Abstract
Objective
To compare postoperative outcomes of hip fracture surgery in patients who were and were not taking clopidogrel at the time of surgery.
Patients and Methods
Using the Rochester Epidemiology Project, we performed a population-based, retrospective cohort study comparing patients who were and were not taking clopidogrel at the time of hip fracture surgery between January 1, 1996 and June 30, 2010. Primary outcomes were perioperative bleeding and mortality. Secondary outcomes were perioperative thrombotic events.
Results
During the study period, 40 residents of Olmsted County, Minnesota (median age, 83 years), who were taking clopidogrel underwent hip fracture repair. These 40 patients were matched 2:1 with 80 controls (median age, 84 years). The groups were similar in age, sex, American Society of Anesthesiologists score, type of surgical procedure, and use of deep vein thrombosis prophylaxis. The mean time from admission to surgery was less than 36 hours for each cohort. Perioperative bleeding complications and mortality were not significantly different between patients who were and were not taking clopidogrel at the time of hip fracture surgery. Combined bleeding outcome criteria was met in 48% of the clopidogrel cohort and 45% of the control cohort (RR 1.06; 95% CI 0.70-1.58; p=0.80). One-year mortality was 28% in the clopidogrel cohort and 29% in the control cohort (hazard ratio, 1.33; 95% CI, 0.84-2.12; p=.23).
Conclusions
Although the small sample size precludes making a definitive conclusion, we found no evidence that prompt surgical treatment of hip fracture in patients on clopidogrel compromises perioperative outcomes.
Since its approval for use by the US Food and Drug Administration in 1997, clopidogrel has been increasingly used as an inhibitor of platelet aggregation, which has made the perioperative management of hip fracture more challenging. The antiplatelet effects of clopidogrel are irreversible for the lifespan of the platelet (5-7 days). Therefore, it is recommended that clopidogrel be discontinued at least 5 days before elective surgery to decrease the risk of bleeding (PLAVIX [package insert]; Bridgewater, NJ: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; 2011). The withdrawal of clopidogrel, however, has been associated with increased thromboembolic events and coronary stent thrombosis.1-3 For patients with acute hip fracture who are taking clopidogrel, the risks of perioperative bleeding must be weighed against the thrombotic risks of clopidogrel discontinuation. There are no clear guidelines on how best to manage this situation; individual surgical practices vary widely.4-6
Small observational studies have reported mixed results with respect to postoperative bleeding complications and mortality in patients with hip fracture taking clopidogrel at the time of hip fracture surgery compared with those who were not taking it. However, these small case series were not population based, had limited follow-up, and were confounded by surgical delay.1, 7-11
We performed an observational, population-based, retrospective cohort study to test the hypothesis that there is no increase in postoperative bleeding complications or mortality in patients who undergo prompt operative treatment of hip fracture while taking clopidogrel.
PATIENTS AND METHODS
Overview and Study Design
This study is a population-based, retrospective cohort study with a 2:1 matched comparison cohort using the database of the Rochester Epidemiology Project (REP). Epidemiologic research in Olmsted County, Minnesota, is possible because the county is geographically isolated from other urban centers, and nearly all medical care is delivered to the population by a limited number of local health care providers. Providers of health care to the community have agreed to share their medical records (with appropriate patient permissions) under the auspices of the REP (AR30582).12
The medical records of the health care institutions participating in the REP contain details of every inpatient hospitalization, every outpatient visit to the offices, clinics, or emergency departments, as well as laboratory results and correspondence concerning each patient. The medical records are easily retrievable because Mayo Clinic has maintained, since the early 1900s, extensive indices based on clinical and histologic diagnoses and surgical procedures. The REP has developed a similar index for the records of other providers of medical care to Olmsted County residents. The result is the linkage of medical records of care available to and used by the Olmsted County population.
Patients
After obtaining institutional review board approval, REP data retrieval specialists identified all patients hospitalized in Olmsted County from January 1, 1996, to June 30, 2010, with the primary or secondary discharge diagnosis of hip fracture (ICD-9-CM codes 820-9). Patients taking clopidogrel at the time of admission for hip fracture were identified by searching the electronic clinical notes through the Mayo Clinic Life Science Systems data warehouse. We excluded patients with nonsurgical management of hip fracture and age younger than 18 years. We also excluded patients with pathologic hip fracture and patients with multitrauma as their surgical interventions can be more involved, multiple, and their comorbid conditions might alter outcomes (e.g. increase risk of thromboembolism). For patients with more than one admission for hip fracture during the study period, only the most recent fracture was considered. For each incident case in the group taking clopidogrel, 2 controls from the remaining hip fracture patients were matched on the basis of age at surgery (±5 years), sex, American Society of Anesthesiologists score, and surgery date (±3 years) to construct the comparison cohort.
Outcomes and Data Collection
The primary investigator and trained nurse abstractors abstracted data from patient medical records using a standardized, computer-based data abstraction form. The abstractors were not blinded to clopidogrel use. Primary outcome measures for the study were perioperative bleeding and mortality. Perioperative bleeding was measured by hemoglobin nadir during the hospital stay, maximum absolute decrease in hemoglobin during hospitalization (admission hemoglobin minus hemoglobin nadir), number of blood transfusions required during hospitalization, or a major bleeding event. A major bleeding event was defined as hemorrhage resulting in another surgical procedure or endoscopic procedure or intracranial, intraperitoneal, or retroperitoneal hemorrhage as identified on radiographic imaging. Because we anticipated few major bleeding events, we also reported a composite bleeding outcome defined as any 1 of the following: hemoglobin nadir less than 7 g/dL, absolute hemoglobin decrease of more than 4 g/dL, transfusion of more than 3 units of packed red blood cells, or a major bleeding event as defined above. We chose these 4 events in composite because we believed that any of these occurrences would be atypical for hip fracture surgery and, therefore, indicative of a serious, unexpected bleeding event. Mortality data were obtained from death certificates through the REP. Using the data from the REP, we could account for the death or whereabouts of all study subjects.
Secondary outcomes were related to perioperative morbidity: in-hospital thromboembolic event (deep venous thrombosis [DVT] or pulmonary embolism), thromboembolic event within 6 weeks of hospital discharge, perioperative cardiovascular event (troponin leak, angina, acute coronary syndrome, myocardial infarction, stroke, transient ischemic attack, limb ischemia) within 30 days of surgery, infection within 30 days of surgery, and reoperation on the affected hip within 1 year of surgery. Because we anticipated few events, we also reported a composite morbidity outcome if patients had any 1 of the above individual events.
Statistical Analysis
Continuous data were summarized as mean (SD), and categorical data were summarized as frequency (percentage). Comparisons of demographics, bleeding outcomes, and secondary outcomes between patients in the control and clopidogrel cohorts were evaluated using 2-sample t, Wilcoxon rank sum, χ2, and Fisher exact tests. The association of clopidogrel use with bleeding and secondary outcomes was summarized as relative risk and 95% CIs. Overall survival was estimated using the Kaplan-Meier method. The association of clopidogrel use with time to death from any cause was summarized as hazard ratio and 95% CI from a Cox proportional hazards regression model. Statistical analyses were performed using the SAS software package (SAS Institute, Inc, Cary, NC). All statistical tests were 2-sided and P<.05 was considered statistically significant
RESULTS
Our search of the REP identified 2,061 patients hospitalized for hip fracture during the study period, 40 of whom were identified as taking clopidogrel and made up the clopidogrel cohort. The control group comprised 80 patients matched 2:1 from among the remaining 2,021 patients with hip fracture. Baseline characteristics are summarized in Table 1. Median age at hip fracture surgery was 83 years for the clopidogrel group and 84 years in the control group. The percentage of men was 45% in both groups. There was no difference in time to surgery between the 2 cohorts. Patients in the clopidogrel cohort were less likely to be taking warfarin at admission (3% vs 19%; P=.01) and were significantly more likely to have a history of a coronary stent placement (33% vs 6%; P<.001) compared with controls. There were no statistically significant differences in the type of hip fracture, type of hip fracture treatment, type of anesthesia used, or admission laboratory results between the clopidogrel and control cohorts.
TABLE 1.
Baseline Characteristics
| Characteristic | Patient groupa
|
P value | |
|---|---|---|---|
| Clopidogrel (n=40) | Control (n=80) | ||
| Age, y | 82.0 (8.7) | 82.3 (8.3) | .68 |
| Men | 18 (45) | 36 (45) | 1.00 |
| Raceb | 1.00 | ||
| White | 37 (93) | 76 (95) | |
| Not white | 0 (0) | 1 (1) | |
| Not reported | 3 (7) | 3 (4) | |
| Previous coronary stent | 13 (33) | 5 (6) | <.001 |
| ASA score | 1.00 | ||
| 3 | 32 (80) | 64 (80) | |
| 4 | 8 (20) | 16 (20) | |
| Type of fracture | .71 | ||
| Femoral neck | 20 (50) | 38 (48) | |
| Intertrochanteric | 15 (38) | 36 (45) | |
| Subtrochanteric | 2 (5) | 2 (3) | |
| More than one | 3 (8) | 4 (5) | |
| Type of fracture repair | .97 | ||
| Screws | 4 (10) | 10 (13) | |
| Plate & screws | 9 (23) | 17 (21) | |
| Intramedullary rod | 12 (30) | 25 (31) | |
| Hemiarthroplasty | 15 (38) | 28 (35) | |
| Anesthesia type | .18 | ||
| Regional | 0 (0) | 6 (8) | |
| General | 40 (100) | 74 (93) | |
| Laboratory values at admission | |||
| Hemoglobin, g/dL | 12.0 (1.5) | 12.5 (1.6) | .14 |
| Platelets, ×109/L | 226 (63) | 234 (89) | .85 |
| Creatinine, mg/dL | 1.5 (1.1) | 1.2 (0.6) | .34 |
| INR | 1.0 (0.1) (n=34) | 1.3 (0.7) (n=69) | .83 |
| aPTT, s | 26.5 (4.4) (n=25) | 27.0 (4.3) (n=54) | .99 |
| Medications at admission | |||
| Warfarin | 1 (3) | 15 (19) | .01 |
| Aspirin | 26 (65) | 50 (63) | .79 |
| Time from admission to surgery, d | 1.1 (0.7) | 1.3 (1.3) | .77 |
| Thromboembolic prophylaxis | 40 (100) | 79 (99) | 1.00 |
| Warfarin | 2 (5) | 17 (21) | .02 |
| Aspirin | 0 (0) | 4 (5) | .30 |
| LMWH | 27 (68) | 49 (61) | .50 |
| Unfractionated heparin | 8 (20) | 7 (9) | .08 |
| SCD use | 38 (95) | 75 (94)c | .77 |
| Other | 0 (0) | 1 (1) | 1.00 |
| Dose reduction of DVT prophylaxis | 16 (40) | 39 (49) | .36 |
aPTT = activated partial thromboplastin time; ASA = American Society of Anesthesiologists; DVT = deep venous thrombosis; INR = international normalized ratio; LMWH = low-molecularweight heparin; SCD = sequential compression device.
Values are mean (SD) or No. of patients (%).
Race was self-reported.
One patient received only SCDs as the thromboembolic prophylaxis regimen.
Bleeding outcomes are summarized in Table 2. There were 3 major bleeding events in the control cohort: 1 intracerebral hemorrhage, 1 hemorrhage resulting in an endoscopic procedure, and 1 subdural hematoma. There were 2 major bleeding events in the clopidogrel cohort: 1 hemorrhage resulting in an endoscopic procedure and 1 gastrointestinal tract bleed that would have resulted in an endoscopic procedure if the patient had not refused. Patients in the clopidogrel cohort were significantly less likely to have been given vitamin K during hospitalization or prescribed warfarin for DVT prophylaxis. None of the patients in either cohort required cryoprecipitate or thrombin glue. None of the other bleeding outcomes were significantly associated with use of clopidogrel.
TABLE 2.
Bleeding Outcomes
| Characteristic | Patient groupa
|
P value | RR (95% CI)b | |
|---|---|---|---|---|
| Clopidogrel (n=40) | Control (n=80) | |||
| Hemoglobin values | ||||
| Nadir, g/dL | 9.1 (1.2) | 9.0 (1.1) | .64 | |
| Delta, g/dL | 3.0 (1.7) | 3.5 (1.6) | .08 | |
| Delta >4 g/dL | 11 (28) | 25 (31) | .67 | 0.88 (0.48-1.60) |
| Major bleeding event | 2 (5) | 3 (4) | 1.00 | 1.33 (0.23-7.66) |
| Combined bleeding outcome | 19 (48) | 36 (45) | .80 | 1.06 (0.70-1.58) |
| pRBCs | ||||
| Total units | 2.1 (2.1) | 2.1 (3.5) | .49 | |
| Patients requiring >3 units | 9 (23) | 14 (18) | .51 | 1.29 (0.61-2.71) |
| Total units of platelets | 0 (0) | 0.1 (0.5) | .32 | |
| Total units of FFP | 0 (0) | 0.5 (1.9) | .04 | |
| Vitamin K during hospitalization | 0 (0) | 12 (15) | .008 | NR |
cryo = cryoprecipitate; FFP = fresh frozen plasma; pRBCs = packed red blood cells; RR = relative risk.
Values are mean (SD) or No. of patients (%).
RR only provided for categorical data.
NR: Relative risk not reported since no patient in the clopidogrel group received vitamin K during hospitalization
None of the differences in secondary morbidity outcomes reached statistical significance (Table 3). Of the 6 control patients with reoperation, 4 had infection and 2 had implant failure. Of the 3 clopidogrel patients with reoperation, 2 had infection and 1 had implant failure. There was no significant difference in the composite morbidity outcome.
TABLE 3.
Secondary Outcomes
| Outcome | Patient groupa
|
P value | RR (95% CI) | |
|---|---|---|---|---|
| Clopidogrel (n=40) | Control (n=80) | |||
| Coronary ischemia | ||||
| Any coronary ischemia | 8 (20) | 10 (13) | .28 | 1.60 (0.68-3.74) |
| Angina | 1 (3) | 0 (0) | .33 | NR |
| Acute coronary syndrome | 0 (0) | 0 (0) | ||
| Troponin leak | 0 (0) | 4 (5) | .30 | NR |
| NSTEMI | 4 (10) | 5 (6) | .48 | 1.60 (0.45-5.63) |
| STEMI | 1 (3) | 0 (0) | .33 | NR |
| Other | 2 (5) | 1 (1) | .26 | 4.00 (0.37-42.80) |
| Cerebral ischemia | ||||
| Stroke | 1 (3) | 0 (0) | .33 | NR |
| TIA | 0 (0) | 0 (0) | ||
| Limb ischemia | 1 (3) | 0 (0) | .33 | NR |
| Venous thromboembolic disease | ||||
| In-hospital DVT | 1 (3) | 0 (0) | .33 | NR |
| In-hospital PE | 0 (0) | 0 (0) | ||
| DVT within 6 wks of discharge | 1 (3) | 2 (3) | 1.00 | 1.00 (0.09-10.70) |
| PE within 6 wks of discharge | 0 (0) | 1 (1) | 1.00 | NR |
| Infectionb | 2 (5) | 2 (3) | .60 | 2.00 (0.29-13.68) |
| Reoperationc | 3 (8) | 6 (8) | 1.00 | 1.00 (0.26-3.79) |
| Combined complication outcomed | 12 (30) | 18 (23) | .38 | 1.33 (0.71-2.49) |
DVT = deep venous thrombosis; NSTEMI = non–ST-segment elevation myocardial infarction; PE = pulmonary embolism; RR = relative risk; STEMI = ST-segment elevation myocardial infarction; TIA = transient ischemic attack.
Values are No. of patients (%).
Septic arthritis within 30 days of surgery.
Reoperation on affected hip within 1 year of surgery.
Patients could have more than 1 complication. In the clopidogrel cohort, 12 patients had 17 complications. In the control cohort, 18 patients had 21 complications.
NR: Relative risk not reported since either no patient in the clopidogrel cohort or no patient in the control cohort had the outcome of interest
At last follow-up, 51 patients in the control cohort had died at a mean of 1.9 years after surgery (median, 1.2; range, 0.1-6.6 years). Among the 29 controls still alive at last follow-up, the mean duration of follow-up was 4.0 years (median, 3.9; range, 0.3-9.1 years). Estimated overall survival rates for control patients at 1, 3, and 5 years after surgery were 71%, 49%, and 37%, respectively. At last follow-up, 28 patients in the clopidogrel cohort had died, at a mean of 2.0 years after surgery (median, 1.2; range, 0.0-6.6 years). Among the 12 patients still alive at last follow-up, the mean duration of follow-up was 2.5 years (median, 2.0; range, 0.8-5.5 years). Estimated overall survival rates at 1, 3, and 5 years after surgery in the clopidogrel group were 72%, 40%, and 23%, respectively. There was no significant difference in mortality between patients who were and were not taking clopidogrel at the time of hip fracture surgery (hazard ratio, 1.33; 95% CI, 0.84-2.12; P=.23) (Figure).
Figure.
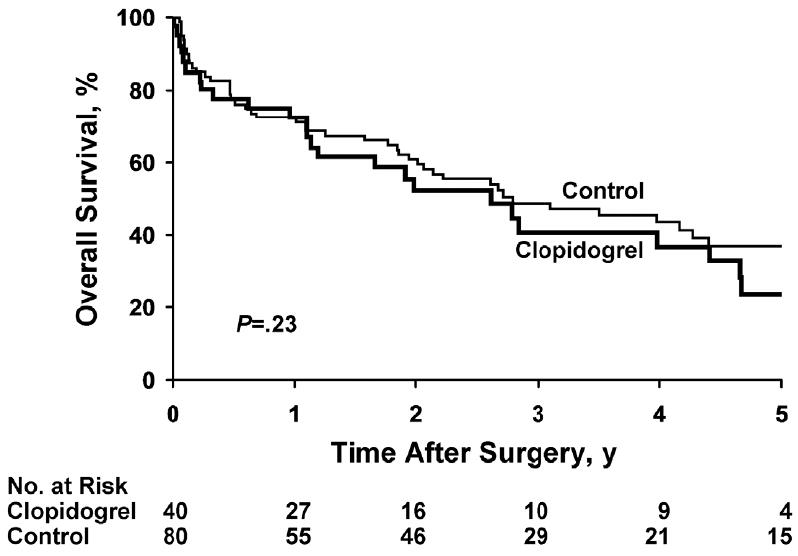
Kaplan-Meier Survival Curves. Overall survival after hip fracture surgery is shown for patients taking clopidogrel (n=40) and controls (n=80).
DISCUSSION
This observational study supports our hypothesis that there is no difference in perioperative bleeding complications or mortality in patients who undergo prompt operative treatment of hip fracture while taking clopidogrel compared with patients not taking clopidogrel.
Our clopidogrel cohort and control cohort were similar. There was no significant operative delay in either cohort: on average, both groups had surgical intervention within 36 hours of admission. This is important because most prior studies were confounded by operative delay. Both cohorts had the same percentage of patients taking aspirin at the time of admission; thus, aspirin use should not explain any differences in outcomes. The rates of pharmacologic DVT prophylaxis overall and dose reduction of DVT prophylaxis were no different between the groups. Thus, the clopidogrel cohort got appropriate pharmacologic DVT prophylaxis at the appropriate dose just as often as the control cohort. This suggests that inadequate DVT prophylaxis does not explain any differences in outcomes.
The differences that were seen between the 2 cohorts can be explained clinically. The control group had a higher percentage of warfarin use on admission. This makes clinical sense; patients who are taking antiplatelet agents are less likely to also be on warfarin because of the increased risk of bleeding when combining clopidogrel and warfarin. It follows, then, that patients in the control cohort perioperatively used more fresh frozen plasma and vitamin K, which are often used to reverse warfarin effects in the perioperative period. It further follows that more patients in the control cohort used warfarin as DVT prophylaxis postoperatively because they were already taking the medication preoperatively. Patients in the clopidogrel cohort were more likely to have had cardiac stents. This also makes clinical sense since a major indication for clopidogrel use is prophylaxis against in-stent thrombosis of cardiac stents.
However, the increased use of warfarin in the control group does raise a potential confounding variable. Perhaps the increased use of warfarin in the control group increased the control group’s bleeding making the two cohorts look more similar. To address this we reanalyzed our data removing patients on warfarin from both the clopidogrel and the control groups (Table 4). We were left with 39 patients in the clopidogrel cohort and 65 patients in the control cohort. There was no significant increase in bleeding outcomes in the clopidogrel cohort compared to the control cohort. However, the maximum hemoglobin delta was larger for the control group than the clopidogrel group. This discrepancy in the hemoglobin delta did not result in any difference in number of units of blood transfused per patient, number of patients requiring more than 3 units of blood, major bleeding events or the combined bleeding outcome. Thus, even when correcting for warfarin use, our study revealed no evidence of clinically significant difference in bleeding between patients on clopidogrel and those not taking any anticoagulant other than aspirin.
Table 4.
Bleeding Outcomes in Subset of Patients Not Taking Warfarin
| Characteristic | No Warfarin at Admission Patient Groupa
|
P value | RR (95% CI)b | |
|---|---|---|---|---|
| Clopidogrel (n=39) | Control (n=65) | |||
| Hemoglobin values | ||||
| Nadir, g/dL | 9.0 (1.2) | 8.9 (1.0) | .43 | |
| Delta, g/dL | 2.9 (1.7) | 3.6 (1.5) | .04 | |
| Delta >4 g/dL | 11 (28) | 23 (35) | .45 | 0.80 (0.44-1.45) |
| Major bleeding event | 2 (5) | 2 (3) | .63 | 1.67 (0.24-11.36) |
| Combined bleeding outcome | 19 (49) | 32 (49) | .96 | 0.99 (0.66-1.48) |
| pRBCs | ||||
| Total units | 2.1 (2.1) | 1.8 (2.2) | .50 | |
| Patients requiring >3 units | 9 (23) | 12 (18) | .57 | 1.25 (0.58-2.70) |
Values are mean (SD) or No. of patients (%).
RR only provided for categorical data.
NR: RR not reported since no patient in the clopidogrel group received vitamin K during hospitalization.
We found no difference in perioperative bleeding between the groups. Because perioperative bleeding can have delayed adverse outcomes in the setting of orthopedic surgery, we evaluated for repeat surgical interventions in the first year after surgery, either for implant failure or infectious complications. Although we saw no difference in the need for a repeat operation, the number of events was too low to draw definite conclusions. Conclusions from other studies regarding perioperative bleeding in patients taking clopidogrel are mixed. A recent study in vascular surgery patients showed no evidence of increased bleeding risk for patients taking clopidogrel at the time of vascular surgery.13 However, a recent meta-analysis noted a significant increase in the rate of reoperation for bleeding (odds ratio, 2.62; 95% CI, 1.96-3.49) in cardiac surgery patients taking clopidogrel at the time of surgery.14 It is unclear whether these results apply to orthopedic surgery.
Conclusions from other small observational studies of hip fracture are also conflicting. Sim and Gonski10 found no difference in rates of bleeding between patients who were and were not taking clopidogrel before hip fracture surgery; however, the clopidogrel group had significant surgical delay (3.5 days) compared with the controls (0.7 day). In a similar study, Chechik et al15 reported that patients taking clopidogrel at the time of hip fracture surgery had greater blood loss than those not taking clopidogrel. That study was also confounded by surgical delay and it did not assess clinically significant bleeding parameters. A more recent study, also by Chechik et al,16 compared patients with hip fracture taking clopidogrel who underwent prompt vs delayed surgery and found no difference in clinically significant bleeding parameters. In our study, there was no difference in time to surgery, which eliminates the confounding variable of surgical delay. We assessed perioperative bleeding with various parameters, removed warfarin use as a confounding variable, evaluated a composite bleeding end point and potential delayed complications from bleeding, and found no significant differences in any of the bleeding-related outcomes between the clopidogrel group and the control group. While the 95% confidence intervals for the bleeding outcomes are relatively wide reflecting our small sample size, our finding of no clinically significant difference in perioperative bleeding between the clopidogrel cohort and the control cohort concurs with the only other small study that controlled for surgical delay16. Our study showed no difference in mortality between the patient groups. Conclusions from other small observational studies are conflicting with respect to perioperative mortality. Harty et al7 reported increased perioperative mortality after hip fracture surgery in those taking clopidogrel compared with the control group (29% vs 4%), which they attributed to a surgical delay of 7.2 days (vs 2.1 days). In a study of 27 patients with hip fracture taking clopidogrel, the average time from admission to operative repair was 8 days for the clopidogrel group and 2 days for the control group, but there was no significant difference in mortality.9 Minimal information on the differences in baseline characteristics of the 2 groups was given however, making it difficult to determine if other confounders existed. We matched patients to minimize differences in variables known to affect morbidity and mortality in hip fracture patients. The patients were matched to the date of their surgery within a 3-year window to account for potential advances in surgical techniques over the time frame of the study. Our 1-year mortality rates of 28% for the clopidogrel group and 29% for the control group are comparable to published norms for patients with hip fracture, which suggests that our patient population was neither sicker nor healthier than other epidemiologic samples.17
We assessed secondary outcomes in both groups for multiple different perioperative complications. There was no significant difference in composite perioperative morbidity between the 2 groups. At baseline, more patients in the clopidogrel cohort had a history of cardiac stents. We did not specifically measure rates of heart disease between the 2 groups to know if the clopidogrel group also had higher rates of cardiac disease. However, even if we assume that the clopidogrel group had a higher rate of premorbid cardiac disease, the observed rate of cardiovascular events was no different between the 2 groups. Collyer et al1 reported a significant increase in the risk of acute coronary syndrome in patients with hip fracture in whom clopidogrel was withheld before surgery compared with controls, with the peak risk occurring between 4 and 8 days after clopidogrel withdrawal. Perhaps the lack of difference in cardiovascular outcomes in our study is because our clopidogrel patients had surgery within 36 hours while the drug effect was still present. However, our overall number of thrombotic events was small limiting our ability to make definitive conclusions.
Our study has several limitations. Despite a large population-based cohort of patients with hip fracture, only 2% (n=40) were taking clopidogrel at the time of admission. We also had a small number of outcomes. The small sample size and low number of outcomes limits our ability to interpret the results definitively. Our study also lacks racial heterogeneity, which, although accurately reflecting the population of Olmsted County, may limit its generalizability. It was not possible to blind the abstractors to clopidogrel use in our study as we needed access to the medication administration record as part of the abstraction process. As such, exposure bias or diagnostic suspicion bias might be considered a limitation of this study. However, our primary endpoints were not subject to interpretation. Additionally, both exposure bias and diagnostic suspicion bias would be expected to increase the relative risk or effect size and our study favors a null hypothesis.
Our study has several strengths. We identified a population-based cohort of patients with hip fracture, so our results reflect less referral bias than in other retrospective studies. In addition, the REP allows for complete, long-term follow-up of patients, thereby allowing us to assess long-term outcomes and mortality. Previous studies have not used population-based cohorts or reported long-term outcomes. Furthermore, there was no significant difference in time to surgery between cohorts in our study. This allows us to minimize the confounding effect of surgical delay that is present in other studies. Thus, while our small sample sizes preclude any definitive conclusions, the population based nature of our study, the successful long-term follow up and the lack of surgical delay add significantly to the existing data regarding the perioperative management of clopidogrel in hip fracture. Further larger studies and/or meta-analyses are needed.
CONCLUSION
Although the small sample size precludes making a definitive conclusion, we found no evidence that prompt surgical treatment of hip fracture in patients on clopidogrel compromises perioperative outcomes. Further larger studies are needed
Acknowledgments
The authors thank Donna K. Lawson, LPN, and Kathleen E. Wolfert, RN, BSN, for their help in data collection and management.
Data were analyzed by Christine Lohse, MS.
This study was made possible by the Rochester Epidemiology Project (Grant #R01 AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The study was funded by an internal grant from the Mayo Clinic Division of General Internal Medicine and a Wrap Up and Publish grant from the Mayo Clinic Department of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the study sponsors.
Abbreviations
- DVT
deep venous thrombosis
- REP
Rochester Epidemiology Project
Footnotes
Financial Support and Disclosure:
Drs. Feely and Mauck and Ms. Lohse have no financial disclosures to report. Dr. Mabry has a pending contract with Zimmer to provide consultation regarding a new total knee prosthesis. Dr. Sems receives royalties from Depuy for a hip implant he designed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collyer TC, Reynolds HC, Truyens E, Kilshaw L, Corcoran T. Perioperative management of clopidogrel therapy: the effects on in-hospital cardiac morbidity in older patients with hip fractures. Br J Anaesth. 2011;107(6):911–915. doi: 10.1093/bja/aer288. [DOI] [PubMed] [Google Scholar]
- 2.Chassot PG, Delabays A, Spahn DR. Perioperative antiplatelet therapy: the case for continuing therapy in patients at risk of myocardial infarction. Br J Anaesth. 2007;99(3):316–328. doi: 10.1093/bja/aem209. [DOI] [PubMed] [Google Scholar]
- 3.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 4.Inman DS, Michla Y, Partington PF. Perioperative management of trauma patients admitted on clopidogrel (Plavix). A survey of orthopaedic departments across the United Kingdom. Injury. 2007;38(5):625–630. doi: 10.1016/j.injury.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Lavelle WF, Demers Lavelle EA, Uhl R. Operative delay for orthopedic patients on clopidogrel (plavix): a complete lack of consensus. J Trauma. 2008;64(4):996–1000. doi: 10.1097/TA.0b013e3180485d23. [DOI] [PubMed] [Google Scholar]
- 6.Palan J, Odutola A, White SP. Is clopidogrel stopped prior to hip fracture surgery--A survey of current practice in the United Kingdom. Injury. 2007;38(11):1279–1285. doi: 10.1016/j.injury.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Harty JA, McKenna P, Moloney D, D’Souza L, Masterson E. Anti-platelet agents and surgical delay in elderly patients with hip fractures. J Orthop Surg (Hong Kong) 2007;15(3):270–272. doi: 10.1177/230949900701500304. [DOI] [PubMed] [Google Scholar]
- 8.Johansen A, White J, Turk A. Clopidogrel therapy--implications for hip fracture surgery. Injury. 2008;39(10):1188–1190. doi: 10.1016/j.injury.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Leonidou A, Cam NB, Chambers IR. Femoral neck fractures in patients on Clopidogrel. The effect of delaying surgery and the introduction of the new SIGN guidelines. Surgeon. 2011;9(6):318–321. doi: 10.1016/j.surge.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Sim W, Gonski PN. The management of patients with hip fractures who are taking Clopidogrel. Australas J Ageing. 2009;28(4):194–197. doi: 10.1111/j.1741-6612.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- 11.Nydick JA, Farrell ED, Marcantonio AJ, Hume EL, Marburger R, Ostrum RF. The use of clopidogrel (Plavix) in patients undergoing nonelective orthopaedic surgery. J Orthop Trauma. 2010;24(6):383–386. doi: 10.1097/BOT.0b013e3181c3f3d9. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 13.Stone DH, Goodney PP, Schanzer A, et al. Clopidogrel is not associated with major bleeding complications during peripheral arterial surgery. J Vasc Surg. 2011;54(3):779–784. doi: 10.1016/j.jvs.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au AG, Majumdar SR, McAlister FA. Preoperative thienopyridine use and outcomes after surgery: a systematic review. Am J Med. 2012;125(1):87–99. e81. doi: 10.1016/j.amjmed.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Chechik O, Thein R, Fichman G, Haim A, Tov TB, Steinberg EL. The effect of clopidogrel and aspirin on blood loss in hip fracture surgery. Injury. 2011;42(11):1277–1282. doi: 10.1016/j.injury.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Chechik O, Amar E, Khashan M, Kadar A, Rosenblatt Y, Maman E. In support of early surgery for hip fractures sustained by elderly patients taking clopidogrel: a retrospective study. Drugs Aging. 2012;29(1):63–68. doi: 10.2165/11598490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290–1299. doi: 10.1093/aje/kwp266. [DOI] [PMC free article] [PubMed] [Google Scholar]


