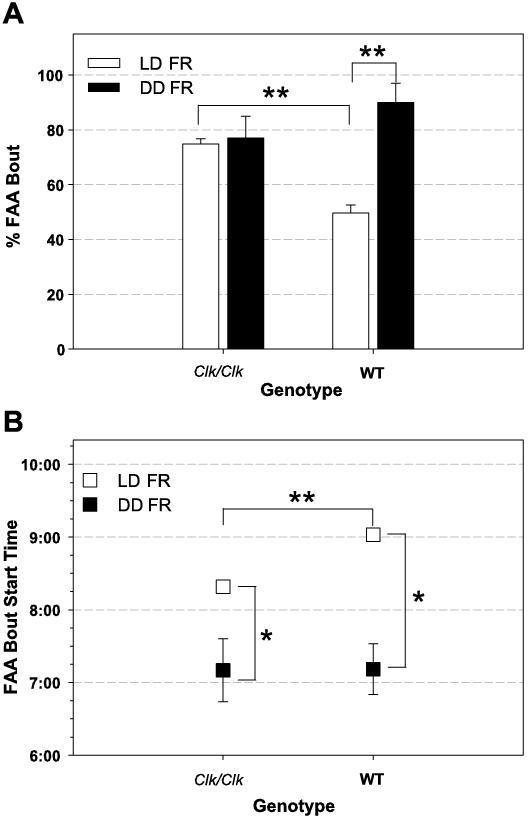
B depicts the average FAA bout start time over FR days. During LD, Clk/Clk FAA bout start times were earlier than those of WT mice, [Clk/Clk 8:19 ± 0:05 SE; WT 9:02 ± 0:04 SE]. Two-way repeated-measures ANOVA confirmed that start times of the two genotypes are significantly different [F(1,32) = 6.02, P < 0.05]. Differences in start times over days is not significant when WT and Clk/Clk data are combined [ F(15,263) = 0.8, P = NS], but one-way repeated-measures ANOVA indicated that WT FAA start times become significantly later over FR days [F(15,90) = 2.01, P < 0.05; see Fig. 2]. However, Clk/Clk FAA start times did not significantly change over FR days [F(15,173) = 1.2, P = NS].
Under DD (Fig. 6A), Clk/Clk mice had nearly the same FAA bout frequency as during LD [77 vs. 75%]. In contrast, the number of FAA bouts significantly increased in WT mice during DD compared with LD [90 vs. 50%]. The difference in FAA bout frequency in DD between genotypes was not significant [t(11) = 1.7, P = NS]. However, the frequency of WT animals demonstrating FAA bouts in the first 15 days of DD FR was significantly greater than in the 15 days of LD FR [t(18) = 5.4, P < 0.001].
During DD, the two genotypes had very similar FAA bout start times [Fig. 6B, Clk/Clk 7:10 ± 0:26 SE; WT 7:11 ± 0:21 SE]. Two-way repeated-measures ANOVA verified that WT and Clk/Clk mice did not differ in FAA bout start time [F(1,10) = 0.01, P = NS] but showed that differences by FR day were significant [F(24,178) = 2.3, P < 0.001]. One-way ANOVA confirmed that the significant difference in start time over days of FR was true for both genotypes [WT: F(24,60) = 2.1, P < 0.01; Clk/Clk: F(24,118) = 1.7, P < 0.05]. As can be seen in the actograms (Fig. 3), FAA tended to start earlier over the course of food restriction. FAA bout start times were earlier in the day during DD food restriction than during LD food restriction. The first 15 days of DD food restriction were compared with the 15 days of LD food restriction in a Mann-Whitney rank sum test (because the data were not normally distributed). FAA bout start times were significantly earlier under DD conditions for both WT [P < 0.01] and Clk/Clk [P < 0.01] mice.
TD
We examined daytime activity during 2 days of TD following LD food restriction and 2 days of free access to food (Fig. 7). FAA was rapidly extinguished by return to AL feeding, and activity was negligible throughout the daylight hours. However, daytime activity spontaneously returned under TD in both WT and Clk/Clk mice. The increase in daytime activity was elevated around the former mealtime and declined thereafter, before lights off.
Fig. 7.
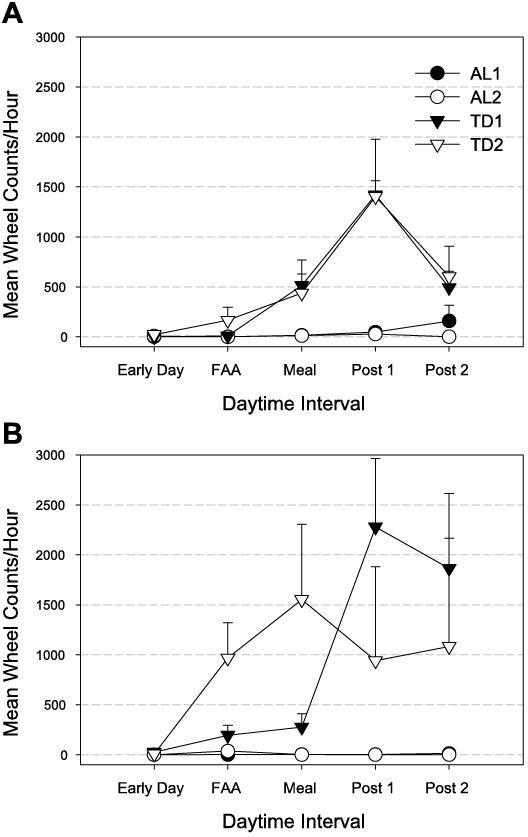
Hourly wheel counts (means ± SE) during the daytime for the 2 days following return to AL food access (AL1, AL1,2) and for the 2 days of TD (TD1, TD1,2) after LD FR for WT (A) and Clk/Clk(B) mice. Daytime activity is averaged over 5 different periods: Early Day, zeitgeber time (ZT) 0–3; FAA, ZT 3–6; Meal, ZT 6–10; Post 1, ZT 10–11; Post 2, ZT 11–12. Where error bars are absent, SE was smaller than the data point symbol for the mean.
Daytime activity (expressed as a ratio of total daily activity) was the same during last 2 days of FR as the 2 days of complete food deprivation (paired t-test, P = NS). Specifically, WT mice demonstrated 11 ± 2% of total activity during the daytime of FR(14,15) and 16 ± 3% during TD(1,2). Similarly, Clk/Clk daytime activity was 35 ± 10% of the daily total during the last 2 days of FR, and 28 ± 10% during TD.
Importantly, FAA and nocturnal bout start times phase-shifted in opposite directions during TD in both genotypes (Fig. 8). In WT and Clk/Clk mice, FAA bout start times were phase delayed by 115 and 17 min, respectively, during TD, resulting in FAA bouts starting closer to the time of former food availability. In contrast, nocturnal bout start times were phase advanced in both WT and Clk/Clk mice 50 and 75 min, respectively, before FR14,15 start times. During TD, nocturnal bouts started before lights off in the LD cycle. The difference in phase shifts of FAA and nocturnal bout start times was significantly different in WT mice, [t(4) = 9.7, P < 0.001]. The same trend was seen in Clk/Clk mice {although the finding did not reach statistical significance [t(4) = 2.1, P = 0.099]}.
Fig. 8.
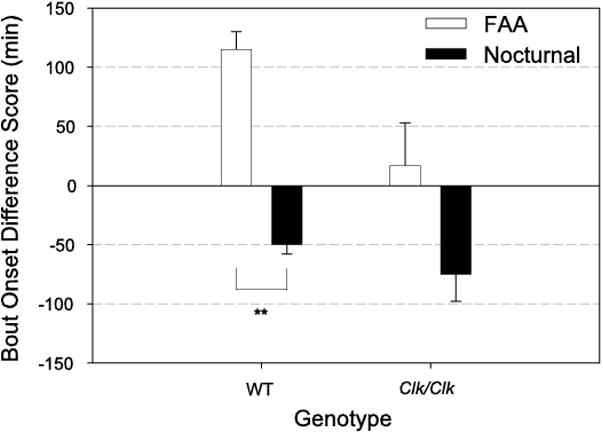
Difference scores (means ± SE) for FAA and nocturnal bout onset times for each genotype. FAA start time during the last 2 days of LD FR (FR14,15) was subtracted from the FAA start time during TD (TD1,2). Difference scores for nocturnal bout start times were similarly calculated. FAA bouts phase-shifted later and nocturnal bout start times phase-shifted earlier during TD in both WT and Clk/Clk mice. **Significantly different groups P < 0.001.
Discussion
Clk/Clk mice show FAA in LD and DD
This is the first demonstration that the Clock gene is not necessary for the expression of a circadian food-entrained behavior. Clk/Clk mice sustain FAA after prolonged housing in DD, when they have become arrhythmic in SCN-dependent locomotor activity. Thus, although CLOCK protein is a core positive regulatory element of the molecular circadian pacemaker and Clk/Clk mutant mice have damped or arrhythmic circadian gene expression in the SCN (14, 32) and throughout the periphery (19, 25, 26), the present studies show that Clk/Clk mice are able to maintain food-entrained circadian rhythms.
It might be argued that the capacity of Clk/Clk mice to predict scheduled feedings in an LD cycle does not preclude the possibility that they use the LD cycle rather than an endogenous FEO as a timing cue. The delivery of food or its consumption could be associated with a specific phase of the LD cycle. However, the ability of arrhythmic Clk/Clk mice to predict scheduled feedings in DD and produce rhythmic FAA argues against this alternative and instead suggests that the FEO is based on a different molecular mechanism than that in the SCN.
FAA might also be attributed to a response to food deprivation itself, but FAA reemerges at the time of the former mealtime after four consecutive days without a temporal food cue (see Figs. 2 and 7). The recurrence of FAA cannot be ascribed to a generalized increase in daytime activity caused by hunger, because activity decreased 2 h after the former mealtime, demonstrating the temporal control of the food-entrained clock. These results demonstrate that, in Clk/Clk mice, as in other rodents (19), FAA is the product of a circadian timer and not a food deprivation-related cue or an interval timer that is reset by food. Taken together, the data suggest that a functional CLOCK-based oscillator is not necessary for food-entrained circadian locomotor rhythms.
FEO and LEO compete for control of behavior
In addition to demonstrating that CLOCK is not necessary for FAA, the present results also indicate a novel relationship between the FEO and SCN in the regulation of circadian activity. In both WT and Clk/Clk mice, there is no overall difference in total amount of activity during AL and FR stages in either LD or DD conditions (Fig. 5, A and B). Nevertheless, FAA magnitude increased over consecutive days of FR, resulting in a corresponding decrease in nocturnal activity (Figs. 2 and 3 and 4, A and B). This shift in the temporal distribution of activity suggests that locomotor activity is homeostatically regulated, as has previously been indicated (27).
Wheel-running activity can be controlled by food (via the FEO) as FAA, and also by light (via the SCN) as nocturnal activity, a finding consistent with a substantial literature indicating that the SCN and FEO are anatomically separate oscillators (19). In both WT and Clk/Clk animals, our results support the duality of circadian control of behavior. Under conditions of food restriction, the influence of both oscillators can be seen, resulting in temporally distinct nocturnal and diurnal patterns of wheel-running behavior. In the absence of light (i.e., in DD), more of the activity is controlled by the FEO. In the presence of light, FAA is reduced, which could be a consequence of masking by light in nocturnal rodents, or it may be due to a reduced effect of the temporal cue provided by a food signal (and the FEO) in the presence of a photic signal (and the SCN). More specifically, FAA magnitude was greater and FAA bouts started earlier in DD compared with LD (Figs. 4, A and B and 6B). Additionally, FAA bout occurrence increased in WT animals in DD compared with LD, whereas Clk/Clk mice seemed to reach a ceiling level and maintained a high number of bouts under both photic conditions (Fig. 6A). There were also differences in FAA expression after a return to AL feeding depending on the lighting conditions. FAA persisted for one more cycle in DD, whereas it damped immediately in LD (Figs. 2 and 3, second AL stages). This may be a consequence of the masking effects of light.
Another indication that the FEO and LEO are distinct oscillators is the finding that the start time of the FAA bout phase-shifts later and the nocturnal bout phase-shifts earlier in the daytime during 2 days of total food deprivation in LD (Fig. 8). It is intriguing that the start of the nocturnal activity component shifts during total food deprivation, even though animals were maintained on an LD cycle. This phase advance of the nocturnal bout is likely to be downstream of the LEO, since food restriction does not phase-shift circadian gene expression in the SCN (7, 37). Similar examples of separate control by light and food can be seen in the behaviors of other rodents. Rats food restricted at various times during the light cycle display FEO-dependent FAA before meals as well as SCN-dependent nocturnal activity (5). Food-restricted rats have a bimodal pattern of corticosterone secretion, in which the first peak depends on feeding time and the second peak is at the same time as in ad libitum-fed animals (17). In summary, there is substantial evidence that both food (or FEO output) and light (or SCN output) temporally control locomotor behavior and that each circadian clock system can operate independently of the other. Under normal ad libitum conditions, these two systems are in phase with each other, but daytime food restriction forces the two out of synchrony and reveals their separate control.
As depicted in Fig. 9, the timing of wheel-running activity depends on the photic and food cues available. When feeding is restricted to day (in LD) or subjective day (in DD), FAA can be distinguished from other locomotor activity in both WT and Clk/Clk mice. In both LD and DD, the SCN in WT mice signals nocturnal locomotor activity, and food-related cues entrain the FEO. In the same conditions in the Clk/Clk mouse, FEO entrains FAA, whereas the contribution of the SCN to activity (if any) is unknown. We do know that, in DD, the behavior of the genotypes differs, with WT mice exhibiting FAA and a free-running, SCN-dependent nocturnal bout of activity; in contrast, Clk/Clk mice can maintain only FAA, whereas the remainder of their activity is arrhythmic.
Fig. 9.
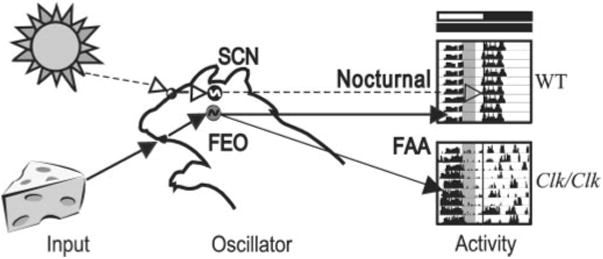
Schematic depicting control of locomotor activity by light- and food-related cues in WT and Clk/Clk mutant mice. Photic cues (denoted by dashed lines and open arrowheads) travel from the eyes via the retinohypothalamic tract to the light-entrainable oscillators in the suprachiasmatic nucleus (SCN). Food-related cues (denoted by solid lines and filled arrowheads) reach a different set of oscillators, the food-entrainable oscillator (FEO), whose locus (i) remains to be determined. Normally, under AL conditions, SCN and FEO are in synchrony, because the LD cycle sets the phase of activity and thus animals will eat during the night or subjective night. When food is restricted to the daytime or formerly inactive period, SCN and FEO are out of phase with each other, as shown here. The 2 bars above the actograms denote former LD cycle and current DD cycle, respectively. Shaded area within each actogram indicates time of food availability. In DD, WT mice express both nocturnal SCN-dependent activity and FEO-dependent FAA, whereas Clk/Clk mice exhibit only FAA. Their remaining locomotor activity is arrhythmic in DD.
The SCN and FEO compete for control of locomotor behavior, and when they are in conflict, the genotypically dependent oscillator strength and the type of entraining cue determine the phase of circadian locomotor rhythms. Although both genotypes show food-entrained rhythms, the presence of a photic temporal cue has different effects on WT and Clk/Clk mice. In LD, Clk/Clk mice demonstrate greater FAA magnitude and FAA bout frequency and earlier FAA bout start times than WT mice (Figs. 4A and 6, A and B). This greater FAA response in Clk/Clk mice in LD suggests that these mice have a more effective FEO and a stronger response to food. Another possibility is that Clk/Clk mice are less masked by light than their WT controls. In contrast, in DD, FAA bout frequency and start times are the same in the two genotypes.
Our results elucidate previous reports of strain and species differences in FAA magnitude, suggesting underlying differences in the effectiveness of the FEO (19, 27, 28, 31), in its output signal, or in SCN-FEO coupling (26, 19). For example, BALB/c mice display rapid induction and greater magnitude of diurnal FAA compared with C57BL/6j mice studied in the same laboratory (13). The C3H mouse is a rigidly nocturnal feeder and can adapt to daytime food restriction only if food access is shifted very gradually (20). Clock/+ heterozy-gotes do not phase-shift after daytime FR in LD and return to free feeding in DD, whereas WT mice phase-advance by ∼1 h (4). Intact rats exhibit less FAA than SCN-lesioned rats (8). WT Long-Evans controls and Brattleboro rats (vasopressin deficient) do not differ under ad libitum food and water conditions under various LD cycles (28). However, under daytime food restriction, WT rats have a peak in body temperature and heart rate in both day and night, whereas Brattle-boros have only the daytime peak (23). The present study extends these findings of strain and species differences in FAA expression by providing evidence that the SCN and FEO compete for control of locomotor behavior.
FEO may depend on another bhlh-PAS domain transcription factor
Gene expression rhythms, such as Per1, Per2, BMAL1, and Pai-1 in the heart, are damped or attenuated under ad libitum conditions in Clk/Clk mice relative to WT controls, but expression becomes diurnally rhythmic after daytime-restricted feeding (19, 26). This increase in rhythmicity and amplitude due to scheduled meals indicates that functional CLOCK protein is not necessary for resetting peripheral clocks. Restricted feeding time could synchronize peripheral oscillators via any of a number of chemical signals (food metabolites, blood-borne molecules). CLOCK protein is but one member of a family of bhlh-PAS domain transcription factors. Another member of the transcription family, neuronal PAS domain protein-2 (NPAS2) could be an analog of CLOCK in the mammalian forebrain. This function is suggested by the relatively damped oscillation of Per2 in homozygous NPAS2 mutant mice in brain areas that normally express NPAS2 and rhythmic Per2(29). Furthermore, coinduction of NPAS2 and BMAL1 activates transcription of endogenous Per and Cry genes in cultured neuroblastoma cells (29). The metabolic state of cells may link environmental conditions to circadian molecular oscillators through changes in redox potential and may also influence circadian gene expression more directly (31). Changes in redox potential affect the DNA binding of CLOCK-BMAL1 and NPAS2-BMAL1 heterodimers (29). If applicable, this mechanism may underlie the synchronization process of peripheral oscillators and/or the FEO not only under restricted feeding conditions but also during typical ad libitum feeding.
This study demonstrates that genotype influences relative oscillator strength and thereby affects the extent to which specific temporal cues influence behavioral rhythms. It remains to be determined whether the FEO is based on a bhlh-PAS domain transcription factor, such as NPAS2, or on a novel, yet-to-be-discovered mechanism. The present results show that the molecular basis of the FEO differs from that known in the SCN.
Acknowledgments
We thank J. S. Takahashi for his gift of Clk/Clk mutant breeding pairs, D. R. Weaver for primers and protocol for Clock genotyping, and J. Tang for development of analysis software. We would also like to thank L. J. Kriegsfeld, M. Antle, and J. LeSauter for their helpful comments on earlier drafts of the manuscript.
This study was supported by National Institutes of Health Grants NS-37919 and NS-41069 (to R. Silver) and 5T32 D0–7328 (to S. Pitts).
References
- 1.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challet E, Pevet P, Vivien-Roels B, Malan A. Phase-advanced daily rhythms of melatonin, body temperature, and locomotor activity in food-restricted rats fed during daytime. J Biol Rhythms. 1997;12:65–79. doi: 10.1177/074873049701200108. [DOI] [PubMed] [Google Scholar]
- 3.Challet E, Solberg LC, Turek FW. Entrainment in calorie-restricted mice: conflicting zeitgebers and free-running conditions. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1751–R1761. doi: 10.1152/ajpregu.1998.274.6.R1751. [DOI] [PubMed] [Google Scholar]
- 4.Challet E, Takahashi JS, Turek FW. Nonphotic phase-shifting in clock mutant mice. Brain Res. 2000;859:398–403. doi: 10.1016/s0006-8993(00)02040-0. [DOI] [PubMed] [Google Scholar]
- 5.Coleman GJ, Harper S, Clarke JD, Armstrong S. Evidence for a separate meal-associated oscillator in the rat. Physiol Behav. 1982;29:107–115. doi: 10.1016/0031-9384(82)90373-0. [DOI] [PubMed] [Google Scholar]
- 6.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- 7.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson AJ, Stephan FK. Feeding-entrained circadian rhythms in hypophysectomized rats with suprachiasmatic nucleus lesions. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1376–R1384. doi: 10.1152/ajpregu.1999.277.5.R1376. [DOI] [PubMed] [Google Scholar]
- 9.Edmonds SC. Food and light as entrainers of circadian running activity in the rat. Physiol Behav. 1977;18:915–919. doi: 10.1016/0031-9384(77)90201-3. [DOI] [PubMed] [Google Scholar]
- 10.Escobar C, Diaz-Munoz M, Encinas F, Aguilar-Rob-lero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1309–R1316. doi: 10.1152/ajpregu.1998.274.5.R1309. [DOI] [PubMed] [Google Scholar]
- 11.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 12.Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmes MM, Mistlberger RE. Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol Behav. 2000;68:655–666. doi: 10.1016/s0031-9384(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 14.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 15.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- 17.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesauter J, Silver R. Heavy water lengthens the period of free-running rhythms in lesioned hamsters bearing SCN grafts. Physiol Behav. 1993;54:599–604. doi: 10.1016/0031-9384(93)90255-e. [DOI] [PubMed] [Google Scholar]
- 19.Minami Y, Horikawa K, Akiyama M, Shibata S. Restricted feeding induces daily expression of clock genes and Pai-1 mRNA in the heart of Clock mutant mice. FEBS Lett. 2002;526:115–118. doi: 10.1016/s0014-5793(02)03159-9. [DOI] [PubMed] [Google Scholar]
- 20.Mistlberger R. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 21.Mistlberger RE, Marchant EG, Kippin TE. Food-entrained circadian rhythms in rats are insensitive to deuterium oxide. Brain Res. 2001;919:283–291. doi: 10.1016/s0006-8993(01)03042-6. [DOI] [PubMed] [Google Scholar]
- 22.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 23.Murphy HM, Wideman CH, Nadzam GR. The interaction of vasopressin and the photic oscillator in circadian rhythms. Peptides. 1996;17:467–475. doi: 10.1016/0196-9781(95)02129-9. [DOI] [PubMed] [Google Scholar]
- 24.Nelson W, Scheving L, Halberg F. Circardian rhythms in mice fed a single daily meal at different stages of lighting regimen. J Nutr. 1975;105:171–184. doi: 10.1093/jn/105.2.171. [DOI] [PubMed] [Google Scholar]
- 25.Oishi K, Fukui H, Ishida N. Rhythmic expression of BMAL1 mRNA is altered in Clock mutant mice: differential regulation in the suprachiasmatic nucleus and peripheral tissues. Biochem Biophys Res Commun. 2000;268:164–171. doi: 10.1006/bbrc.1999.2054. [DOI] [PubMed] [Google Scholar]
- 26.Oishi K, Miyazaki K, Ishida N. Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2 and BMAL1 mRNAs in the heart of Clock mutant mice on Jcl:ICR background. Biochem Biophys Res Commun. 2002;298:198. doi: 10.1016/s0006-291x(02)02427-0. [DOI] [PubMed] [Google Scholar]
- 27.Osiel S, Golombek DA, Ralph MR. Conservation of locomotor behavior in the golden hamster: effects of light cycle and a circadian period mutation. Physiol Behav. 1998;65:123–131. doi: 10.1016/s0031-9384(98)00140-1. [DOI] [PubMed] [Google Scholar]
- 28.Peterson GM, Watkins WB, Moore RY. The suprachiasmatic hypothalamic nuclei of the rat. VI. Vasopressin neurons and circadian rhythmicity. Behav Neural Biol. 1980;29:236–245. doi: 10.1016/s0163-1047(80)90573-7. [DOI] [PubMed] [Google Scholar]
- 29.Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 30.Richter CP. A behavioristic study of the activity of the rat. Comp Psych Monographs. 1922;1:1–55. [Google Scholar]
- 31.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 32.Silver R, Sookhoo AI, LeSauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport. 1999;10:3165–3174. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- 33.Steeves TD, King DP, Zhao Y, Sangoram AM, Du F, Bow-cock AM, Moore RY, Takahashi JS. Molecular cloning and characterization of the human CLOCK gene: expression in the suprachiasmatic nuclei. Genomics. 1999;57:189–200. doi: 10.1006/geno.1998.5675. [DOI] [PubMed] [Google Scholar]
- 34.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 35.Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979;25:346–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- 36.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 38.Van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res Brain Res Rev. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- 39.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Low-rey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilsbacher LD, Takahashi JS. Circadian rhythms: molecular basis of the clock. Curr Opin Genet Dev. 1998;8:595–602. doi: 10.1016/s0959-437x(98)80017-8. [DOI] [PubMed] [Google Scholar]
- 41.Yagita K, Tamanini F, van der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibro-blasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]


