Abstract
Campylobacteriosis is the most frequent food-borne human enteritis. The major source for infection with Campylobacter spp. is broiler meat. Risk assessments consider the reduction of Campylobacter in primary production to be most beneficial for human health. The aim of this study was to test the efficacy of a bacteriophage application under commercial conditions which had proved to be effective in previous noncommercial studies under controlled experimental conditions. A phage cocktail for Campylobacter reduction was tested on three commercial broiler farms each with a control and an experimental group. Colonization of Campylobacter was confirmed prior to phage application in fecal samples. Subsequently, a phage cocktail was applied via drinking water in the experimental group (log10 5.8 to 7.5 PFU/bird). One day after phage application, Campylobacter counts of one experimental group were reduced under the detection limit (<50 CFU/g, P = 0.0140) in fecal samples. At slaughter, a significant reduction of >log10 3.2 CFU/g cecal content compared to the control was still detected (P = 0.0011). No significant reduction was observed in the experimental groups of the other trials. However, a significant drop in cecal Campylobacter counts occurred in a phage-contaminated control. These results suggest that maximum reduction of Campylobacter at the slaughterhouse might be achieved by phage application 1 to 4 days prior to slaughter.
INTRODUCTION
Campylobacteriosis is a common food-borne zoonosis worldwide. In 2012, it was the most frequent food-borne bacterial enteritis in Germany, with more than 62,000 reported cases (1), and in 2010, there were 212,064 cases in the European Union (EU) (2). The thermotolerant Campylobacter species C. jejuni and C. coli are the most frequently isolated agents, and symptoms in humans range from watery to hemorrhagic diarrhea. These generally self-limiting infections are occasionally followed by severe complications such as Guillain-Barré syndrome and reactive arthritis (3, 4).
Campylobacter spp. are part of the normal intestinal flora of many livestock animals, especially birds. In the EU, 71% of broilers in slaughterhouses harbor Campylobacter spp. in their intestine, and due to fecal pollution, broiler meat becomes contaminated (5). Subsequent human infections arise from uncooked poultry meat, hand-to-mouth transfer in the kitchen, and cross-contamination of other foods. In contrast to other bacterial food-borne zoonoses like salmonellosis, there was an increase of 8.5% in reported cases in Germany in 2011 and of 7% from 2008 to 2010 in the EU (2, 6), posing a serious threat to public health. Risk assessments have been carried out, and control options at different levels of the food chain are under discussion (7).
Of all human cases, 50 to 80% are believed to be attributed to chicken as a whole, including direct spread from farms into the environment. Therefore, the Panel on Biological Hazards of the European Food Safety Authority regards the reduction at farm level to be most effective for public health benefits (8). These measures could reduce the number of cases of human campylobacteriosis considerably (7).
Microbiological criteria and performance objectives in primary production for Campylobacter are currently under discussion in Europe (7). Therefore, additional measures to reduce the Campylobacter load are necessary to meet these criteria, which can be established at different stages in the food chain (9).
Bacteriophages have a long history of use in Eastern European countries (10), and phage-based biocontrol of food-borne pathogens is a promising approach (11). Their use for reducing Campylobacter in the chicken gut has been investigated in studies with different phages, doses, experimental settings, and application routes. All currently published studies showed promising results with reductions of Campylobacter in the chicken gut of 0.5 to 5.0 log10 CFU/g (12–16). Adjusting the dosing methods and timing of previous studies to the conditions in commercial broiler houses plays a major role in further developing bacteriophage-mediated biocontrol of Campylobacter (17).
Inoculum size and timing as well as phage host range and density of target bacteria are key elements in the success of phage therapy against Campylobacter in broiler chickens (18). Phage numbers reaching the site of bacterial colonization have to be sufficiently high to reduce bacterial numbers. All studies published to date have used oral doses of log10 5 to 11 PFU/bird (12–15). There are two ways of bacterial reduction by phages. Passive reduction refers to the reduction of bacteria by the initial phage dose and therefore implies a high number of applied viruses per bacterial cell. Active reduction, in contrast, can take place with a lower initial dose when phages reach sufficient numbers for bacterial reduction by replication (19).
For phage replication, a threshold density of bacteria is necessary (20). In all previous studies, birds were infected at the same time and were Campylobacter positive at comparable colonization levels when phages were administered (12–15). In commercial broiler flocks, most birds become infected via horizontal transmission, and groups involve more than 10,000 birds. It is hard to estimate the time when threshold levels are met, although Campylobacter is considered to spread rapidly in an infected flock (21). Under current biosecurity measures, most flocks become Campylobacter positive at an age of 3 weeks and older (22). Therefore, waiting too long in order to meet bacterial threshold densities in field trials would involve the risk of birds being slaughtered before maximum reduction had occurred.
Yet another challenge is that phages are highly specific for a certain host. Very few phages are able to infect different species, and the host range of most of them includes just a number of strains of one bacterial species (23). For the application of phages in commercial broiler houses, the presence of susceptible host bacteria is of great importance for phages in order to affect Campylobacter load. To increase the host range of applied phages, cocktails of phages with different host ranges were used successfully in some studies (12, 24, 25).
These considerations support the use of broad-spectrum cocktails in high doses and a timing that allows Campylobacter to be spread sufficiently for meeting threshold densities before phage application (15). However, field trials are urgently needed for further assessment of these considerations (7, 18, 26, 27).
Thus, the aim of this study was to assess the effects and dynamics of well-characterized phages in field trials in commercial broiler houses. In this trial, we applied phages earlier than recommended for commercial use, because a long observation period is needed for results on phage host interaction and maximum reduction.
MATERIALS AND METHODS
Field trials.
Application of phages in the field trials was performed according to German law and was acknowledged by the Animal Welfare Committee of the University of Veterinary Medicine Hannover. In accordance with German law, permission to apply bacteriophages as feed additive was given by the competent authority (LAVES-Lower Saxony State Office for Consumer Protection and Food Safety, reference number 41.3-63003-13/2011).
Broiler flocks and farm management.
Three field trials under commercial rearing conditions were carried out on broiler farms in the northwest of Germany. The first and second field trials were carried out on the same farm but in different sheds. The third trial was performed on another farm. Further information is given in Table 1 on flock size, breeding line, and season as well as biosecurity, vaccines, and feed additives used. In all trials, birds of the control group received the same feed and feed additives as well as vaccinations and were taken care of by the same person, the same biosecurity measures being applied as in the experimental group (Table 1).
Table 1.
Field trial rearing conditions
| Type of condition | Field trial 1 | Field trial 2 | Field trial 3 |
|---|---|---|---|
| Farm | 1 | 1 | 2 |
| Flock size/group | 13,400a,b | 10,100a/21,500b | 13,500a,b |
| Breed | Rossa/Cobb + Rossb | Cobba,b | Ross 308a,b |
| Season | Autumn | Summer | Summer |
| Biosecurity | |||
| Stables of groups | Different buildings | Different buildings | Same building |
| Rubber boots | Changed | Changed | Not changed |
| Clothes | Not changed | Not changed | Not changed |
| Thinning | Yes | No | No |
| Vaccination (first 15 days of life) | Gumboro disease, Newcastle disease, infectious bronchitis | Gumboro disease, Newcastle disease, infectious bronchitis | Gumboro disease, Newcastle disease, infectious bronchitis |
| Feed additives | |||
| First 15 days of life | Vitamins A, D3, E | Vitamins A, D3, E | Vitamins A, D3, E |
| Whole rearing period | Buffered acidsc | Buffered acidsc | |
| Antibiotic therapy | Exptl group: penicillin for 3 days until 3 days before start of expt | None | None |
Experimental group.
Control group.
Ammonium formate, lactic acid, citric acid, acetic acid, copper, and zinc; dosing: both groups, 1% in drinking water, stopped during phage application.
In the first trial, birds were reared under commercial conditions on a farm including 11 sheds in 7 buildings. The two groups of trial 1 were located in two houses of the same construction type, each containing the respective flock. The houses were located adjacent to one another with a corridor of approximately 6 m in between. Both groups were of the same age and housed on the same day. Pet birds and horses were kept next to the farm. Both bird groups tested negative for Campylobacter spp. at an age of 29 days but tested positive by real-time PCR (DNA extraction and real-time PCR detection kit; AniCon Labor GmbH, Germany) of cloacal swabs at day 34 (Table 2). Phage application was carried out at day 36. The concentration of the phage suspension was log10 7.4 PFU/ml before transport, and a volume of 9.2 liters was dosed for 2.6 h into the drinking line of the experimental group using the standard dosing equipment available on the farm. This corresponds to a dose of log10 7.2 PFU/bird in the experimental group (calculated by log10 7.4 PFU/ml × 9,200 ml/13,400 birds). Drinking water supply was stopped 1.5 h before dosing in order to make the birds thirsty and accelerate the uptake of phages.
Table 2.
Time scale of phage application and samples
| Age of birds (days) | Step(s) in field triala: |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| 27 | C | ||
| 28 | C | ||
| 30 | |||
| 31 | x, P | ||
| 32 | x, P | x | |
| 33 | x | ||
| 34 | C | x | |
| 35 | x | ||
| 36 | x, P | x | |
| 37 | x | ||
| 38 | X | X | |
| 42 | X | ||
Abbreviations: P, phage cocktail application (after fecal sampling); x, fecal samples for phage and Campylobacter counts; X, cecal samples at slaughter for phage and Campylobacter counts; C, initial sampling to confirm Campylobacter presence.
The second trial was performed on the same farm as field trial 1 but in different buildings. The two trial groups were located in two houses, each containing just the respective flock. The houses were located directly opposite with a corridor of 50 m in between. Birds of both groups were of the same age and housed on the same day. Horses were housed next to the house of the control group. Both groups tested positive for Campylobacter spp. at 27 days of age by incubation of cloacal swabs in Campyfood broth and subsequent plating on Campyfood agar (bioMérieux, France). Phage application was carried out at day 32. The concentration of the phage suspension was log10 8.1 PFU/ml before transport, and a volume of 7 liters was dosed for 2 h into the drinking line using the standard dosing equipment available on the farm. This corresponds to a dose of log10 7.9 PFU/bird (calculated by log10 8.1 PFU/ml × 7,000 ml/10,100 birds). The drinking water supply was stopped 1 h before dosing.
In the third trial, birds were reared under commercial conditions on a farm including 8 sheds in 2 buildings 8 m apart. Each building included two floors and two sheds on each floor. The groups of trial 3 were located on different floors in one building. The farmers were told to look after the control group first in order to prevent carryover of phages. Birds in the two groups were of the same age and housed on the same day. Both groups tested positive for Campylobacter spp. at 28 days of age by PCR. Phage application was carried out in the experimental group at day 31 as indicated in Table 2. The concentration of the phage suspension was log10 7.3 PFU/ml before transport, and a volume of 20 liters was dosed for 6.3 h into the drinking line using the standard dosing equipment available on the farm. This corresponds to a dose of log10 7.5 PFU/bird (calculated by log10 7.3 PFU/ml × 20,000 ml/13,500 birds). Drinking water supply was stopped 2 h before dosing.
In all trials, buffered acids in drinking water and drinking water supply of the control were stopped in the same way as in the experimental group whereas no phages were administered. AviBlue (Lohmann Animal Health GmbH, Germany) was added for detecting blue staining in the drinking water of the experimental groups in all trials. Samples of 10 ml were taken from the suspensions prior to dosing and from the drinking water in the experimental groups for enumeration of phages.
Per group, 9 fecal samples were taken directly before dosing and at different sampling times after application as indicated in Table 2. For the final sampling of each trial, 9 cecal samples per group were taken from broilers in the slaughterhouse as a spot check of the contamination level on the farm (Table 2).
Preparation of cultures.
The obligate lytic and well-characterized type III phages NCTC 12672, 12673, 12674, and 12678 of the British phage typing scheme (28) were kindly provided by Lohmann Animal Health GmbH.
For propagation of phage strains, Campylobacter was grown on Mueller-Hinton (MH) blood agar (Oxoid, Germany) for 18 to 20 h and suspended in 10 mmol MgSO4. Density was adjusted to McFarland standard 3 (Densimat; bioMérieux). All cultures containing Campylobacter were incubated in a CO2 incubator (Binder, Germany) under microaerobic conditions (5% O2, 10% CO2).
Phages were propagated on their C. jejuni host strains NCTC 12661, 12664, and 12660 as described elsewhere (29), 100 μl each of the phage suspension and host Campylobacter being added to 5 ml of liquefied NZCYM agar (Carl Roth GmbH & Co KG, Germany; 0.7% agar-agar; Merck). The agar was poured onto NZCYM plates containing 1.5% agar-agar. The plates were incubated for 24 h (48 h in the case of phage NCTC 12672).
Subsequently, 5 ml SM buffer (5.8 g NaCl, 2.0 g MgSO4 · 7H2O, 50 ml 1 M Tris [Sigma-Aldrich, Germany], pH 7.5, 5 ml 2% gelatin) was added. After swaying the plates on an orbital shaker (120 rpm) overnight at 4°C, 5% (wt/vol) chloroform (Sigma-Aldrich) was added to the recovered SM buffer and incubated for 15 min. Samples were centrifuged at 4°C and 13,000 × g for 20 min, and the supernatant was filtered through a 0.22-μm filter.
Portions of 1.8 ml of the phages were stored in tubes after adding 1 drop of glycerin at −20°C. Working cultures were stored at 4°C. Campylobacter stock cultures were stored at −80°C (Cryobank vials; mast or skimmed milk).
For the field trials, phage suspension was prepared separately for each phage in batches of 5 liters Standard I nutrient broth (Merck), inoculated each with 100 ml of phages (log10 7 PFU/ml) and host (McFarland standard 3). The suspension was incubated, centrifuged, and filtered as described above. During incubation, microaerobic conditions were ensured by aerating the broth with 10% CO2 and 5% O2. Ten liters of each phage suspension was stored at 4°C. A cocktail was mixed in the adequate concentration and volume directly prior to the trials.
Sampling methods.
In each field trial, 9 fresh (warm and not stepped upon by birds) excreta were collected from both the control and the experimental group from different locations in the sheds. Each sample was taken with a clean pair of laboratory gloves and transported in sterile plastic bags. From the processing lines in German slaughterhouses, 9 cecal samples per group were taken and each transferred to a sterile plastic bag. All samples were transported under chilled conditions, at approximately 4 ± 2°C (up to 10 to 15°C for field trial 1). Laboratory testing of all samples took place within 24 h after sampling.
Laboratory testing.
For serial dilutions of fecal samples, excreta were aseptically transferred to the medium. For preparing cecal samples, the tip of the cecum was decontaminated by dipping it in boiling water for a few seconds. Then, the tip was aseptically removed and the luminal content was transferred to a sodium chloride peptone buffer without coming into contact with the outside of the gut.
Campylobacter bacteria were enumerated by preparing log10 serial dilutions in sodium chloride peptone buffer (NaCl, 8.5 g/liter; peptone, 1 g/liter) of 1 g of cecal content or feces, respectively. From the dilutions 10−1 to 10−8, 0.1 ml was plated on Karmali agar (Oxoid, Germany) in duplicate. After an incubation period of 48 h, the colonies were counted and the concentration was calculated. Presumptive Campylobacter colonies were confirmed by positive oxidase and catalase testing and their typical cell morphology and motility under the microscope.
Species identification.
Species identification of the isolates was performed by testing representative isolates of all trials, groups, and samplings by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) analysis (Biotyper software 3.0; Bruker, Germany).
Typing of Campylobacter isolates.
From each trial, 3 to 8 representative isolates were characterized by multilocus sequence type (MLST) analysis. This analysis was done according to the method of Dingle et al. (30, 31). The amplification and sequencing primers were obtained from the Campylobacter jejuni PubMLST webpage. All seven loci, aspA (aspartase), glnA (glutamine synthetase), gltA (citrate synthase), glyA (serine hydroxyl methyltransferase), pgm (phosphor glucomutase), tkt (transketolase), and uncA (ATP synthase alpha subunit), were amplified, and then purification and sequencing reactions were performed by Eurofins MWG Operon (Ebersberg, Germany). Sequence files were read, assembled, evaluated, aligned, and compared to the reference set of alleles using BioNumerics 7.1 (Applied Maths, Belgium). Sequence types (STs) and clonal complexes (CCs) were evaluated with the MLST online plugin function via the official PubMLST database (http://pubmlst.org/campylobacter/).
For biochemical characterization, 22 isolates per group were taken randomly from the samples, transferred to Karmali plates, and after 24 h of incubation stored at −80°C. They were cultivated in Preston broth (Oxoid, Germany) prior to characterization to prevent possible contamination. Isolates from the first field trial were directly plated on MH blood agar, but subcultivation was done before storage in order to obtain pure cultures. APIcampy tests (bioMérieux, France) were performed according to the manufacturer's instructions. For analysis, APIweb (bioMérieux, France) was used.
Enumeration of phages.
Campylobacter strain NCTC 12662 was used for the enumeration of phages. One gram of cecal content or feces was diluted 1:10 with SM buffer and shaken overnight at 4°C. The sample was centrifuged at 4°C and 13,000 × g for 10 min and filtered through a 0.22-μm filter (Rotilabo syringe filter; Carl Roth GmbH & Co KG, Germany). Drinking water and suspension samples were filtered only. A 10-fold dilution series was prepared, and phages were enumerated using the double agar overlay method described by Connerton et al. (32). Instead of 0.6% agar for the overlay, 0.7% was used. After this initial screening of the dilution series, we determined the exact concentration of phages by adding 100 μl of the corresponding dilutions to the molten agar as described for phage propagation. All dilutions were prepared in duplicate, and the plaques were evaluated after 24 h of incubation.
The number of phages applied per bird was calculated by the measured concentration in the drinking water and the volume of phage-dosed drinking water.
In vitro phage susceptibility testing.
The susceptibility of Campylobacter isolates from the samples was tested in vitro separately for each phage and the whole cocktail. Tests included spots of phage suspension on overlays of representative Campylobacter isolates as described by Connerton et al. (32) and plaque formation on overlay, adding phages to the agar as described for enumeration of phages. Susceptibility tests for each trial were carried out twice on different days using three representative isolates per biotype and group.
Data analysis.
Necessary sample size was calculated by a program for calculating optimal sample size for t test according to the instructions of Dufner et al. (33) in cooperation with the Department of Biometry, Epidemiology and Information Processing of the University of Veterinary Medicine Hannover, using SAS 9.1 and 9.3 software. A standard deviation of 1 was estimated from results of previous experimental trials (15), and a detection level of 1.5 log10 was used. Standard values were used for α and β (α = 0.05, β = 0.20). Significances of differences between the control and experimental groups were calculated with SAS 9.1 and 9.3 software using the Wilcoxon rank sum test.
RESULTS
Field trials. (i) Field trial 1.
No phages were isolated from fecal samples taken directly before dosing. An administered dose of log10 7.5 PFU/bird was calculated from PFU/ml in drinking water samples and dosed drinking water volume per bird.
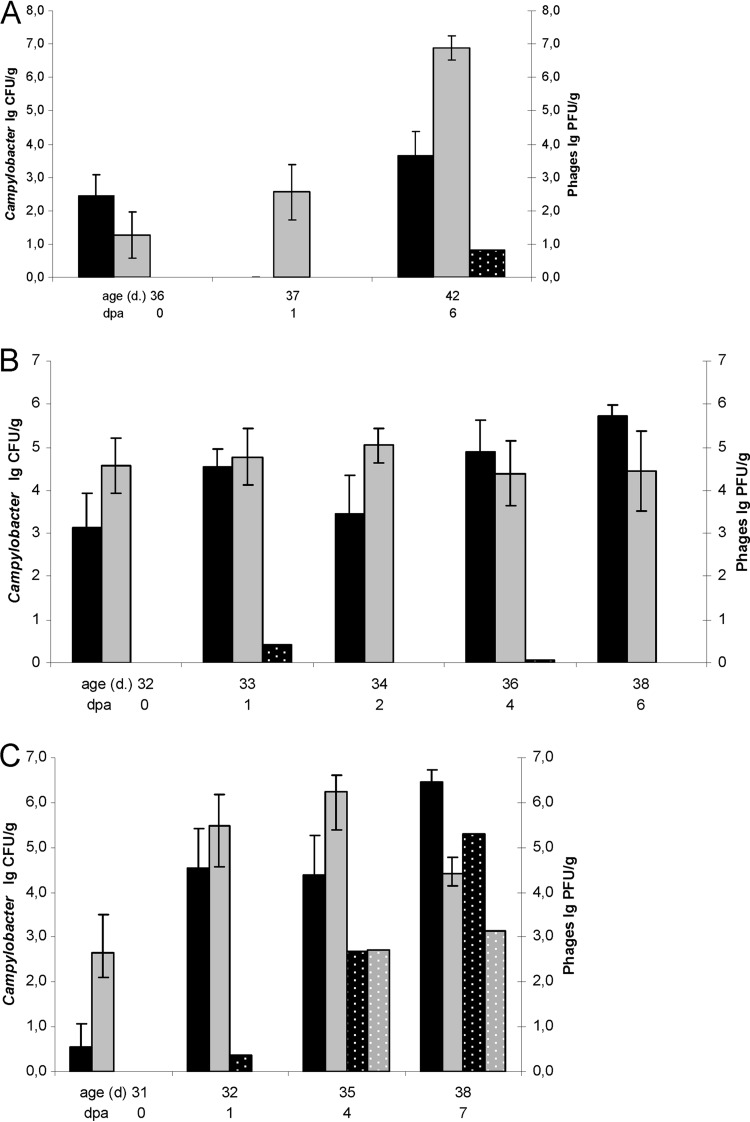
Results of field trial 1 are shown in Fig. 1A. Campylobacter counts in feces of the experimental group were significantly reduced under the detection limit 1 day after phage application (with a detection limit of 50 CFU/g). In cecal samples of the experimental group, colonization remained about log10 3.2 CFU/g lower (P = 0.0011) than in the control until slaughter. In the control group, a rise of Campylobacter counts occurred. Campylobacter isolates from samples of field trial 1 were identified as Campylobacter jejuni subsp. jejuni by MALDI-TOF.
Fig 1.
Comparison of Campylobacter counts with and without phage application in three in vivo field trials. (A) Field trial 1; (B) field trial 2; (C) field trial 3. Black bars, experimental group; gray bars, control group; solid black and gray bars, Campylobacter counts (log10 CFU/g); stippled bars, phage counts. Error bars show standard errors of the means (n = 9); dpa, days postapplication.
In field trial 1, mean counts of phages did not exceed log10 1 PFU/g over the whole period of 6 days (Fig. 1A). Phages could be detected at day 6 after application in four cecal samples, and counts in these samples ranged from 1.66 to 2.14 PFU/g.
(ii) Field trial 2.
No phages were isolated from fecal samples taken directly before dosing in field trial 2. An administered dose of log10 5.8 PFU/bird was calculated for field trial 2 by measuring phage numbers in drinking water samples and the volume of phage-dosed drinking water per bird. In contrast, measuring the concentration of the phage cocktail after transport directly prior to dosing and number of birds resulted in a calculated dose of log10 7.9 PFU/bird.
The Campylobacter counts dropped (Fig. 1B, 1 day postapplication [dpa]) log10 1.1 CFU/g from 1 to 2 days after phage application in fecal samples, but the resulting difference of log10 1.6 CFU/g compared to the control was not significant (P = 0.09). In the control group, Campylobacter counts rose continuously. Campylobacter isolates from samples were identified as Campylobacter jejuni subsp. jejuni by MALDI-TOF.
Phages could be reisolated 1 day after application but could not be detected again during the following 5 days (Fig. 1B), except in the case of one bird where a single plaque could be isolated from feces at day 4 postapplication.
(iii) Field trial 3.
In field trial 3, a dose of log10 7.6 PFU/bird was calculated from measured phage numbers in the drinking water sample and the volume of phage-dosed drinking water per bird. No phages were isolated from the fecal samples taken directly before dosing.
No reduction of Campylobacter counts was observed in the experimental group of this trial. A nonsignificant stagnation of Campylobacter counts from day 1 to day 4 postapplication was observed in feces within a similar time scale compared to the nonsignificant drop in the experimental group of field trial 2 (Fig. 1B and C). As in the other trials in the control group of field trial 3, Campylobacter counts rose continuously. However, in trial 3 an entry of phages to the control occurred in between the sampling days 1 and 4 postapplication. It was followed by a significant drop of cecal Campylobacter counts in the control group 7 dpa compared to fecal counts 4 dpa (P = 0.00078). These cecal counts were also significantly lower than counts in the experimental group 7 dpa (P = 0.0020). Campylobacter isolates from samples were identified as Campylobacter jejuni subsp. jejuni by MALDI-TOF.
In field trial 3, a clear increase in phage counts could be seen in both groups (Fig. 1C), indicating a replication of at least one of the cocktail phages.
Typing of Campylobacter isolates.
In order to assess whether different Campylobacter strains were present during the trial, we carried out a multilocus sequence typing analysis (MLST) of 15 isolates isolated from all groups and trials (Table 3). Additionally, biochemical differentiation of >400 Campylobacter isolates of both groups in all three trials was conducted. Results are presented in Fig. 2A to D. Campylobacter jejuni subsp. jejuni was the only isolated subspecies in all field trials.
Table 3.
Multilocus sequence typing analysis (MLST), biotypes, and susceptibilities of Campylobacter jejuni isolates selected from the field trials
| Isolate from trial | MLST |
Biotype | In vitro susceptibility | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aspA | glnA | gltA | glyA | pgm | tkt | uncA | STc | CCd | |||
| 1a | 8 | 28 | 4 | 243 | 23 | 29 | 35 | 4819 | No match | 2 | No |
| 1a | 8 | 28 | 4 | 243 | 23 | 29 | 35 | 4819 | No match | 1 | No |
| 1b | 8 | 28 | 4 | 243 | 23 | 29 | 35 | 4819 | No match | 1 | No |
| 2a | 2 | 15 | 4 | 3 | 154 | 25 | 35 | 905 | No match | 2 | No |
| 2a | 7 | 17 | 2 | 15 | 23 | 3 | 12 | 51 | ST-443 complex | 2 | No |
| 2a | 7 | 17 | 2 | 15 | 23 | 3 | 12 | 51 | ST-443 complex | 1 | No |
| 2b | 7 | 17 | 2 | 15 | 23 | 3 | 12 | 51 | ST-443 complex | 2 | No |
| 3a | 22 | 15 | 4 | 64 | 23 | 25 | 23 | New | 1 | No | |
| 3a | 22 | 15 | 4 | 64 | 23 | 25 | 23 | New | 1 | No | |
| 3a | 2 | 15 | 4 | 64 | 74 | 25 | 23 | 4755 | ST-1034 complex | 2 | Yes |
| 3a | 2 | 15 | 4 | 64 | 74 | 25 | 23 | 4755 | ST-1034 complex | 2 | Yes |
| 3b | 2 | 15 | 4 | 64 | 74 | 25 | 23 | 4755 | ST-1034 complex | 2 | Yes |
| 3b | 2 | 15 | 4 | 64 | 74 | 25 | 23 | 4755 | ST-1034 complex | 2 | Yes |
| 3b | 2 | 15 | 4 | 64 | 74 | 25 | 23 | 4755 | ST-1034 complex | 1 | No |
| 3b | 2 | 15 | 4 | 64 | 74 | 25 | 23 | 4755 | ST-1034 complex | 1 | No |
Experimental group.
Control group.
ST, sequence type.
CC, clonal complex.
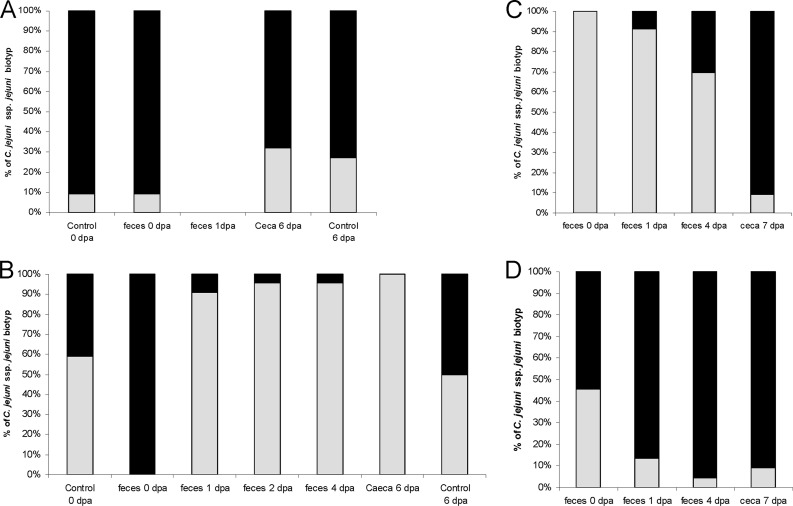
Fig 2.
Proportions of Campylobacter jejuni subsp. jejuni subtypes 1 and 2 at different days post-phage application (dpa). In field trials 1 (A) and 2 (B), experimental group proportions are shown for every sampling, whereas for the control just first and last samples are shown (first and last bars). For field trial 3, experimental (C) and control (D) groups are shown separately because in both groups phages were reisolated. For each bar, 22 isolates were analyzed. Gray bars, C. jejuni subsp. jejuni subtype 1; black bars, C. jejuni subsp. jejuni subtype 2.
In field trial 1, MLST analysis revealed sequence type (ST) 4819 to be present in both groups (Table 3, field trial 1). Results of biotyping, in contrast, found both biotypes 1 and 2 to be present. None of the three tested isolates in vitro was found to be susceptible to the cocktail phages.
In field trial 2, sequence type 51 was found in both the control and the experimental group. Additionally, sequence type 905 was found in the experimental group. Both biotypes were present in the control and found in almost equal amounts at the first and the last sampling. In contrast, in the experimental group of this trial all examined isolates were biotype 2 before phage application, while 1 day after phage application 91% were biotype 1 and 6 days after phage application all examined isolates belonged to biotype 1 (Fig. 2B). None of the tested isolates in vitro was found to be susceptible to the cocktail phages (n = 3 for each biotype).
In field trial 3, sequence type 4755 was present in both groups. In the experimental group, a second sequence type was found that has not been previously reported in the PubMLST database. A similar pattern of biotypes as in field trial 2 with exchanged types was seen in the experimental group (Fig. 2C). In the contaminated-control group of field trial 3, both biotypes were present at all sampling times (Fig. 2D). In in vitro susceptibility tests, biotype 2 was found to be susceptible to the cocktail phages, whereas biotype 1 was not (n = 3 for each biotype).
In all trials, different biotypes were found to belong to one sequence type. In field trial 3, biotypes coincided with phage susceptibility of the isolates.
DISCUSSION
Reduction of Campylobacter.
Previous studies have shown that phages significantly reduce the colonization level of Campylobacter spp. in the avian gut. However, the extent and duration of reduction after dosing were highly variable (12–16). The field trials were carried out to reassess the results of these studies and to account for the demands of the conditions in commercial broiler production (e.g., no CaCO3 could be used as buffer in the drinking line). A stagnation (Fig. 1C) or decrease of Campylobacter was seen in all field trials in the period of 1 to 4 days after phage application (Fig. 1A to C). However, only in field trial 1 and in the contaminated control of field trial 3 was a significant reduction detected. The reduction of Campylobacter counts in the experimental group of field trial 1 resulted in a >log10 3.2 reduction at slaughter. Reducing the Campylobacter load by log10 3 CFU/g in the intestines at slaughter is considered to reduce public health risk by at least 90% (7).
The results of the field trials are in approximate agreement with other experimental studies (12, 13, 15). Consistent colonization could be observed in all control groups (12). We can therefore assume that the different conditions in the field trials (Table 2) had no influence on Campylobacter colonization. In field trial 3, entry and subsequent replication of phages in the contaminated-control group occurred, this probably being responsible for the significant terminal drop of Campylobacter counts at day 7 postapplication in this group (19). The phages contaminating this group could have derived from the environment of the shed or from the experimental group. Since the change was considerable, we assume that entry of phages to the contaminated-control group may have occurred via rubber boots which were not changed when going between the sheds of the control and the experimental groups in field trial 3 (Table 1).
Phage reisolation rates.
In field trial 1, phage isolation rates were low (Fig. 1A). A possible explanation for this is the fact that, in contrast to field trials 2 and 3, the transport from broiler houses to the laboratory took longer in trial 1 (up to 8 h compared to 2 h in field trials 2 and 3). Although overall few phages were reisolated in field trial 1, in 4 samples plaques were observed 6 days postapplication with concentrations ranging from log10 1.66 to log10 2.14 PFU/g. We suppose that phages replicated at least in some animals of field trial 1 because without replication we would expect phages to be excreted at this time (13). Results of subsequent in vitro tests concerning the effects of storage conditions on phage numbers suggested that phage concentration decreased more rapidly in feces than in ceca under the applied conditions (data not shown). This could be a possible explanation for the fact that, 24 h after applying the phage cocktail, no phages were found in the feces while in cecal samples phages were able to be isolated.
According to these results, cooling conditions were changed and times of transport were reduced for field trials 2 and 3, using a transportable refrigerator set at 4°C instead of an insulated box with cool packs.
In field trial 2, a mean of log10 0.4 PFU/g feces after 24 h was calculated with concentrations ranging from log10 0.48 to log10 2 PFU/g feces, which is in accordance with concentrations 24 h after application in field trial 3. However, in field trial 2 phages dropped below the detection limit afterwards except for one sample where 2 plaques were found 3 days later. Assuming that these two plaques were due to ingested droppings, no phage replication took place in field trial 2 (13, 15).
In field trial 3, two samples were phage positive 24 h after application, each containing log10 1.71 PFU/g feces. In the following 3 days, massive phage replication occurred (Fig. 1C). This raises the question why no reduction in Campylobacter counts was observed in the experimental group.
An explanation may be found in studies with Escherichia coli where a minimal effect of phages in vivo was found, notwithstanding that isolates were susceptible in in vitro tests. These studies assume a different physiological state of bacterial cells or external factors which prevent phage infection in vivo (34).
Bacteriophage-host dynamics.
The probability of phage adsorption to bacteria is mainly influenced by the density of bacteria and phages (35). Phage replication becomes possible at a certain bacterial density, commonly referred to as proliferation threshold, and time plays a major role in the likelihood of phage adsorption occurring (20). The proportion of viruses to host cells is commonly referred to as multiplicity of infection (MOI) (36).
A distinction has to be made between passive and active reduction of Campylobacter. If Campylobacter counts are reduced in one infection cycle, passive reduction has occurred (19). This approach requires an MOI of >10 for substantial reduction without phage replication, while active reduction relies on low doses and replication of phages at the site of the Campylobacter colonization.
Early phage application in the field trials interfered with completed Campylobacter colonization of the flocks and thus with the presence of bacterial threshold densities (Fig. 1A to C). A critical host cell concentration of 105 CFU/ml was stated in the publication of Hagens and Loessner (35). However, the study published by Bigwood et al. (37) indicates that reduction of Campylobacter through phages is possible at low host cell concentrations, given a sufficiently high concentration of phages. It cannot be demonstrated if an MOI of >10 was met for passive reduction of Campylobacter in our trials. Active reduction of Campylobacter occurred in the contaminated-control group of field trial 3 (19).
Considerations on bacteriophage-host dynamics were developed by in vitro experiments and simulations (38), and the dynamics of phage-host interaction are very much strain, dose, and host dependent. They cannot easily be transferred to in vivo field conditions. However, they should be considered when planning and discussing in vivo trials.
The subsequent rise in Campylobacter counts after reduction in colonization tallies with findings of other studies under experimental conditions (13, 15). This can be explained by acquired resistances of Campylobacter and occurrence and replication of new, per se nonsusceptible strains (39) or effects regarding changed physiological states of the bacteria or external factors as described in trials with E. coli (34). Also, an increased potential of the contaminating phages to kill bacteria cannot be ruled out as a possible reason for these findings.
Susceptible host bacteria.
As stated above, availability of susceptible host bacteria is one of the main influencing factors for the efficacy of phage application. A study carried out by Connerton et al. (32) revealed that succession of Campylobacter during phage infection was due to new genotypes rather than development of resistance of the existing strains. Findings of other authors support these results (39, 40). While susceptible strains might disappear after application of phages, nonsusceptible strains can easily grow and lead to a rise in Campylobacter counts subsequent to an initial reduction. Similar findings were made by Scott et al. (40) in flocks with natural phage infections. Other authors found the coexistence of strains with different phage susceptibilities in broiler flocks (32). We used MLST analysis for characterizing different Campylobacter isolates. Results of typing are presented in Table 3. In both field trials 2 and 3, two sequence types were present in the experimental group. However, these sequence types did not coincide with phage susceptibility in these trials. In total, 413 Campylobacter isolates were typed by the APIcampy test system (Fig. 2A to C) for roughly estimating the proportions of different biotypes. The test divides the subspecies C. jejuni subsp. jejuni into four biotypes, and two of them were found to be present in the field trials. It remains unclear why different biotypes belonged to one sequence type and why biotype and phage susceptibility matched in the third trial. Further research is necessary for better understanding of this issue. The susceptibility tests could be performed only after phage application. Therefore, the results of these tests were not known when phages were applied. Despite the absence of in vitro susceptibility in isolates of field trial 1, a reduction took place. Other experimental studies in chickens found phages hardly ever lysing Campylobacter isolates of the same source in vitro (16, 32, 39). Nevertheless, phage findings are associated with reduced numbers of Campylobacter (16, 39). The susceptible biotype isolated at the end of field trial 3 was, however, more frequently isolated. Differences in the abilities of phages to reduce Campylobacter in vitro and in vivo were mentioned in the study of Loc Carrillo et al. (13), and different colonization potentials of isolates with different genetic backgrounds could explain our findings (41). It has been previously shown that different genetic variants of Campylobacter are present in different flocks or slaughter groups (42, 43).
These first field trials with phage application against Campylobacter in commercial broiler houses suggest that phages can lead to a reduction of up to log10 3.2 CFU in Campylobacter load. Such reductions are postulated to be beneficial for public health (7). Phage application is cost-effective, considered to be safe, and easily carried out by the farmer (44). However, an improved timing and suitable phage cocktails are necessary for reproducible results. The results of our study suggest an application approximately 2 to 4 days prior to slaughter for maximum reduction. In addition, broad-spectrum phage cocktails in sufficient doses for passive reduction of different host strains are needed. For large-scale practice, additional research is required, particularly regarding guaranteeing the absence of virulence genes and monitoring occurring resistances against phages (29).
ACKNOWLEDGMENTS
This work was supported by Lohmann Animal Health GmbH and N-Bank.
We thank Wiebke Jansen for lending her support and the laboratory staff Birgit Führing, Silke Schlote-Kohne, Andreas Schridde, Rouwen Stucke, Ina Vasen, and Sabine Korff for their excellent technical assistance. Our thanks go to Anke Grosse-Herrenthey from Lohmann Animal Health for her assistance with the MALDI-TOF analysis.
Footnotes
Published ahead of print 27 September 2013
REFERENCES
- 1.Robert-Koch-Institut 2013. Aktuelle Statistik meldepfl ichtiger Infektionskrankheiten, Deutschland. Epidemiologisches bulletin, 14.01.2013, vol 2, p 20–22 Robert-Koch-Institut, Berlin, Germany [Google Scholar]
- 2.EFSA 2012. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J. 10:2597. 10.2903/j.efsa.2012.2597 [DOI] [PubMed] [Google Scholar]
- 3.Crushell E, Harty S, Sharif F, Bourke B. 2004. Enteric campylobacter: purging its secrets? Pediatr. Res. 55:3–12 [DOI] [PubMed] [Google Scholar]
- 4.Zilbauer M, Dorrell N, Wren BW, Bajaj-Elliott M. 2008. Campylobacter jejuni-mediated disease pathogenesis: an update. Trans. R. Soc. Trop. Med. Hyg. 102:123–129 [DOI] [PubMed] [Google Scholar]
- 5.EFSA 2010. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008, part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 8:1503. 10.2903/j.efsa.2010.1503 [DOI] [Google Scholar]
- 6.Frank C. (ed). 2012. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2011. Robert-Koch-Institut, Berlin, Germany [Google Scholar]
- 7.EFSA 2011. Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 9:2105. 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- 8.EFSA 2010. Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 8:1437. 10.2903/j.efsa.2010.1437 [DOI] [Google Scholar]
- 9.Ellerbroek L. 2012. Application of microbiological criteria in food processing-metrics. Arch. Lebensmittelhyg. 63:101–106 [Google Scholar]
- 10.Kutateladze M, Adamia R. 2010. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 28:591–595 [DOI] [PubMed] [Google Scholar]
- 11.Goodridge LD, Bisha B. 2011. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 1:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho CM, Gannon BW, Halfhide DE, Santos SB, Hayes CM, Roe JM, Azeredo J. 2010. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 10:232. 10.1186/1471-2180-10-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loc Carrillo C, Atterbury RJ, el-Shibiny A, Connerton PL, Dillon E, Scott A, Connerton IF. 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71:6554–6563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Shibiny A, Scott A, Timms A, Metawea Y, Connerton P, Connerton I. 2009. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 72:733–740 [DOI] [PubMed] [Google Scholar]
- 15.Wagenaar JA, Van Bergen MA, Mueller MA, Wassenaar TM, Carlton RM. 2005. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 109:275–283 [DOI] [PubMed] [Google Scholar]
- 16.Atterbury RJ, Dillon E, Swift C, Connerton PL, Frost JA, Dodd CE, Rees CE, Connerton IF. 2005. Correlation of Campylobacter bacteriophage with reduced presence of hosts in broiler chicken ceca. Appl. Environ. Microbiol. 71:4885–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greer GG. 2005. Bacteriophage control of foodborne bacteria. J. Food Prot. 68:1102–1111 [DOI] [PubMed] [Google Scholar]
- 18.Connerton PL, Timms AR, Connerton IF. 2011. Campylobacter bacteriophages and bacteriophage therapy. J. Appl. Microbiol. 111:255–265 [DOI] [PubMed] [Google Scholar]
- 19.Payne RJ, Jansen VA. 2003. Pharmacokinetic principles of bacteriophage therapy. Clin. Pharmacokinet. 42:315–325 [DOI] [PubMed] [Google Scholar]
- 20.Payne RJ, Jansen VA. 2001. Understanding bacteriophage therapy as a density-dependent kinetic process. J. Theor. Biol. 208:37–48 [DOI] [PubMed] [Google Scholar]
- 21.Evans SJ, Sayers AR. 2000. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 46:209–223 [DOI] [PubMed] [Google Scholar]
- 22.van Gerwe T, Miflin JK, Templeton JM, Bouma A, Wagenaar JA, Jacobs-Reitsma WF, Stegeman A, Klinkenberg D. 2009. Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl. Environ. Microbiol. 75:625–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loc Carrillo CM, Connerton PL, Pearson T, Connerton IF. 2007. Free-range layer chickens as a source of Campylobacter bacteriophage. Antonie Van Leeuwenhoek 92:275–284 [DOI] [PubMed] [Google Scholar]
- 24.O'Flynn G, Ross RP, Fitzgerald GF, Coffey A. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70:3417–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanji Y, Shimada T, Yoichi M, Miyanaga K, Hori K, Unno H. 2004. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 64:270–274 [DOI] [PubMed] [Google Scholar]
- 26.Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I, Pearson D, Salvat G, Allen VM. 2011. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 77:8605–8614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janez N, Loc Carrillo C. 2013. Use of phages to control Campylobacter spp. J. Microbiol. Methods 95:68–75. 10.1016/j.mimet.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 28.Sails AD, Wareing DR, Bolton FJ, Fox AJ, Curry A. 1998. Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. J. Med. Microbiol. 47:123–128 [DOI] [PubMed] [Google Scholar]
- 29.Fischer S, Kittler S, Klein G, Glünder G. 2013. Microplate-test for the rapid determination of bacteriophage-susceptibility of Campylobacter isolates-development and validation. PLoS One 8:e53899. 10.1371/journal.pone.0053899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dingle KE, Colles FM, Falush D, Maiden MC. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dingle KE, Colles FM, Wareing DR, Ure R, Fox AJ, Bolton FE, Bootsma HJ, Willems RJ, Urwin R, Maiden MC. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connerton PL, Loc Carrillo CM, Swift C, Dillon E, Scott A, Rees CE, Dodd CE, Frost J, Connerton IF. 2004. Longitudinal study of Campylobacter jejuni bacteriophages and their hosts from broiler chickens. Appl. Environ. Microbiol. 70:3877–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dufner J, Jensen U, Schumacher E. 1992. Statistik mit SAS. Universität Hohenheim, B. G. Teubner, Stuttgart, Germany [Google Scholar]
- 34.Chibani-Chennoufi S, Sidoti J, Bruttin A, Kutter E, Sarker S, Brussow H. 2004. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob. Agents Chemother. 48:2558–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagens S, Loessner MJ. 2010. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr. Pharm. Biotechnol. 11:58–68 [DOI] [PubMed] [Google Scholar]
- 36.Kasman LM, Kasman A, Westwater C, Dolan J, Schmidt MG, Norris JS. 2002. Overcoming the phage replication threshold: a mathematical model with implications for phage therapy. J. Virol. 76:5557–5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigwood T, Hudson JA, Billington C. 2009. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol. Lett. 291:59–64 [DOI] [PubMed] [Google Scholar]
- 38.Cairns BJ, Timms AR, Jansen VA, Connerton IF, Payne RJ. 2009. Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog. 5:e1000253. 10.1371/journal.ppat.1000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Shibiny A, Connerton PL, Connerton IF. 2005. Enumeration and diversity of campylobacters and bacteriophages isolated during the rearing cycles of free-range and organic chickens. Appl. Environ. Microbiol. 71:1259–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott AE, Timms AR, Connerton PL, El-Shibiny A, Connerton IF. 2007. Bacteriophage influence Campylobacter jejuni types populating broiler chickens. Environ. Microbiol. 9:2341–2353 [DOI] [PubMed] [Google Scholar]
- 41.Ahmed IH, Manning G, Wassenaar TM, Cawthraw S, Newell DG. 2002. Identification of genetic differences between two Campylobacter jejuni strains with different colonization potentials. Microbiology 148:1203–1212 [DOI] [PubMed] [Google Scholar]
- 42.Klein G, Beckmann L, Vollmer HM, Bartelt E. 2007. Predominant strains of thermophilic Campylobacter spp. in a German poultry slaughterhouse. Int. J. Food Microbiol. 117:324–328 [DOI] [PubMed] [Google Scholar]
- 43.Lienau J-A, Ellerbroek L, Klein G. 2007. Tracing flock-related Campylobacter clones from broiler farms through slaughter to retail products by pulsed-field gel electrophoresis. J. Food Prot. 70:536–542 [DOI] [PubMed] [Google Scholar]
- 44.Monk AB, Rees CD, Barrow P, Hagens S, Harper DR. 2010. Bacteriophage applications: where are we now? Lett. Appl. Microbiol. 51:363–369 [DOI] [PubMed] [Google Scholar]