Abstract
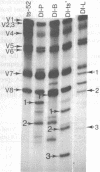
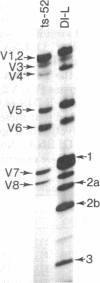
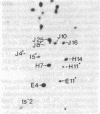
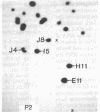
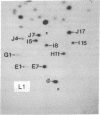
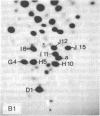
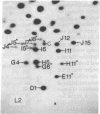
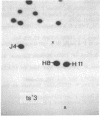
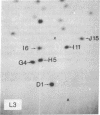
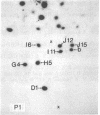
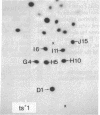
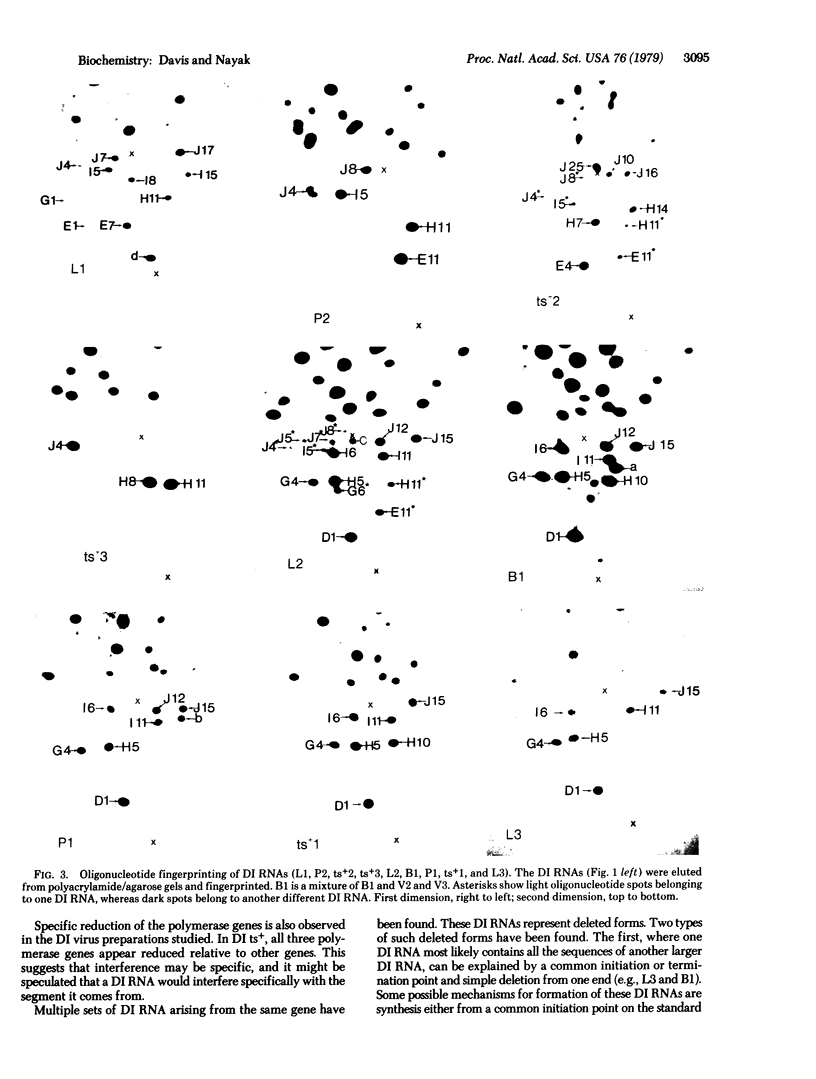
Each clone of ts-52 and ts+ WSN influenza virus, when serially passaged at high multiplicity, gives rise to defective interfering (DI) virus with a unique set of new RNA species. The new RNAs (DI RNA) from several DI viruses were examined by the technique of RNase T1 oligonucleotide two-dimensional electrophoresis. It was found that each DI RNA arises from a specific segment of standard viral RNA. All DI RNA studied arose from the viral polymerase genes (P1, P2, and P3). DI RNAs originating from the same polymerase gene were interrelated. Certain of these DI RNAs appeared to contain completely overlapping nucleotide sequences. Others contained both overlapping and nonoverlapping nucleotide sequences. The latter DI RNAs may be formed from the progenitor viral RNA segment by a mechanism other than a common initiation (or termination) point and a simple deletion from one end.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Crumpton W. M., Dimmock N. J., Minor P. D., Avery R. J. The RNAs of defective-interfering influenza virus. Virology. 1978 Oct 15;90(2):370–373. doi: 10.1016/0042-6822(78)90322-7. [DOI] [PubMed] [Google Scholar]
- Desselberger U., Nakajima K., Alfino P., Pedersen F. S., Haseltine W. A., Hannoun C., Palese P. Biochemical evidence that "new" influenza virus strains in nature may arise by recombination (reassortment). Proc Natl Acad Sci U S A. 1978 Jul;75(7):3341–3345. doi: 10.1073/pnas.75.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U., Palese P. Molecular weights of RNA segments of influenza A and B viruses. Virology. 1978 Jul 15;88(2):394–399. doi: 10.1016/0042-6822(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Glimp T., Clewley J. P., Bishop D. H. Studies on the generation of vesicular stomatitis virus (indiana serotype) defective interfering particles. Virology. 1978 Jan;84(1):142–152. doi: 10.1016/0042-6822(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Ueda M., Sugiura A. Origin of small RNA in von Magnus particles of influenza virus. J Virol. 1979 Mar;29(3):1142–1148. doi: 10.1128/jvi.29.3.1142-1148.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Tobita K., Janda J. M., Davis A. R., De B. K. Homologous interference mediated by defective interfering influenza virus derived from a temperature-sensitive mutant of influenza virus. J Virol. 1978 Oct;28(1):375–386. doi: 10.1128/jvi.28.1.375-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Stark C., Kennedy S. I. The generation and propagation of defective-interfering particles of Semliki Forest virus in different cell types. Virology. 1978 Aug;89(1):285–299. doi: 10.1016/0042-6822(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Ueda M., Tobita K., Enomoto C. Further isolation and characterization of temperature-sensitive mutants of influenza virus. Virology. 1975 Jun;65(2):363–373. doi: 10.1016/0042-6822(75)90042-2. [DOI] [PubMed] [Google Scholar]
- VON MAGNUS P. Propagation of the PR8 strain of influenza A virus in chick embryos. III. Properties of the incomplete virus produced in serial passages of undiluted virus. Acta Pathol Microbiol Scand. 1951;29(2):157–181. doi: 10.1111/j.1699-0463.1951.tb00114.x. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]