Abstract
The study of anterograde and retrograde amnesia (AA and RA) in the laboratory and the clinic has provided important information about the structure and organization of memory. The severity of AA is usually correlated with the severity of RA. Nevertheless, variations in the expression of AA and RA have been reported, which presumably reflect variation in the locus and extent of brain damage. The relationship between AA and RA has rarely been described quantitatively in groups of patients where detailed anatomical information is available. We have quantified the severity of AA and RA for factual information in 11 memory-impaired patients with bilateral medial temporal lobe lesions, including 5 for whom detailed post-mortem neurohistological information was available. The findings describe an orderly relationship between AA and RA, such that patients with more severe AA also had more extensive RA. In addition, RA was measurable only after AA reached a substantial level of severity. This relationship between AA and RA in patients with identified medial temporal lobe lesions appears to describe a general principle, which applies to a range of etiologies, including traumatic amnesia, where the locus and extent of brain damage is less well understood. Whenever patients deviate substantially from the relationship described here, one should be alert to the likelihood that significant damage has occurred outside or in addition to the structures in the medial temporal lobe.
Keywords: anterograde amnesia, retrograde amnesia, memory, medial temporal lobe
1. Introduction
The phenomena of anterograde and retrograde amnesia have been described in the laboratory and clinic for more than 100 years (Ribot, 1881) and have been an important source of information about the structure and organization of memory. Anterograde amnesia (AA) refers to an impaired capacity for new learning. Retrograde amnesia (RA) refers to the loss of information that was acquired before the onset of amnesia. It has long been recognized that AA and RA tend to occur together in the same patients (Barbizet, 1970; Rose & Symonds, 1960; Russell, 1971; Victor, 1969). In addition, the severity of AA is usually correlated with the severity of RA (Kopelman, 1989; Squire & Alvarez, 1995; Wickelgren, 1979). Yet it is also true that RA can sometimes appear disproportionately severe in comparison to AA (Barr, Goldberg, Wasserstein, & Novelly, 1990; Bright et al., 2006; Hornberger et al., 2010; Kapur, Ellison, Smith, McLellan, & Burrows, 1992; Milton et al., 2010; O'Connor, Butters, Miliotis, Eslinger, & Cermak, 1992; Reed & Squire, 1998; Sehm et al., 2011). Moreover, AA can sometimes occur in the absence of RA (Corkin, Hurt, Twitchell, Franklin, & Yin, 1987; Russell & Nathan, 1946). These examples illustrate variations in the expression of AA and RA, which presumably depend on the locus and extent of brain injury or disease. Detailed neuroanatomical information should clarify the relationship between AA and RA.
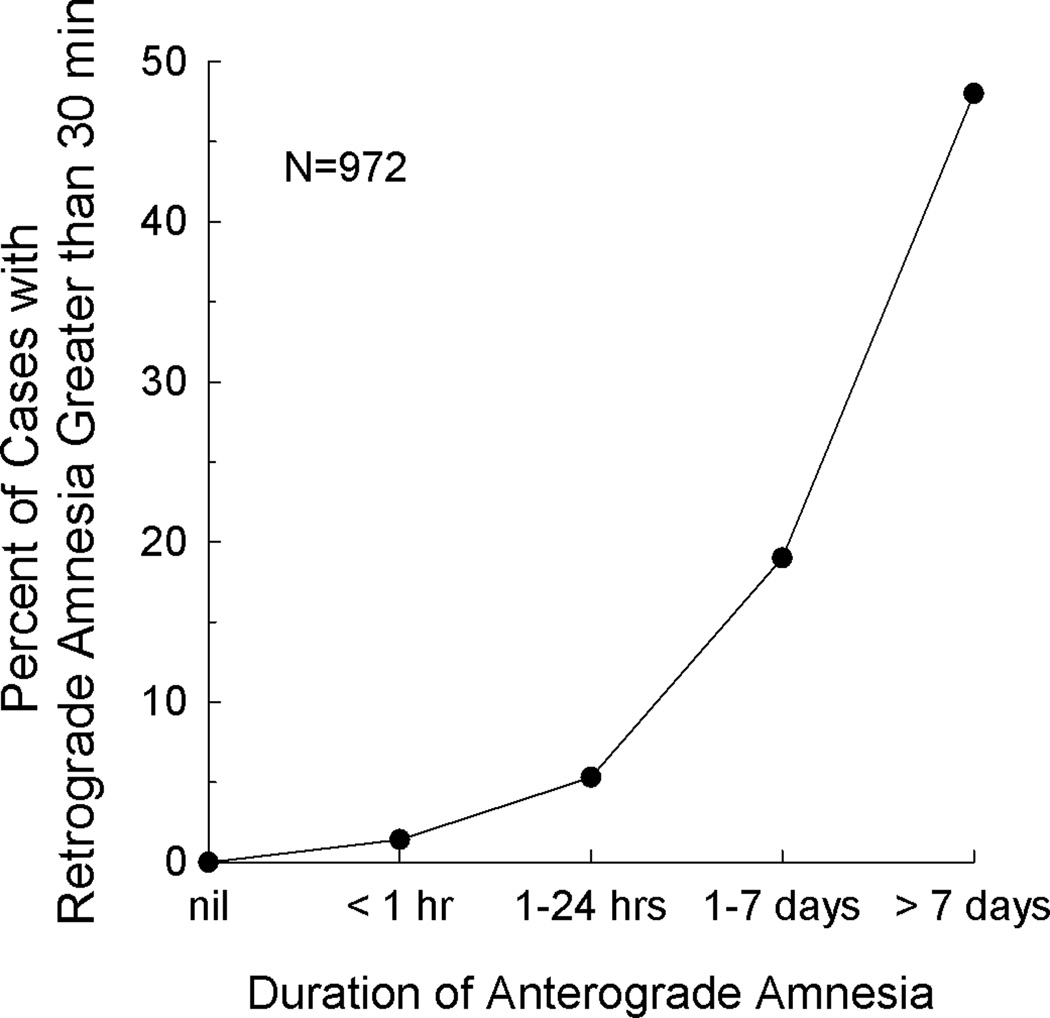
The most comprehensive study of the relationship between AA and RA was carried out in more than 1000 patients who had sustained closed head injury (Russell & Nathan, 1946). Figure 1 shows the relationship between the duration of post-traumatic amnesia and the extent of RA for 972 cases. RA was assessed informally, usually by querying about autobiographical information. We take the duration of AA to be indicated by the time after injury when post-traumatic amnesia resolves. As the duration of AA increased, the number of cases exhibiting pronounced RA also increased. Interestingly, when AA covered one day or less, only a small number of cases (N=19) exhibited substantial RA (Russell & Nathan, 1946; Table 4a). In fact, out of the 503 cases exhibiting AA of one day or less, 32 had no RA at all. These data describe an orderly relationship between AA and RA and also suggest that AA may need to reach some threshold of severity before RA is observed. Put differently, it appears to be easier to disrupt new learning ability and harder to disrupt already acquired information, presumably because some fixation or consolidation of memory has occurred (McGaugh, 2000; Squire & Alvarez, 1995). Unfortunately, in cases of (non-penetrating) traumatic brain injury, the locus and extent of damage is often difficult to determine. Information is not available about how the severity of AA relates to the severity of RA in patient groups with identified neuroanatomical damage.
Figure 1.
The relationship between the duration of anterograde amnesia (AA) and the severity of retrograde amnesia (RA) in 972 cases of traumatic brain injury. Note the orderly relationship between AA and RA. Data from Russell and Nathan (1946; Table 4). We have considered the duration of AA to be the time after injury when post-traumatic amnesia resolves.
To obtain quantitative information about AA and RA in patients with identified neuropathological change, we have determined the severity of AA and RA in 11 memory-impaired patients with identified bilateral lesions within the medial temporal lobe. For five of the patients, detailed post-mortem neurohistological information was available. Patients were assessed with five measures of AA and one measure of RA. The AA measures assessed both verbal and nonverbal learning ability. For measures of RA, we considered tests of autobiographical information and tests of factual information. Whereas early studies of RA depended on informal interviews that focused on autobiographical information, beginning in the 1970s methods were developed to assess RA quantitatively by asking factual questions about news events or famous persons from different past time periods (Albert, Butters, & Levin, 1979; Marslen-Wilson & Teuber, 1975; Sanders & Warrington, 1971). Quantitative assessments of autobiographical memory were also subsequently developed (Crovitz & Schiffman, 1974; Kopelman, Wilson, & Baddeley, 1989), but these do not easily achieve the kind of temporal resolution that is afforded, for example, by news events tests. Accordingly, to explore the quantitative relationship between AA and RA, we assessed RA with a test of approximately 100 news events that covered most of the life span prior to the onset of amnesia.
2. Materials and Methods
2.1 Participants
Data are presented for eleven memory-impaired patients with damage limited to the MTL (Table 1). For the first five patients described below, the description of damage was based on post-mortem neurohistological analysis. Patient RB became amnesic in 1978 at age 52 as the result of an ischemic event that occurred as a complication of open heart surgery. After his death in 1983, he was found to have bilateral damage limited to the CA1 field of the hippocampus (Zola-Morgan, Squire, & Amaral, 1986). Patient GD became amnesic in 1983 at age 43 following a period of hypotension that occurred during major surgery. After his death in 1992, he was also found to have bilateral damage limited to the CA1 field of the hippocampus (Rempel-Clower, Zola, Squire, & Amaral, 1996). Patient WH became amnesic in 1986 at age 57 possibly due to cerebral ischemia. After his death in 1993, he was found to have bilateral damage involving all fields of the hippocampus as well as damage to the dentate gyrus, subiculum, and some of entorhinal cortex (Rempel-Clower, et al., 1996). Patient LM became amnesic in 1984 at age 54 as the result of respiratory arrest that occurred during an epileptic seizure. After his death in 1990 he was found to have bilateral damage involving all fields of the hippocampus as well as damage to the dentate gyrus (Rempel-Clower, et al., 1996). Patient EP became amnesic in 1992 at age 70 after contracting viral encephalitis. After his death in 2008, he was found to have large, bilaterally symmetric lesions of the MTL that eliminated the temporal pole, the amygdala, the entorhinal cortex, the hippocampus, the perirhinal cortex, and rostral parahippocampal cortex. His lesion also extended laterally to substantially involve the fusiform gyrus. Perhaps because of loss of connectivity between medial and lateral structures, there were secondary changes in the superior, middle, and temporal gyri, which were atrophic, and the underlying white matter was gliotic (Insausti, Annese, Amaral, & Squire, 2013).
Table 1.
Characteristics of memory-impaired patients
| WMS-R | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age (yrs) |
Education (yrs) |
Anatomical Findings |
IQ (WAIS-R) |
Attention | Verbal | Visual | General | Delay |
| RB | M | 53 | 10 | H* | 103 | --- | --- | --- | --- | --- |
| GD | M | 45 | 12 | H* | 92 | 109 | 86 | 88 | 85 | 60 |
| WH | M | 64 | 17 | H | 113 | 88 | 72 | 82 | 67 | <50 |
| LM | M | 55 | 15 | H | 109 | 124 | 94 | 82 | 89 | 62 |
| EP | M | 79 | 12 | MTL | 98 | 94 | 59 | 92 | 68 | 56 |
| KE | M | 64 | 13.5 | H | 108 | 114 | 64 | 84 | 72 | 55 |
| LJ | F | 68 | 12 | H | 101 | 104 | 85 | 87 | 81 | 54 |
| GP | M | 57 | 16 | MTL | 98 | 102 | 79 | 62 | 66 | <50 |
| RS | M | 49 | 12 | H | 99 | 99 | 85 | 81 | 82 | <50 |
| GW | M | 46 | 12 | H | 108 | 105 | 67 | 86 | 70 | <50 |
| JRW | M | 42 | 12 | H | 90 | 87 | 65 | 95 | 70 | <50 |
Note. The Wechsler Adult Intelligence Scale-Revised (WAIS-R) and the Wechsler Memory-Scale Revised (WMS-R) yield mean scores of 100 in the normal population, with a SD of 15. The WMS-R does not provide numerical scores for individuals who score < 50. KE, LJ, EP, GP, and GW were given the WAIS-III rather than the WAIS-R. RB was not given the WMS-R. Age indicates the age of the patient when given the News Events Test. Neurohistological information is available for the first five patients listed. Asterisk indicates a lesion limited to the CA1 field of the hippocampus. H, bilateral damage to the hippocampus with minimal damage to adjacent cortex; MTL, bilateral damage to the hippocampus and parahippocampal gyrus; IQ, intelligence quotient.
Of the remaining six patients, five have damage thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex), and one has large bilateral lesions of the MTL. Patient KE became amnesic in 2004 at age 63 after an episode of ischemia associated with kidney failure and toxic shock syndrome. LJ (the only female) became amnesic at age 51 during a 6-month period in 1988 with no known precipitating event. Her memory impairment has been stable since that time. Patient GP became amnesic in 1987 at age 41 after contracting viral encephalitis. Patients RS and GW became amnesic in 1998 and 2001, respectively (ages 41 and 42), after drug overdoses and associated respiratory failure. Patient JRW became amnesic in 1990 at age 27 following an anoxic episode associated with cardiac arrest.
Estimates of MTL damage for these six patients were based on magnetic resonance images from 19 age-matched, healthy males for KE, GP, RS, GW, and JRW, and 11 age-matched, healthy females for patient LJ (Gold & Squire, 2005). Patients KE, LJ, RS, GW, and JRW have an average bilateral reduction in hippocampal volume of 49, 46, 33, 48 and 44% respectively (all values > 2.9 SDs from the control mean). On the basis of patients LM and WH, who had similar bilateral volume loss in the hippocampus (estimated from magnetic resonance images) and for whom detailed postmortem neurohistological information was obtained (Rempel-Clower, et al., 1996), the degree of volume loss in these five patients likely reflects nearly complete loss of hippocampal neurons. For these same five patients, the volume of the parahippocampal gyrus (temporopolar, perirhinal, entorhinal, and parahippocampal cortices) is reduced by 11, −5, 10, 12 and −17%, respectively (all values within 2 SDs of the control mean). The post-encephalitic patient GP has a 96% reduction in hippocampal volume bilaterally and a 94% reduction in parahippocampal gyrus volume. Eight coronal magnetic resonance images from each of these patients, together with detailed descriptions of the lesions, can be found in Supplemental Material. The volumes for parahippocampal gyrus differ a little from volumes reported previously for these patients and are based on recently published, more detailed guidelines for identifying the caudal border of the gyrus (Franko, Insausti, Artacho-Perula, Insausti, & Chavoix, epub 2012).
Additional measurements, based on four controls for each patient, were carried out for the frontal lobes, lateral temporal lobes, parietal lobes, occipital lobes, insular cortex, and fusiform gyrus (see Bayley, Gold, Hopkins, & Squire, 2005). For patients KE, LJ, RS, GW, and JRW, volumes of these regions are within 16% of control values. The only volume reduction greater than 1.3 SDs of the control mean was the parietal lobe for RS (Bayley et al., 2005). For patient GP, the volumes of the insular cortex and the fusiform gyrus were reduced bilaterally by 65% and 49%, respectively.
Forty-two healthy volunteers served as controls for the News Events Test (12 female, 60.4 ± 1.8 yrs of age, 13.6 ± 0.5 years of education; means ± SEMs). Eleven of these also served as controls for four of the anterograde memory tests (2 female, 61.3 ± 3.3 yrs of age, 15.7 ± 1.1 years of education; means ± SEMs). A separate group of eight volunteers (Squire & Shimamura, 1986) served as controls for one anterograde memory test: Rey Auditory Verbal Learning Test (5 female, 50.9 ± 1.2 yrs of age, 14.8 ± 0.7 years of education; means ± SEMs). All procedures were approved by the Institutional Review Board at the University of California San Diego. Participants gave written informed consent prior to participation in accordance with the Declaration of Helsinki.
2.2 Measuring Anterograde Amnesia
2.2.1. Delayed Recall of a Complex Figure
Participants copied a complex diagram (Rey-Osterrieth figure; Osterrieth, 1944) and then reproduced it from memory after a 10–15 min delay. The copy and the reproduction of the figure were each scored on a 36-point scale (Taylor, 1998).
2.2.2. Paired-Associate Learning
Participants completed three study-test trials with a list of 10 unrelated word pairs (Squire & Shimamura, 1986). To begin, the word pairs were displayed one at a time while the experimenter read each pair aloud. Immediately after all 10 pairs were presented, participants were shown the first word from each pair and asked to recall the second word. This procedure was repeated two more times with the same pairs in different orders, and the score was the total number of pairs recalled (maximum = 30).
2.2.3. Rey Auditory Verbal Learning Test (RAVLT)
For the recall portion of the RAVLT, 15 words were presented orally, and then recall was tested. The study-test sequence was then repeated four times. The recognition portion of the test used 15 different words. Five successive study-test trials were given, and testing on each trial followed a yes-no format with 30 words (15 old 15 new). The score was the average percent correct score across all 10 trials. RB was not given this test.
2.2.4. Dementia Rating Scale
Participants were administered the Memory Subscale from the Dementia Rating Scale (Mattis, 1976). The maximum score is 25 points. RB was not given this test.
2.2.5. Wechsler Memory Scale-Revised (WMS-R) Logical Memory Subtest
Recall was tested for two short prose passages, each consisting of 25 segments. The first passage was read aloud to the participant, followed by an immediate recall test. The second passage was then read aloud, also followed by an immediate recall test. Recall of both passages was then tested 30 min later. The score was the sum of segments recalled from both passages at the 30-min test. Patient RB received the WMS rather than the WMS-R.
Based on the performance of controls, z-scores were calculated for each patient for each of the five anterograde memory tests. The five z-scores were then averaged to create a measure of AA for each patient.
2.3 Measuring Retrograde Amnesia
News Events Test. The test was constructed from a pool of 289 questions covering notable news events that occurred in a specific year between 1938 and 2004. For each patient, the questions spanned from the onset of amnesia to the time when the patient was 15 years old (mean = 105.0 ± 17.5 questions per patient). We did not query earlier time periods, because even for healthy individuals performance is poor for news events that occurred during childhood or early adolescence (Squire, 1974). The News events Test was administered in a free recall format (e.g., What caused a suspension bridge to collapse over the Narrows at Tacoma, Washington? [1940]; Who killed John Lennon? [1980], Who is Elizabeth Smart? [2003], the year for each event was not included as part of the question). The score was the percentage of questions answered correctly from each 5-year time interval covered by the test. An average of 13.1 questions (range 7.5 to 31.1 questions) was available for each 5-year time interval.
Eight to sixteen controls for each patient were identified from a pool of 42 controls, based on age, education, and when they took the test relative to the patient (within about 1 year). Some controls were matched to more than one patient. For each control, the questions were assigned to 5-year time intervals according to the time of onset of amnesia for the patient to whom the control was matched. The extent of RA for each patient was defined as the number of 5-year time intervals where the patient’s score was significantly below the score of the controls for the same time interval (one-sample t-test). The AA and RA measures were obtained at roughly similar times (mean = 2.8 ± 0.9 years apart). This interval was calculated by averaging the dates when the five AA tests were given and subtracting this value from the date when the RA test was given.
Data for patients and the news events test have been published previously in a different format (Bayley, Hopkins, & Squire, 2006; Squire, Haist, & Shimamura, 1989; Zola-Morgan, et al., 1986).
3. Results
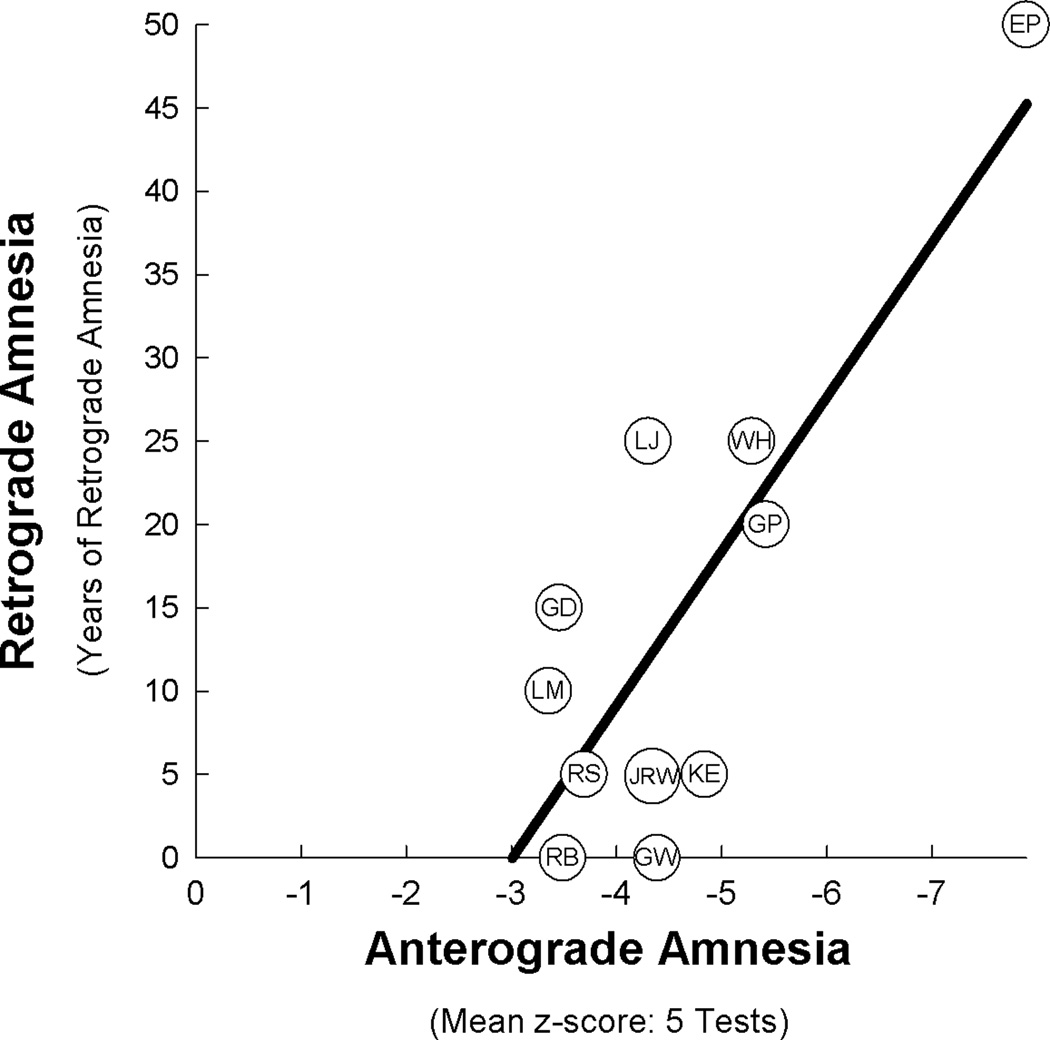
Figure 2 shows the severity of AA and RA for each patient. Across all 11 patients, there was a significant relationship between AA and RA (r = 0.81, p < 0.005), such that patients with more severe AA had more severe RA. Thus, when damage extended outside the hippocampus to substantially involve the parahippocampal gyrus (EP and GP), substantial RA was consistently observed, and AA was very severe (z = −6.7). Patient EP had the most severe AA and also had more RA than any other patient. Finally, when the lesion was limited largely to the hippocampus, RA and AA were less severe (for AA, z = −4.1).
Figure 2.
The relationship between the severity of anterograde amnesia (AA) and the severity of retrograde amnesia (RA) in 11 patients with bilateral damage to the medial temporal lobe (r = 0.81, p < 0.005). Patients are represented by their initials (see Table 1). AA is the mean z-score from five tests of new learning ability (see section 2.2). RA was measured by a test of approximately 100 questions about notable news events that covered most of the lifespan prior to the onset of amnesia. The extent of RA was measured in 5-year intervals (see section 2.3).
We also estimated the severity of RA by calculating each patient’s mean percent correct score across the entire test (instead of calculating the number of 5-year time periods in which patients obtained impaired scores). A significant relationship between AA and RA was found with this method (r = 0.73, p < 0.05; Figure S2A). The relationship was also strong when the mean percent correct scores were converted to z-scores based on how many standard deviations each patient’s score fell below the control group’s score (r = 0.79, p < 0.01; Figure S2B).
It has been suggested that comparisons of AA and RA would be advantaged by using the same kind of test to assess both AA and RA (Kopelman, 2000; Mayes, 2002; Mayes, Daum, Markowisch, & Sauter, 1997). Ten of the 11 patients in our study were tested not only about news events that occurred before they became amnesic but also about news events that occurred after they became amnesic. For these patients a mean of 71.6 questions covered the period after they became amnesic. AA was estimated by calculating a mean percent correct score for each patient. By this method, AA and RA were related (r = 0.73, p < .05; Figure S3A). A marginal relationship between AA and RA was found when the percent correct scores were converted to z-scores (r = 0.59, p < .08; Figure S3B). The results of two additional analyses are illustrated in Figures S4A, B.
It is apparent in Figure 1 that measurable RA was observed only after AA reached a substantial level of severity. While RA covering less than 5 years would not have been detected by our method, which depended on 5-year time intervals, the results indicated that RA does not typically reach back 5 years or more unless AA is quite severe. In addition, one should not conclude that patients without detectable RA (RB and GW) had no RA at all. Rather, RA may not have covered a sufficient number of years in the most recent 5-year interval for impairment to be detected. In fact, GW did exhibit two years of RA on the News Events Test when the 5-year interval immediately prior to the onset of amnesia was rescored year by year.
4. Discussion
We measured AA and RA in 11 patients with damage to the MTL. As the severity of AA increased, so did the severity of RA. Patients with damage to both the hippocampus and parahippocampal gyrus exhibited the most severe AA and the most severe RA. Patients with damage limited largely to the hippocampus exhibited less severe AA and less severe RA. Although there was variability in the severity of RA, the average severity of RA in the two patient subgroups (mean = 10 years, median = 5 years for the hippocampal group and mean = 35 years, median = 35 years for the MTL group) are in line with previous reports (Bayley, et al., 2006; Manns, Hopkins, & Squire, 2003).
Our findings for memory-impaired patients with bilateral MTL lesions parallel the findings from a large study of closed-head injury (Russell & Nathan, 1946) as well as the findings from a smaller study of 25 patients (Blomert & Sisler, 1974). First, for all these patient groups the relationship between AA and RA was similar. RA was substantial only after a threshold of AA was reached (compare Figures 1 and 2). In the case of MTL patients (Figure 2), one could wonder if RA might have been detected in association with less severe AA if the test of RA had been more sensitive (i.e., if our test could have detected an RA of less than five years). Yet, Russell and Nathan also observed a threshold when AA and RA were measured in minutes and days instead of years. For example, when AA was 1 day or less, 6–10% of patients exhibited no RA at all (Russell & Nathan, Tables 4, 5). Moreover, even when AA covered more than 1 day, 27% of patients had RA of less than one minute (Russell & Nathan, Table 5). Lastly, in the case of gunshot wounds to the head, 65 of 185 cases had a definite period of AA but no RA at all (Russell & Nathan). Thus, anterograde memory is easier to disrupt than retrograde memory, and this conclusion does not depend on the sensitivity of the measures.
Second, all these studies found variable severity of RA in patients with a similar severity of AA. For example, in our study RA ranged from no RA to 15 years in patients with similar AA (GD, LM, RB, and RS). In the study of traumatic amnesia, RA ranged from 0 to 12 hours when AA was 1 day or less (Russell & Nathan, Tables 4, 5). Third, RA was consistently observed when AA was sufficiently severe. [Note one report of traumatic amnesia (N = 109) that found no relationship between AA and RA (Corkin, et al., 1987), though in this study patients with the mildest and most severe symptoms were excluded].
Although patients with MTL lesions, as presented here, and patients with traumatic amnesia provide the most complete information about the relationship between AA and RA, the fact that AA and RA are often associated has been noted in patients with a range of etiologies. Thus, AA and RA have been described as appearing and then diminishing together (though not necessarily at the same rate) in cases of transient global amnesia (Evans, 1966; Fisher & Adams, 1964; Kritchevsky & Squire, 1989; Shuttleworth & Morris, 1966), Wernicke’s encephalopathy/Korsakoff’s psychosis (Victor, Adams, & Collins, 1989), tumors or cysts near the third ventricle (Ignelzi & Squire, 1976; Victor, 1969), electroconvulsive therapy (Squire & Chace, 1975; Squire, Slater, & Miller, 1981), and transient epileptic amnesia (Zeman, Boniface, & Hodges, 1998). Note that interpretation of remote memory performance in transient epileptic amnesia can be complicated by the fact that poor memory performance (particularly for recent time periods) may result from impaired new learning (secondary to seizures) as well as from retrograde memory loss itself (Butler & Zeman, 2008; Hornberger, et al., 2010). Note too that this association between AA and RA need not hold in all circumstances, for example, in Korsakoff’s syndrome (Fama, Marsh, & Sullivan, 2004; Mayes, et al., 1997), when remote memory impairment can be extensive and its severity related in part to abnormalities in neocortex (Fama, et al., 2004).
It is worth mentioning that in some of these studies, and in the work by Russell and Nathan (1946) RA was assessed with measures of autobiographical memory. Our study assessed RA by testing semantic memory for public events, so that our conclusions about the relationship between AA, RA, and MTL damage are limited to non-autobiographical material. It would be useful to study the relationship between AA and RA in patients with medial temporal lobe damage using tests of autobiographical memory. Unfortunately, the tools available to measure autobiographical memory do not easily lend themselves to such an analysis. Specifically, most of the tests (Kopelman, et al., 1989; Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002) sample only a few time periods. Better tests could be constructed [see Bayley, Hopkins, and Squire (2003) for use of the method introduced by Crovitz and Schiffman (1974)], but even then it would be difficult to achieve good temporal resolution across the life span.
Patient EP had the most severe AA and the most severe RA, which covered as much as 50 years prior to the onset of his amnesia (Figure 2). Nonetheless, his performance improved somewhat when questions concerned events that had occurred > 30 years before his amnesia and reached normal levels for the period 46–50 years before amnesia when he was 20–24 years old. Although EP’s score for RA did fall within the 95% confidence interval of the regression line (even when his data were not used to construct the regression line), he differed from the other patients in that his neuropathology extended into lateral temporal cortex. These changes appeared to be secondary to the primary focus of his encephalitic lesion (Insausti, et al., 2013). Accordingly it is possible that these changes in lateral temporal cortex made some contribution to the extent and severity of his retrograde memory loss.
Lateral temporal cortex is essential for long-established semantic knowledge about objects and word meanings (Hodges & Graham, 2001; Hodges, Patterson, Oxbury, & Funnell, 1992; Levy, Bayley, & Squire, 2004). Damage to this region has also been associated with cases where RA is disproportionately severe in comparison to AA (Barr, et al., 1990; Bright, et al., 2006; O'Connor, et al., 1992; Reed & Squire, 1998). Particularly notable are cases of what has been termed focal retrograde amnesia (Hornberger, et al., 2010; Kapur, 1993; Kapur, et al., 1992; Kopelman, 2000; Sehm, et al., 2011). [Note that it can be difficult to distinguish focal retrograde amnesia from psychogenic amnesia (Kopelman, 2000; Markowitsch, 2002)].
In summary, we examined the relationship between AA and RA for factual information in patients with MTL lesions where detailed anatomical information was available. There was an orderly relationship between the severity of AA and the extent of RA. A similar relationship between AA and RA appears to hold in the case of patients with a range of etiologies where the locus and extent of brain injury is less well understood. In these cases [for example, in patients with traumatic amnesia from closed head injury (Russell & Nathan, 1946)], we suggest that significant dysfunction has occurred within the MTL. Similarly, for new cases, one might propose that, when AA and RA scores are in a similar relationship to what we report here, significant damage has occurred within the MTL. Furthermore, when patients have AA and RA scores that deviate substantially from the relationship described here, one should be alert to the likelihood that significant damage has occurred outside of or in addition to structures in the MTL.
Supplementary Material
Highlights.
Orderly relationship between the severity of anterograde (AA) and retrograde amnesia (RA)
Easier to disrupt new learning ability; harder to disrupt information already acquired
RA measurable only after AA reaches a substantial level of severity
Severity of AA and RA related to severity of medial temporal lobe damage (MTL)
Deviations from the AA-RA relationship described here suggest damage outside of the MTL
Acknowledgments
We thank Ashley Knutson and Erin Light for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs (VA Merit to L.R.S.), National Institute of Mental Health (MH24600 to L.R.S.), and a National Science Foundation grant (#SMA-1041755) to the Temporal Dynamics of Learning Center, an NSF Science of Learning Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christine N. Smith, Department of Psychiatry, University of California, San Diego, CA 92093; Research Service, Veterans Affairs San Diego Healthcare System, San Diego, CA 92161
Jennifer C. Frascino, Department of Psychiatry, University of California, San Diego, CA 92093; Research Service, Veterans Affairs San Diego Healthcare System, San Diego, CA 92161
Ramona O. Hopkins, Department of Psychology and Neuroscience Center, Brigham Young University, Provo, UT 84143; Department of Medicine, Pulmonary and Critical Care Division, Intermountain Medical Center, Murray, UT 84143
Larry R. Squire, Veterans Affairs San Diego Healthcare System, San Diego, CA 92161; Departments of Psychiatry, Neurosciences, and Psychology, University of California, San Diego, La Jolla, CA 92093
References
- Albert MS, Butters N, Levin J. Temporal gradients in the retrograde amnesia of patients with alcoholic Korsakoff's disease. Archives of Neurology. 1979;36:211–216. doi: 10.1001/archneur.1979.00500400065010. [DOI] [PubMed] [Google Scholar]
- Barbizet J. Human Memory and Its Pathology. San Francisco: W.H. Freeman and Co; 1970. [Google Scholar]
- Barr WB, Goldberg E, Wasserstein J, Novelly RA. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990;28:243–256. doi: 10.1016/0028-3932(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005;46(5):799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38(1):135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26(51):13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomert DM, Sisler GC. Measurement of Retrograde Post-Traumatic Amnesia. Canadian Psychiatric Association Journal. 1974;19(2):185–192. doi: 10.1177/070674377401900215. [DOI] [PubMed] [Google Scholar]
- Bright P, Buckman JR, Fradera A, Yoshimasu H, Colchester ACF, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learning & Memory. 2006;13:545–557. doi: 10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain : a journal of neurology. 2008;131(Pt 9):2243–2263. doi: 10.1093/brain/awn127. [DOI] [PubMed] [Google Scholar]
- Corkin S, Hurt RD, Twitchell TE, Franklin LC, Yin RK. Consequences of nonpenetrating and penetrating head injury: retrograde amnesia, post-traumatic amnesia, and lasting effects on cognition. In: Levin HS, Grafman J, Eisenberg HM, editors. Neurobehavioral recovery from head injury. New York: Oxford University Press; 1987. pp. 318–329. [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- Evans JH. Transient loss of memory, an organic mental syndrome. Brain. 1966;89:539–548. doi: 10.1093/brain/89.3.539. [DOI] [PubMed] [Google Scholar]
- Fama R, Marsh L, Sullivan EV. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. Journal of the International Neuropsychological Society : JINS. 2004;10(3):427–441. doi: 10.1017/S135561770410310X. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Adams RD. Transient Global Amnesia. Acta neurologica Scandinavica. Supplementum. 1964;40(SUPPL 9):1–83. [PubMed] [Google Scholar]
- Franko E, Insausti AM, Artacho-Perula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. doi: 10.1002/hbm.22170. (epub 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15(1):79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Graham KS. Episodic memory: insights from semantic dementia. Philosophical Transactions Royal Society of London Series B356. 2001;356:1423–1434. doi: 10.1098/rstb.2001.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia withtemporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Mohamed A, Miller L, Watson J, Thayer Z, Hodges JR. Focal retrograde amnesia: Extending the clinical syndrome of transient epileptic amnesia. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2010;17(10):1319–1321. doi: 10.1016/j.jocn.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Ignelzi RJ, Squire LR. Recovery from anterograde and retrograde amnesia after percutaneous drainage of a cystic craniopharyngioma. Journal of Neurology, Neurosurgery, and Psychiatry. 1976;39(12):1231–1235. doi: 10.1136/jnnp.39.12.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Annese J, Amaral DG, Squire LR. Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient E.P. Proc Natl Acad Sci U S A. 2013;110(21):E1953–E1962. doi: 10.1073/pnas.1306244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N. Focal retrograde amnesia in neurological disease: A critical review. Cortex. 1993;29:217–234. doi: 10.1016/s0010-9452(13)80177-3. [DOI] [PubMed] [Google Scholar]
- Kapur N, Ellison D, Smith MP, McLellan DL, Burrows EH. Focal retrograde amnesia following bilateral temporal lobe pathology. Brain. 1992;115:73–85. doi: 10.1093/brain/115.1.73. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Remote and autobiographical memory, temporal context memory and frontal atrophy in Korsakoff and Alzheimer patients. Neuropsychologia. 1989;27:437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Focal retrograde amnesia and the attribution of causality: An exceptionally critical review. Cognitive Neuropsychology. 2000;17(7):585–621. doi: 10.1080/026432900750002172. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical and Experimental Neuropsychology. 1989;5:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kritchevsky M, Squire LR. Transient global amnesia: Evidence for extensive, temporally-graded retrograde amnesia. Neurology. 1989;39:213–218. doi: 10.1212/wnl.39.2.213. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: medial vs. lateral temporal lobe. Proc Natl Acad Sci U S A. 2004;101(17):6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;37:127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Functional retrograde amnesia - Mnestic block syndrome. Cortex. 2002;38(4):651–654. doi: 10.1016/s0010-9452(08)70030-3. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Teuber HL. Memory for remote events in anterograde amnesia: Recognition of public figures from news photographs. Neuropsychologia. 1975;13:353–364. doi: 10.1016/0028-3932(75)90013-5. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. In: Bellack R, Keraso B, editors. Geriatric PsychiatryX. New York: Grune and Stratton; 1976. pp. 77–121. [Google Scholar]
- Mayes AR. Does focal retrograde amnesia exist and if so, what causes it? Cortex. 2002;38(4):670–673. doi: 10.1016/s0010-9452(08)70034-0. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Daum I, Markowisch HJ, Sauter B. The relationship between retrograde and anterograde amnesia in patients with typical global amnesia. Cortex. 1997;33(2):197–217. doi: 10.1016/s0010-9452(08)70001-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolitation. 2000;248:51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Milton F, Muhlert N, Pindus DM, Butler CR, Kapur N, Graham KS, Zeman AZ. Remote memory deficits in transient epileptic amnesia. Brain : a journal of neurology. 2010;133(Pt 5):1368–1379. doi: 10.1093/brain/awq055. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- O'Connor M, Butters N, Miliotis P, Eslinger P, Cermak LS. The dissociation of anterograde and retrograde amnesia in a patient with herpes encephalitis. Journal of Clinical and Experimental Neuropsychology. 1992;14:159–178. doi: 10.1080/01688639208402821. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe [The test of copying a complex figure] Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. Journal of Neuroscience. 1998;(18):3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [Case Reports Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot T. Les Maladies de la Memoire [English translation: Diseases of Memory] New York: Appleton-Century-Crofts; 1881. [Google Scholar]
- Rose FC, Symonds CP. Persistent memory defect following encephalitis. Brain. 1960;83:195–212. doi: 10.1093/brain/83.2.195. [DOI] [PubMed] [Google Scholar]
- Russell WR. The Traumatic Amnesias. Oxford: Oxford University Press; 1971. [Google Scholar]
- Russell WR, Nathan PW. Traumatic amnesia. Brain. 1946;69:280–300. doi: 10.1093/brain/69.4.280. [DOI] [PubMed] [Google Scholar]
- Sanders HI, Warrington DK. Memory for remote events in amnesic patients. Brain. 1971;94:661–668. doi: 10.1093/brain/94.4.661. [DOI] [PubMed] [Google Scholar]
- Sehm B, Frisch S, Thone-Otto A, Horstmann A, Villringer A, Obrig H. Focal retrograde amnesia: voxel-based morphometry findings in a case without MRI lesions. PLoS One. 2011;6:10, e26538. doi: 10.1371/journal.pone.0026538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth EC, Morris CE. The transient global amnesia syndrome. A defect in the second stage of memory in man. Archives of Neurology. 1966;15(5):515–520. doi: 10.1001/archneur.1966.00470170069007. [DOI] [PubMed] [Google Scholar]
- Squire LR. Remote memory as affected by aging. Neuropsychologia. 1974;12:429–435. doi: 10.1016/0028-3932(74)90073-6. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Chace PM. Memory functions six to nine months after electroconvulsive therapy. Archives of General Psychiatry. 1975;32:1557–1564. doi: 10.1001/archpsyc.1975.01760300095008. [DOI] [PubMed] [Google Scholar]
- Squire LR, Haist F, Shimamura AP. The neurology of memory: Quantitative assessment of retrograde amnesia in two groups of amnesic patients. Journal of Neuroscience. 1989;9:828–839. doi: 10.1523/JNEUROSCI.09-03-00828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Shimamura AP. Characterizing amnesic patients for neurobehavioral study. Behavioral Neuroscience. 1986;100:866–877. doi: 10.1037//0735-7044.100.6.866. [DOI] [PubMed] [Google Scholar]
- Squire LR, Slater PC, Miller PL. Retrograde amnesia and bilateral electroconvulsive therapy. Long-term follow-up. Arch Gen Psychiatry. 1981;38(1):89–95. doi: 10.1001/archpsyc.1981.01780260091010. [DOI] [PubMed] [Google Scholar]
- Taylor LB. Scoring criteria for the Rey-Osterrieth Complex Figure Test. In: Spreen O, Strauss E, editors. A compendium of neuropsychological tests. Administration, norms, and commentary. New York: Oxford University Press; 1998. pp. 350–351. [Google Scholar]
- Victor M. The amnesic syndrome and its anatomical basis. Canadian Medical Association journal. 1969;100(24):1115–1125. [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders due to Alcoholism and Malnutrition. 2 ed. Philadelphia: F.A. Davis; 1989. [Google Scholar]
- Wickelgren WA. Chunking and consolidation: A theoretical synthesis of semantic networks, configuring, S-R versus cognitive learning, normal forgetting, the amnesic syndrome, and the hippocampal arousal system. Psychological Review. 1979;86:44–60. [PubMed] [Google Scholar]
- Zeman AZ, Boniface SJ, Hodges JR. Transient epileptic amnesia: a description of the clinical and neuropsychological features in 10 cases and a review of the literature. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64(4):435–443. doi: 10.1136/jnnp.64.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. Journal of Neuroscience. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.