Abstract
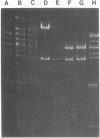
A purified EcoRI restriction endonuclease fragment that determines resistance to kanamycin and is incapable of self-replication was used to select autonomously replicating fragments from an EcoRI digest of a Salmonella typhimurium F' plasmid containing the chromosomal region believed to include the S. typhimurium origin of DNA replication. Both the F factor and S. typhimurium chromosome replication origins were cloned by this procedure. The EcoRI fragmentment containing the S. typhimurium origin of replication is 19.4 kilobase pairs long and includes functional asp+ and uncB+ genes. Restriction endonuclease analysis of deletions obtained from the S. typhimurium origin plasmid indicated that the replication origin (ori region) is contained within a 3.3-kilobase pair region. Comparison with Escherichia coli origin plasmids shows colinearity of gene arrangement on the chromosomes in this region and suggests that some, but not all, regions of the nucleotide sequence in the origin region may be conserved (identical) in these two bacterial species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong K. A., Hershfield V., Helinski D. R. Gene cloning and containment properties of plasmid Col E1 and its derivatives. Science. 1977 Apr 8;196(4286):172–174. doi: 10.1126/science.322277. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. The asparagine synthetase of Escherhic coli. I. Biosynthetic role of the enzyme, purification, and characterization of the reaction products. J Biol Chem. 1969 Aug 10;244(15):4112–4121. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Diaz R., Pritchard R. H. Cloning of replication origins from the E. coli K12 chromosome. Nature. 1978 Oct 12;275(5680):561–564. doi: 10.1038/275561a0. [DOI] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Zyskind J. W., Smith D. W. The Escherichia coli dnaB protein is required for initiation of chromosomal DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):559–562. doi: 10.1101/sqb.1979.043.01.061. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Eisenstark A. Bi-directional chromosomal replication in Salmonella typhimurium. J Bacteriol. 1973 Jul;115(1):168–176. doi: 10.1128/jb.115.1.168-176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Vielmetter W. Bidirectional growth of the E. coli chromosome. Nat New Biol. 1973 Apr 4;242(118):130–132. doi: 10.1038/newbio242130a0. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. A., Russo R., Wilcox G. An improved horizontal slab gel electrophoresis apparatus for DNA separation. Anal Biochem. 1977 Mar;78(1):235–243. doi: 10.1016/0003-2697(77)90028-8. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Construction of plasmid R6K derivatives in vitro: characterization of the R6K replication region. Plasmid. 1978 Sep;1(4):571–580. doi: 10.1016/0147-619x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Lee F., Bertrand K., Bennett G., Yanofsky C. Comparison of the nucleotide sequences of the initial transcribed regions of the tryptophan operons of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):193–217. doi: 10.1016/s0022-2836(78)80005-9. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Genetic relatedness in the family Enterobacteriaceae. Annu Rev Microbiol. 1976;30:327–349. doi: 10.1146/annurev.mi.30.100176.001551. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Adelberg E. A. Antipolarity in the ilv operon of Escherichia coli K-12. J Bacteriol. 1969 Jun;98(3):1179–1194. doi: 10.1128/jb.98.3.1179-1194.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. NOVEL Escherichia coli dnaB mutant: direct involvement of the dnaB252 gene product in the synthesis of an origin-ribonucleic acid species during initiaion of a round of deoxyribonucleic acid replication. J Bacteriol. 1977 Mar;129(3):1476–1486. doi: 10.1128/jb.129.3.1476-1486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]