Abstract
Termite-derived methane contributes 3 to 4% to the total methane budget globally. Termites are not known to harbor methane-oxidizing microorganisms (methanotrophs). However, a considerable fraction of the methane produced can be consumed by methanotrophs that inhabit the mound material, yet the methanotroph ecology in these environments is virtually unknown. The potential for methane oxidation was determined using slurry incubations under conditions with high (12%) and in situ (∼0.004%) methane concentrations through a vertical profile of a termite (Macrotermes falciger) mound and a reference soil. Interestingly, the mound material showed higher methanotrophic activity. The methanotroph community structure was determined by means of a pmoA-based diagnostic microarray. Although the methanotrophs in the mound were derived from populations in the reference soil, it appears that termite activity selected for a distinct community. Applying an indicator species analysis revealed that putative atmospheric methane oxidizers (high-indicator-value probes specific for the JR3 cluster) were indicative of the active nest area, whereas methanotrophs belonging to both type I and type II were indicative of the reference soil. We conclude that termites modify their environment, resulting in higher methane oxidation and selecting and/or enriching for a distinct methanotroph population.
INTRODUCTION
Termites are a natural methane source, contributing about 20 Tg CH4 per year to the total global methane budget (500 to 600 Tg CH4 per year) (1). Emission of termite-derived methane is determined by the balance of methane production in the termite gut and oxidation. Considering that no evidence of termite gut-inhabiting methane-oxidizing microorganisms (methanotrophs) has been found (2), the methane produced is released into the atmosphere unmitigated. However, the mound material can act as a methane sink, where complete oxidation of termite-derived methane has been reported in mounds of the fungus-growing termite Macrotermes (3). Hence, methane emissions would be higher if not for the methanotrophs inhabiting the mound, yet the methanotrophic community in these environments and, more specifically, the response of methane oxidation and community composition to termite activity are largely unknown.
Canonical methanotrophs requiring oxygen can be differentiated into type I (Gammaproteobacteria) and type II (Alphaproteobacteria) on the basis of the pmoA gene phylogeny (4, 5). Type I methanotrophs include 15 genera to date, while 2 other genera, Methylocystis and Methylosinus, are grouped into type II. Methylocella, Methyloferula, and Methylocapsa are alphaproteobacterial methanotrophs, too, but they are phylogenetically distinct, belonging to the family Beijerinckiaceae. The physiology, biochemistry, and phylogeny of type I and type II methanotrophs have been reviewed repeatedly (6, 7). More recently, the physiological characteristics of type I and type II methanotrophs have been correlated to their life strategies (8). Methane monooxygenase (MMO) is the key enzyme in methane oxidation, existing as soluble (sMMO) and particulate (pMMO) forms. The pmoA and mmoX genes encode subunits of pMMO and sMMO, respectively, and have been used to examine methanotroph diversity in culture-independent studies (9, 10). Some methanotrophs have a particularly high affinity for methane and can oxidize methane at low (≤40 ppm by volume [ppmv]) to atmospheric (1.7 ppmv) concentrations (11–14). Besides a few cultivated Methylocystis spp., a plentitude of phylogenetically distinct pmoA sequences typically retrieved from forest, grassland, and meadow soils has been associated with atmospheric methane oxidation (15–17). The respective methanotrophs have so far remained resistant to isolation, but the pmoA sequences form clusters that can be affiliated with type I (upland soil cluster γ [USCγ], JR2, and JR3) and type II (USCα, RA14, and JR1) methanotrophs and a cluster positioned between characterized methane and ammonium monooxygenase (RA21).
In earlier studies of termite-derived methane emission, in situ gas flux was determined from entire termite mounds, implying that microbially mediated processes are homogenously distributed in the mound (18–20). However, termite activity concentrates in the nest area and may modify the immediate mound environment, thus creating different habitats within a mound. These modifications may have an adverse or stimulatory effect on microbial processes. Furthermore, for many fungus-growing termite mounds, in situ gas flux measurement is made challenging by the dimensions (diameter, ∼18 m; height, ∼5 m) of the mounds (21). In this study, we investigated the response of methane oxidation to termite activity along a vertical profile through a termite (Macrotermes falciger) mound covering its base, active nest area, and chimney in order to determine the sites with potential methanotrophic activity. Hypothesizing that termite activity leaves an imprint on the methanotrophic community structure, we determined the community in the active nest area and an adjacent soil which served as a reference site using pmoA-based diagnostic microarray analysis.
MATERIALS AND METHODS
Description of study sites.
The study site is located in the vicinity of Lubumbashi, Katanga Province, Democratic Republic of the Congo. The region forms part of the Miombo ecoregion, an area recognized to be of biological significance since the 2000s (22). The average areal density of M. falciger mounds in the region is about 3 mounds ha−1. The mounds are commonly in excess of 6 m high, have diameters of more than 20 m, and cover approximately 5% of the total land surface. The primary vegetation of the region is represented by Miombo woodlands. The rainfall distribution is unimodal, with the highest monthly averages occurring between November and March; the annual mean precipitation is 1,270 mm (23). The in situ nest temperature in this type of mound is typically 27°C to 30°C (24).
Sampling procedure.
Mound materials were sampled from two active M. falciger mounds (mound 1, 11°33′48.47″S, 27°29′53.79″E; mound 2, 11°29′22.21″S, 27°35′53.96″E) in June 2011. The physiochemical properties of termite mound material from the same area have been documented before (21, 25). The mounds were partially destroyed to sample a vertical profile through the chimney and nest and were left to recover for 3 months. Topsoil approximately 10 m from the mound served as a reference. Mound material was air dried, sieved at 2 mm, and stored at room temperature. In situ gas was sampled from different parts of the vertical profile (Table 1) in October 2011. To assess the active mound areas, holes were drilled to insert plastic tubes (diameter, 7 mm) fitted with filters (synthetic mesh) at the end. The tube was connected to a 60-ml syringe and a needle via a three-way valve acting as a sampling port. After inserting the tube, the hole was sealed with wet mound material (clay), the tubes were flushed using the syringe, and the setup was left to equilibrate for 1 h before sampling. Gas was collected in a preevacuated 12-ml gas-tight glass vial topped with a butyl rubber stopper in triplicate. The glass vials were transported back to the laboratory to determine CH4 and CO2 concentrations.
Table 1.
Sample description, characteristics, and methane uptake rates of termite mound materials and reference soilsf
| Sampling site | Sample description |
Nutrient content (mg kg−1 soil) |
Mean ±SD in situ gas mixing ratio (ppmv) |
Methane uptake rate (nmol g soil [dw]−1 h−1)d |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ht from surface (cm) | pH | TOCa (%) | Resin Pb | Total N | NH4+c | NO3−c | CO2 | CH4 | Preincubation | Subsequent incubation | |
| Termite mound 1 | |||||||||||
| Central chimney | 600 | 5.0 | 0.8 | 0.5 | 770.1 | 2.9 | 3.0 | 14,206 ± 994 | 21.2 ± 0.1 | NA | NA |
| Nest chimney | 500 | 8.2 | 0.5 | 3.2 | 668.5 | 2.1 | 2.5 | 15,322 ± 485 | 22.1 ± 0.4 | NA | NA |
| Active nest area | 450 | 8.2 | 0.6 | 10.6 | 669.3 | 2.1 | 21.2 | 11,044 ± 333 | 18.4 ± 2.0 | 0.052 ± 0.015 | 0.049 ± 0.003 |
| Below nest area | 400 | 7.9 | 0.5 | 4.4 | 1228.2 | 6.1 | 481.7 | NA | NA | NA | NA |
| Below nest area | 250 | 4.3 | 0.7 | 1.8 | 1611.5 | 3.0 | 1068.8 | NA | NA | NA | NA |
| Reference soile | 5.4 | 1.5 | 0.6 | 1069.6 | 7.0 | 0.6 | NA | NA | 0.019 ± 0.004 | 0.017 ± 0.003 | |
| Termite mound 2 | |||||||||||
| Central chimney | 300 | 6.6 | 0.6 | 0.5 | 733.9 | 2.9 | 9.8 | 23,968 ± 1,498 | 33.9 ± 2.0 | NA | NA |
| Nest chimney | 200 | 7.5 | 1.0 | 2.1 | 1037.5 | 2.4 | 26.2 | 22,830 ± 3,354 | 31.3 ± 3.8 | NA | NA |
| Active nest area | 150 | 8.1 | 1.3 | 2.5 | 957.5 | 2.2 | 45.0 | 17,936 ± 1,197 | 20.6 ± 1.5 | 0.085 ± 0.024 | 0.090 ± 0.008 |
| Below nest area | 100 | 8.4 | 0.7 | 4.4 | 710.2 | 1.9 | 48.9 | NA | NA | NA | NA |
| Mound base | 0 | 7.7 | 0.7 | 6.3 | 856.1 | 1.7 | 339.0 | NA | NA | NA | NA |
| Reference soile | 5.0 | 1.8 | 0.3 | 1195.5 | 3.7 | 1.2 | NA | NA | 0.023 ± 0.004 | 0.021 ± 0.004 | |
TOC, total organic carbon content.
Resin P, resin-extractable P, which is a good indicator of bioavailable P.
Total N determination (mg N kg−1 soil).
The methane uptake rate was determined in incubations with in situ methane concentrations. NA, data not available.
Reference soil was classified as a ferralsol according to the World Reference Base for Soil Resources of the Food and Agricultural Organization (FAO-WRB).
Additional physiochemical parameters of comparable mound material have been reported before (21).
Preliminary on-site flux measurements.
Preliminary batch incubations were performed in triplicate on-site using the active nest mound material, fungus comb (Termitomyces microcarpus), and worker termites, which were placed in 260-ml serum bottles at weights normalized to equal fresh weights. In all incubations, a moist filter paper was placed at the bottom of the serum bottle to increase humidity. Prior to incubation, ambient air was sampled for reference, the headspace was flushed, and the bottle was topped with a Teflon-coated rubber stopper. The bottles were incubated statically at 28°C in the dark. Changes in gaseous CH4 and CO2 concentrations were monitored over time (16 h). Gas was collected (volume, 13 ml) and stored in preevacuated glass vials for transport back to the laboratory to determine the gas concentrations.
Experimental setup.
Soil slurry batch incubations were set up in triplicate in 120-ml bottles containing 5 g mound material and 5 ml autoclaved deionized water. Methane was added to the headspace at 12% (vol/vol) in air, and the bottles were incubated at 28°C on a shaker (120 rpm) in the dark. In incubations under conditions with methane concentrations comparable to the in situ level, 5 g mound material and 5 ml autoclaved deionized water were mixed in 260-ml bottles, and methane was added at a concentration of approximately 40 ppmv (0.004% [vol/vol] methane) in the headspace. An additional incubation without mound material and with methane served as a control. Each incubation was performed in triplicate.
Determination of methane uptake and soil chemical parameters.
The amount of methane in the headspace was measured using a compact gas chromatograph (Covenant Analytical Solutions, Belgium) and gas chromatography with a flame ionization detector (Shimadzu, Japan) in incubations under conditions with high and in situ methane concentrations, respectively. In incubations under conditions with the in situ methane concentration, the methane uptake rate was determined by linear regression by following methane depletion over time (26 days) after preincubation (26 days). Preincubation was performed under the same conditions with 0.004% (vol/vol) methane in the headspace.
Resin-extractable P was determined using resin-impregnated membrane strips (26). Total N was determined using an elemental analysis-isotope ratio mass spectrometer (2020; SerCon, United Kingdom), whereas NH4+ and NO3− were determined in a 1 M KCl extract (ratio 2:1) using a continuous-flow autoanalyzer (Skalar, Chemlab, The Netherlands). Total organic carbon content (TOC) was determined using a TOC analyzer (TOC5050A; Shimadzu, Japan).
DNA extraction.
DNA was extracted from the starting material and the same material after incubation under conditions with the in situ methane concentration in triplicate using a Q-Biogene soil extraction kit (MP Biomedicals), according to the manufacturer's instructions, with a minor modification (27): three additional washing steps with 5.5 M guanidine thiocyanate (Sigma-Aldrich) were introduced after elution with the binding buffer to minimize coextraction of humic acids. DNA extracts were stored at −20°C until further analysis.
pmoA-based microarray analysis.
The microarray analysis was performed as described before (4, 27), with a minor modification. We performed a nested PCR to prepare the target for the microarray probes: the first PCR (30 cycles) was performed using the A189f/A682r primer combination, and 1 μl of PCR product was then used as the template for the subsequent PCR (30 cycles) using the A189f/T7_A682r primer combination. PCR was carried out in duplicate reactions for each DNA extract, and the PCR products were pooled during the cleanup step to minimize random errors. PCR was performed with three DNA extracts of each sample obtained from the starting material and after incubation (three independent batch incubations). Results are given as the averages of these triplicate analyses.
The microarray data were standardized against the mean total array intensity and then against the reference value for positive detection (4). The standardized microarray data were visualized as a heatmap, produced in R software, version 2.10.0 (28), using the heatmap.2 package implemented in the gplots package, version 2.7.4. Nonmetric multidimensional scaling (vegan package [29]) was used to summarize overall differences. An indicator species analysis (labdsv package [30]) helped identify probes indicative of specific habitats with a high probability (P < 0.05; Table 2).
Table 2.
Probes and corresponding taxonomic affiliations indicative of the active nest area and reference soil with a high probability (P ≤ 0.05), revealed by indicator species analysisa
| Sampling site, methanotroph type, and probe (taxonomic affiliation) | Indicator value |
|---|---|
| Active nest area, type I | |
| P_JR3.505 (upland grassland soil cluster) | 1.00 |
| O_501.286 (Methylococcus-like) | 0.97 |
| P_JR3.593 (upland grassland soil cluster) | 0.94 |
| O_BB51.299 (Methylobacter) | 0.91 |
| P_ML_SL.3.300 (Methylobacter) | 0.91 |
| LF1a.456 (Methylobacter-like) | 0.91 |
| DS2.287 (deep sea cluster) | 0.90 |
| Ib453 (type Ib, general) | 0.87 |
| P_Mb_LW12.211 (Methylobacter) | 0.85 |
| LP21.436 (pmoA2) | 0.85 |
| Kuro18.205 (deep sea cluster) | 0.73 |
| P_LK580 (Lake Konstanz sediment cluster) | 0.72 |
| Reference soil | |
| Type I | |
| P_OSC220 (Finnish soil clones) | 0.98 |
| P_MmES543 (Methylomonas) | 0.96 |
| LW14.639 (Methylosarcina-like) | 0.86 |
| Alp7.441 (Methylomonas-like) | 0.85 |
| P_JRC3.535 (Japanese rice cluster) | 0.83 |
| JHTY1.267 (Methylogaea-like) | 0.82 |
| P_Mb_C11.403 (Methylobacter) | 0.78 |
| MsQ290 (Methylosarcina-like) | 0.78 |
| O_fw1.641 (Methylococcus-like and Methylocaldum-like) | 0.56 |
| Type II | |
| O_II509 (type II, general) | 0.87 |
| P_McyM309 (Methylocystis) | 0.74 |
| P_Mcy270 (Methylocystis) | 0.63 |
Only data for probes targeting methanotrophs are shown.
Detection of other methanotrophs.
Besides the microarray analysis, PCRs targeting Methylocella-like mmoX (31), “Candidatus Methylomirabilis oxyfera”-like pmoA (32), and Methylacidiphilum-like pmoA belonging to the phylum Verrucomicrobia (33) were performed (Table 3).
Table 3.
Primer combinations used for detection of methanotrophs
| Primer set | PCR or methodology | Target gene | Target microorganism | Reference(s) |
|---|---|---|---|---|
| mmoXLF/mmoXLR | Nested PCR | mmoX | Methylocella genus specific | 31 |
| A189_b/cmo682 | Nested PCR | pmoA | “Candidatus Methylomirabilis oxyfera” specific (phylum NC10) | 32, 54 |
| Cmo182/cmo568 | ||||
| V170f/V613b | Direct PCR | pmoA | Methylacidiphilum specific (phylum Verrucomicrobia) | 33 |
| A189f/T7_A682r | Diagnostic microarray analysis | pmoA | Aerobic methanotrophs (general probe) | 4, 27 |
RESULTS AND DISCUSSION
The abiotic environment and methane uptake.
The methane concentrations in the mounds (20 to 35 ppmv) were higher than atmospheric levels (Table 1), as expected, and were comparable to the concentrations detected in other termite mounds (∼2 to 50 ppmv [20]). The carbon dioxide concentrations (1.1 × 104 to 2.4 × 104 ppmv) in the mounds were in the range detected in other Macrotermes mounds (0.25 × 104 to 5.2 × 104 ppmv), but the carbon dioxide concentrations have been demonstrated to fluctuate diurnally in these types of mounds (34, 35).
Preliminary batch measurements verified that M. falciger termites are a net methane source, while the mound material acted as a net methane sink (see Fig. S1 in the supplemental material). It remains unknown if any termites harbor methanotrophs, but so far, methane oxidizers have not been detected in the termite gut (2). Hence, we focused on the methanotrophic potential in the mound material. Methane uptake showed a biphasic pattern (Fig. 1) when mound material was incubated under conditions with high (12% [vol/vol]) methane concentrations, suggesting induced methanotrophic activity (36). Under these conditions, the potential for methane oxidation was higher around the active nest area (mound 1, 450 cm; mound 2, 150 cm; Fig. 1) and was detected only after 5 to 6 days in the reference soils. Although methane uptake was detected for other mound layers (Fig. 1), the material from the active nest reacted faster (shorter lag phase; ≤2 days), reflecting a higher abundance of viable methanotrophs. In termite mound 1, material from layers below the active nest did not exhibit methane uptake even after 26 days (Fig. 1). This coincides with the higher ammonium concentrations (Table 1) but does not explain the lack of activity, as methane uptake was detected in the reference soil containing even higher total ammonium. While it has often been reported that ammonium inhibits methane oxidation (37, 38), the reverse is true in some situations (39). Indeed, sensitivity to ammonium differs among methanotrophs (40, 41). The methanotrophic community compositions in termite mound and reference soils were dissimilar (Fig. 2 and 3; Table 2) and may explain the different responses of methane oxidation to soil ammonium concentrations.
Fig 1.
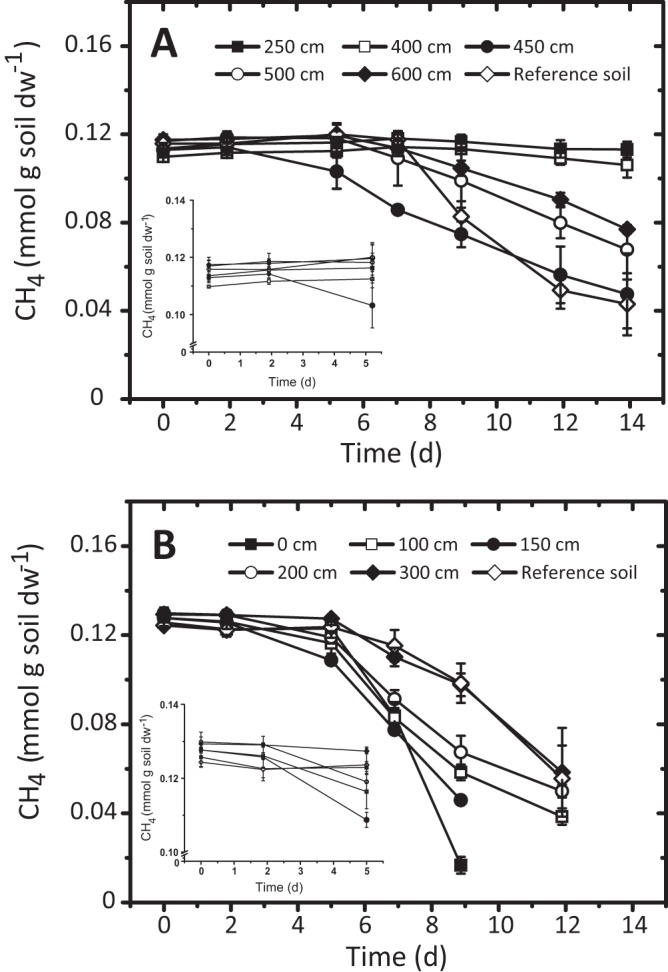
Methane uptake in incubations under conditions with high methane concentrations (12% [vol/vol]) in termite mounds 1 (A) and 2 (B). Sample positions through the vertical profiles are indicated by height (cm) above the ground (see the height for each sampling site in Table 1). The inset shows data for the first 5 days, demonstrating an earlier onset of methane uptake in the active nest material. Incubations for each profile were performed in triplicate, and the results are means ± standard deviations.
Fig 2.
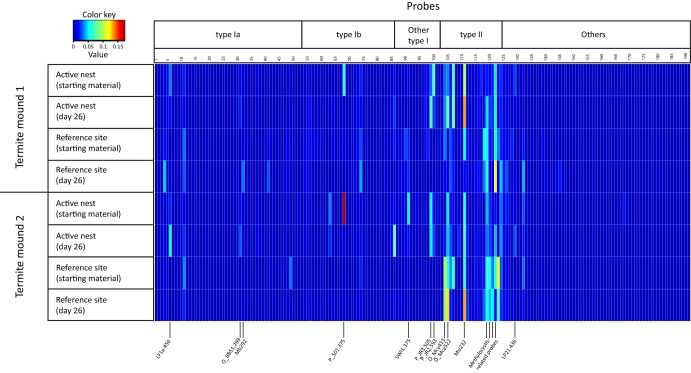
pmoA-based microarray analysis, visualized as a heatmap showing the diversity of the methanotroph community in the starting material and after incubation under conditions with in situ methane concentrations (26 days) for samples from the active nest area and reference soils, respectively. Probe names and their corresponding specificity are given elsewhere (54). The microarray analysis was performed in triplicate for each sample, and the results are shown here as averages. The color code indicates relative abundance, with red indicating a higher abundance. The probe covers type I and type II methanotrophs. Probes designated “others” are those that indicate amoA (encoding ammonia monooxygenase), pmoA2, verrucomicrobial methanotroph, and environmental sequences without known affiliations (between pmoA and amoA).
Fig 3.
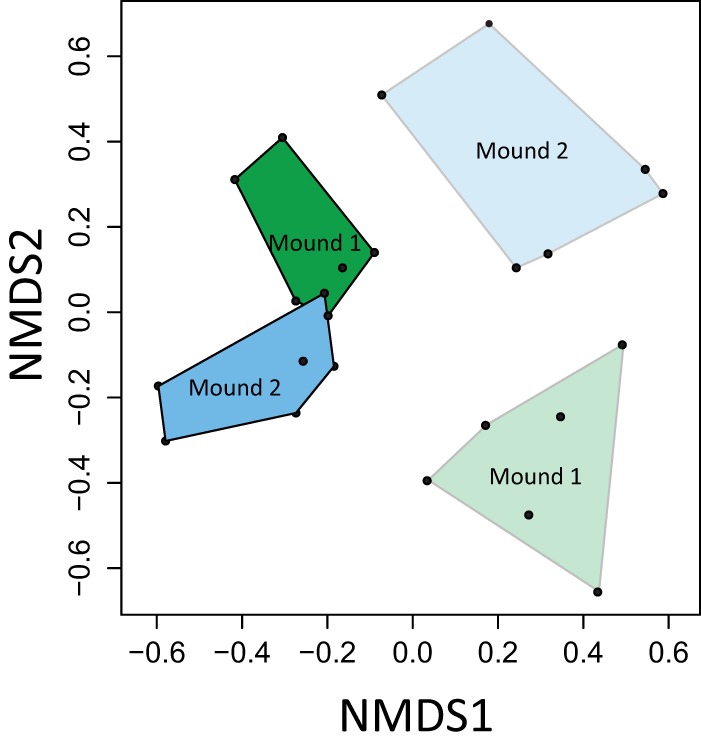
Nonmetric multidimensional scaling (NMDS) analysis of standardized microarray data (stress = 0.17). Green and blue, termite mounds 1 and 2, respectively. The light and dark shades indicate reference soil and active nest material, respectively.
Methane uptake rates: incubation under conditions with in situ methane concentrations.
Further incubations were performed using material from the active nest and the reference site under conditions with low methane concentrations (0.003% to 0.004% [vol/vol]) comparable to the in situ concentrations after preincubation under the same conditions (Fig. 4). The methane concentration showed a linear decrease during preincubation and subsequent incubation, indicating steady state, and reflects the in situ uptake trends. Methane uptake rates, determined by linear regression, were nearly identical during preincubation and subsequent incubation in both mounds (Table 1). The active nest materials showed different methane uptake rates, with mound 2 exhibiting values twice as high as those found in mound 1 (Table 1). However, in both mounds, the methane uptake rate was significantly higher in the active nest material than in the reference soil (t test; P ≤ 0.05). The methane uptake rates in the active nest materials and reference soils were determined to be 0.05 to 0.09 nmol g (dry weight [dw])−1 h−1 and about 0.02 nmol g (dw)−1 h−1, respectively. Hence, it appears that termite activity modifies the mound environment, enabling higher methane uptake.
Fig 4.
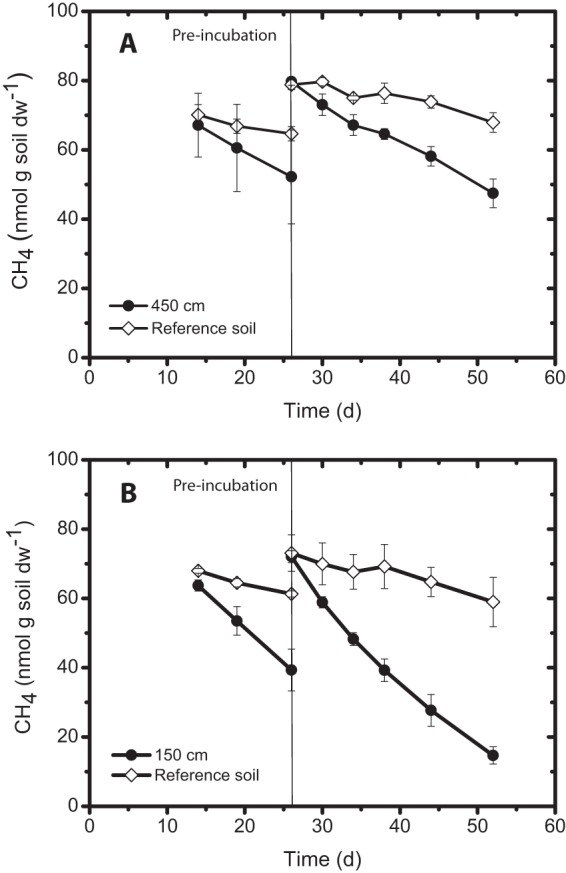
Methane uptake in incubations under conditions with in situ methane concentrations (0.004% [vol/vol]) in termite mounds 1 (A) and 2 (B). Incubations were performed with samples from the active nest area and their respective reference soils in triplicate, the the results are means ± standard deviations. Samples are indicated by height (cm) above the ground (see the height for each sampling site in Table 1). Incubation conditions were similar during preincubation and the subsequent incubation. The methane uptake rates determined from linear regression during these incubations are given in Table 1.
Consistent with previous studies (11, 42), methane uptake rates in incubations under conditions with in situ concentration (Fig. 4) were in the range (0.01 to 0.75 nmol g [dw]−1 h−1) found in various forest soils incubated under conditions with atmospheric methane concentrations. With largely comparable pHs, TOCs, and ammonium concentrations in mound materials exhibiting activity (Table 1), termites increase soil moisture and elevate methane levels in the mound. Higher methane availability has an additional effect on population dynamics. While the methane concentration itself may favor some methanotrophs, according to the affinity of their MMO, higher concentrations also increase the energy flow through a population, affecting the dynamics of the community (43). Methane was consumed in incubations under conditions with high methane concentrations. Besides atmospheric methane oxidizers, the mound material also harbored low-affinity methanotrophs, as confirmed by the microarray analysis (Fig. 2). These methanotrophs may benefit during the wet season, when the increased soil water content may stimulate methane production. Hence, the mound material harbored a versatile methanotroph community capable of methane oxidation both at high and at low concentrations.
Methanotrophic community composition.
A diagnostic microarray was used to determine the composition of methanotrophic communities in the starting material and after incubation (26 days) under conditions with in situ methane concentrations. The microarray analysis detects a wide range of known methanotrophs (44), including species belonging to Verrucomicrobia (45) and the enigmatic methane oxidizers (Crenothrix ployspora [46]). However, the microarray cannot detect methanotrophs lacking the pmoA gene; Methylocella and Methyloferula possess only the sMMO (47, 48). Hence, in addition to pmoA, the mmoX gene belonging to Methylocella-like methanotrophs was targeted (Table 3) but was not detected (data not shown). Furthermore, we could not amplify the pmoA gene of verrucomicrobial methanotrophs and anaerobic methane oxidizers belonging to the phylum NC10 (Table 3; data not shown). Therefore, we focused on the pmoA gene, amplified using the A189f/T7_A682r primer combination, which covers the vast majority of methanotrophs (49).
The methanotroph communities in the reference soils from both sites were dissimilar, but the compositions in the active nest material converged (Fig. 3). The ordination suggests the selection of a specific community, likely as a consequence of termite activity. Furthermore, we performed an indicator species analysis (Table 2) (50) to identify methanotrophs that are indicative of the mound material. This analysis considered the relative abundance and frequency, among other parameters (50), of probes occurring in the different sites. Interestingly, type I methanotrophs represented by upland grassland soil clusters (high-indicator-value probes P_JR3.505 and P_JR3.593 [15]), Methylobacter-like and Methylococcus-like methanotrophs, and pmoA2 were indicative of the active nest material. Previously, the upland grassland soil clusters were detected in other upland soils (15, 51) and thought to form the dominant population responsible for atmospheric methane oxidation in a desert soil (52). However, they were rarely detected in methane-emitting environments and are not as strictly correlated with environments which act as a sink for atmospheric methane as the USC groups (5, 16, 27, 53–55). Although putative atmospheric methane oxidizers (USCγ) (13) cluster within the Methylococcaceae, cultured Methylobacter and Methylococcus species have not been shown to oxidize methane at low or atmospheric concentrations; their role as atmospheric methane oxidizers remains elusive. However, it is not entirely unusual to codetect pmoA sequences belonging to these type I methanotrophs alongside putative atmospheric methane oxidizers, as was observed before (56, 57). pmoA2, an isozyme of pmoA belonging to type II methanotrophs (Methylocystis-Methylosinus group [58]), is also indicative of the mound, but the corresponding probe (LP21.436) had a relatively low indicator value (Table 2). In contrast, the presence of a higher diversity of type I methanotrophs (Methylomonas-, Methylobacter-, Methylosarcina-, Methylogaea-, Methylococcus-, and Methylocaldum-like methanotrophs and other uncultured soil clusters; Table 2) and type II methanotrophs, mainly characterized by Methylocystis species, was indicative of the reference soil. Furthermore, a discrepancy within the genus Methylobacter was detected in the active nest and reference soils (type Ia; probes LF1a.456, O_BB51.299, and Mb292; Table 2 and Fig. 2), but these methanotrophs represented only a minor fraction. Although the methanotrophs in the mound had developed from the indigenous methanotrophic community represented by the reference soil, it appears that termite activity selected for a specific community structure.
Overall, we show that termites modify their environment, allowing higher methane uptake. However, it is unclear if activity was confined to specific areas in the mound, but there was a tendency for higher activity in the active nest area. While the responses of methanotrophs to N amendments, methane, oxygen, and copper have been widely documented (7, 38, 59, 60), their responses to biotic factors are less well known (8, 61). Exemplifying the interaction of methanotrophs with their biotic environment, we provide a first insight into the methanotroph community and evidence for termite-facilitated selection/enrichment of the methanotroph community in M. falciger mounds. Hence, future studies resolving the active population which facilitates methane mitigation from termite mounds warrant attention.
Supplementary Material
ACKNOWLEDGMENTS
We extend our gratitude to Florias Mees (Royal Museum for Central Africa, Tervuren, Belgium) for proofreading the manuscript. We thank Levente Bodrossy (CSIRO, Tasmania, Australia) for introducing us to the microarray analysis and Claudia Lüke and Andreas Reim (Max Planck Institute, Marburg, Germany) for assistance with this type of analysis.
A.H. and N.B. are supported by research grants from Geconcerteerde Onderzoeksactie (GOA) project BOF09/GOA/005 of the Ghent University Special Research Fund. H.E. and E.V.R. are supported by the Fund for Scientific Research (FWO Flanders; G.0011.10N).
Footnotes
Published ahead of print 13 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02785-13.
REFERENCES
- 1.Intergovernmental Panel on Climate Change 2007. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 2.Pester M, Tholen A, Friedrich MW, Brune A. 2007. Methane oxidation in termite hindguts: absence of evidence and evidence of absence. Appl. Environ. Microbiol. 73:2024–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugimoto A, Inoue T, Kirtibutr N, Abe T. 1998. Methane oxidation by termite mounds estimated by the carbon isotopic composition of methane. Global Biogeochem. Cycles 12:595 [Google Scholar]
- 4.Bodrossy L, Stralis-Pavese N, Murrell JC, Radajewski S, Weilharter A, Sessitsch A. 2003. Development and validation of a diagnostic microbial microarray for methanotrophs. Environ. Microbiol. 5:566–582 [DOI] [PubMed] [Google Scholar]
- 5.Lüke C, Frenzel P. 2011. Potential of pmoA amplicon pyrosequencing for methanotroph diversity studies. Appl. Environ. Microbiol. 77:6305–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotsenko YA, Murrell JC. 2008. Metabolic aspects of aerobic obligate methanotrophy. Adv. Appl. Microbiol. 63:183–229 [DOI] [PubMed] [Google Scholar]
- 7.Semrau JD, DiSpirito AA, Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol. Rev. 34:496–531 [DOI] [PubMed] [Google Scholar]
- 8.Ho A, Kerckhof F-M, Luke C, Reim A, Krause S, Boon N, Bodelier PLE. 2013. Conceptualizing functional traits and ecological characteristics of methane-oxidizing bacteria as life strategies. Environ. Microbiol. Rep. 5:335–345 [DOI] [PubMed] [Google Scholar]
- 9.McDonald IR, Bodrossy L, Chen Y, Murrell JC. 2008. Molecular ecology techniques for the study of aerobic methanotrophs. Appl. Environ. Microbiol. 74:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebner S, Svenning MM. 2013. Environmental transcription of mmoX by methane-oxidizing proteobacteria in a subarctic palsa peatland. Appl. Environ. Microbiol. 79:701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender M, Conrad R. 1994. Methane oxidation activity in various soils and freshwater sediments: occurrence, characteristics, vertical profiles, and distribution on grain size fractions. J. Geophys. Res. 99:531–540 [Google Scholar]
- 12.Knief C, Dunfield PF. 2005. Response and adaptation of different methanotrophic bacteria to low methane mixing ratios. Environ. Microbiol. 7:1307–1317 [DOI] [PubMed] [Google Scholar]
- 13.Kolb S, Knief C, Dunfield PF, Conrad R. 2005. Abundance and activity of uncultured methanotrophic bacteria involved in the consumption of atmospheric methane in two forest soils. Environ. Microbiol. 7:1150–1161 [DOI] [PubMed] [Google Scholar]
- 14.Baani M, Liesack W. 2008. Two isozymes of particulate methane monooxygenase with different methane oxidation kinetics are found in Methylocystis sp. strain SC2. Proc. Natl. Acad. Sci. U. S. A. 105:10203–10208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horz H-P, Rich V, Avrahami S, Bohannan BJM. 2005. Methane-oxidizing bacteria in a California upland grassland soil: diversity and response to simulated global change. Appl. Environ. Microbiol. 71:2642–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolb S. 2009. The quest for atmospheric methane oxidizers in forest soils. Environ. Microbiol. Rep. 1:336–346 [DOI] [PubMed] [Google Scholar]
- 17.Shrestha PM, Kammann C, Lenhart K, Dam B, Liesack W. 2012. Linking activity, composition and seasonal dynamics of atmospheric methane oxidizers in a meadow soil. ISME J. 6:1115–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdonald J, Eggleton P, Bignell DE, Forzi F, Fowler D. 1998. Methane emission by termites and oxidation by soils, across a forest disturbance gradient in the Mbalmayo Forest Reserve, Cameroon. Glob. Change Biol. 4:409–418 [Google Scholar]
- 19.Jamali H, Livesley SJ, Dawes TZ, Hutley LB, Arndt SK. 2011. Termite mound emissions of CH4 and CO2 are primarily determined by seasonal changes in termite biomass and behaviour. Oecologia 167:525–534 [DOI] [PubMed] [Google Scholar]
- 20.Jamali H, Livesley SJ, Hutley LB, Fest B, Arndt SK. 2013. The relationships between termite mound CH4/CO2 emissions and internal concentration ratios are species specific. Biogeoscience 10:2229–2240 [Google Scholar]
- 21.Mujinya BB, Mees F, Erens H, Dumon M, Baert G, Boeckx P, Ngongo M, Van Ranst E. 2013. Clay composition and properties in termite mounds of the Lubumbashi area, D.R. Congo. Geoderma 192:304–315 [Google Scholar]
- 22.WWF 2012. Miombo eco-region “home of the Zambezi” conservation strategy: 2011–2020 WWF, Washington, DC: http://awsassets.panda.org/downloads/miombo_conservation_strategy_2011_2020.pdf [Google Scholar]
- 23.Malaisse F. 2010. How to live and survive in Zambezian open forest (Miombo ecoregion). Les Presses Agronomiques de Gembloux, Gembloux, Belgium [Google Scholar]
- 24.Korb J. 2003. Thermoregulation and ventilation of termite mounds. Naturwissenschaften 90:212–219 [DOI] [PubMed] [Google Scholar]
- 25.Mujinya BB, Van Ranst E, Verdoodt A, Baert G, Ngongo LM. 2010. Termite bioturbation effects on electro-chemical properties of ferralsols in the Upper Katanga (D.R. Congo). Geoderma 158:233–241 [Google Scholar]
- 26.Sharpley A. 2009. Bioavailable phosphorus in soil, p 38–41 In Kovar JL, Pierzynski GM. (ed), Methods of phosphorus analysis for soils, sediments, residuals, and waters, 2nd ed. Virginia Polytechnic and State University, Blacksburg, VA [Google Scholar]
- 27.Ho A, Lüke C, Frenzel P. 2011. Recovery of methanotrophs from disturbance: population dynamics, evenness and functioning. ISME J. 5:750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Development Core Team R 2012. R: a language and statistical computing environment, 2.15.1. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 29.Oksanen J, Blanchet F, Kindt R, Legendre P, O'Hara R, Simpson G. 2010. Vegan: community ecology package, 1.18-12. http://r-forge.r-project.org/projects/vegan/ [Google Scholar]
- 30.Roberts DW. 2013. Ordination and multivariate analysis for ecology: R package labdsv, 1.5-0. R Foundation for Statistical Computing; Vienna, Austria [Google Scholar]
- 31.Rahman MT, Crombie A, Chen Y, Stralis-Pavese N, Bodrossy L, Meir P, McNamara NP, Murrell JC. 2011. Environmental distribution and abundance of the facultative methanotroph Methylocella. ISME J. 5:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luesken FA, Zhu B, Van Alen TA, Butler MK, Diaz MR, Song B, Op den Camp HJM, Jetten MSM, Ettwig KF. 2011. pmoA primers for detection of anaerobic methanotrophs. Appl. Environ. Microbiol. 77:3877–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharp CE, Stott MB, Dunfield PF. 2012. Detection of autotrophic verrucomicrobial methanotrophs in a geothermal environment using stable isotope probing. Front. Microbiol. 3:303. 10.3389/fmicb.2012.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto T, Abe T. 1979. The role of termites in an equatorial rain forest ecosystem of West Malaysia. Oecologia 38:261–274 [DOI] [PubMed] [Google Scholar]
- 35.Korb J, Lisenmair KE. 2000. Ventilation of termite mounds: new results require a new model. Behav. Ecol. 11:486–494 [Google Scholar]
- 36.Steenbergh AK, Meima MM, Kamst M, Bodelier PLE. 2010. Biphasic kinetics of a methanotrophic community is a combination of growth and increased activity per cell. FEMS Microbiol. Ecol. 71:12–22 [DOI] [PubMed] [Google Scholar]
- 37.King G. 1997. Responses of atmospheric methane consumption by soils to global climate change. Glob. Change Biol. 3:351–362 [Google Scholar]
- 38.Bodelier PLE, Laanbroek HJ. 2004. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 47:265–277 [DOI] [PubMed] [Google Scholar]
- 39.Bodelier PL, Roslev P, Henckel T, Frenzel P. 2000. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 403:421–424 [DOI] [PubMed] [Google Scholar]
- 40.Poret-Peterson AT, Graham JE, Gulledge J, Klotz MG. 2008. Transcription of nitrification genes by the methane-oxidizing bacterium, Methylococcus capsulatus strain Bath. ISME J. 2:1213–1220 [DOI] [PubMed] [Google Scholar]
- 41.Noll M, Frenzel P, Conrad R. 2008. Selective stimulation of type I methanotrophs in a rice paddy soil by urea fertilization revealed by RNA-based stable isotope probing. FEMS Microbiol. Ecol. 65:125–132 [DOI] [PubMed] [Google Scholar]
- 42.Holmes A, Roslev P, McDonald IR, Iversen N, Henriksen K, Murrell JC. 1999. Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl. Environ. Microbiol. 65:3312–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krause S, Lüke C, Frenzel P. 2012. Methane source strength and energy flow shape methanotrophic communities in oxygen-methane counter-gradients. Environ. Microbiol. Rep. 4:203–208 [DOI] [PubMed] [Google Scholar]
- 44.Stralis-Pavese N, Abell GCJ, Sessitsch A, Bodrossy L. 2011. Analysis of methanotroph community composition using a pmoA-based microbial diagnostic microarray. Nat. Protoc. 6:609–624 [DOI] [PubMed] [Google Scholar]
- 45.Op den Camp HJM, Islam T, Stott MB, Harhangi HR, Hynes A, Schouten S, Jetten MSM, Birkeland N-K, Pol A, Dunfield PF. 2009. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 1:293–306 [DOI] [PubMed] [Google Scholar]
- 46.Stoecker K, Bendinger B, Schöning B, Nielsen PH, Nielsen JL, Baranyi C, Toenshoff ER, Daims H, Wagner M. 2006. Cohn's Crenothrix is a filamentous methane oxidizer with an unusual methane monooxygenase. Proc. Natl. Acad. Sci. U. S. A. 103:2363–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dedysh SN, Liesack W, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Bares A, Panikov MNS, Tiedje JM. 2000. Methylocella palustris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int. J. Syst. Evol. Microbiol. 50:955–969 [DOI] [PubMed] [Google Scholar]
- 48.Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN. 2011. Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int. J. Syst. Evol. Microbiol. 61:2456–2463 [DOI] [PubMed] [Google Scholar]
- 49.Bourne D, McDonald IR, Murrell JC. 2001. Comparison of pmoA PCR primer sets as tools for investigating methanotroph diversity in three Danish soils. Appl. Environ. Microbiol. 67:3802–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67:345–366 [Google Scholar]
- 51.Bissett A, Abell GCJ, Bodrossy L, Richardson AE, Thrall PH. 2012. Methanotrophic communities in Australian woodland soils of varying salinity. FEMS Microbiol. Ecol. 80:685–695 [DOI] [PubMed] [Google Scholar]
- 52.Angel R, Conrad R. 2009. In situ measurement of methane fluxes and analysis of transcribed particulate methane monooxygenase in desert soils. Environ. Microbiol. 11:2598–2610 [DOI] [PubMed] [Google Scholar]
- 53.Henneberger R, Lüke C, Mosberger L, Schroth MH. 2012. Structure and function of methanotrophic communities in a landfill-cover soil. FEMS Microbiol. Ecol. 81:52–65 [DOI] [PubMed] [Google Scholar]
- 54.Ho A, Vlaeminck SE, Ettwig KF, Schneider B, Frenzel P, Boon N. 2013. Revisiting methanotrophic communities in sewage treatment plants. Appl. Environ. Microbiol. 79:2841–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bodelier PLE, Meima-Franke M, Hordijk CA, Steenbergh AK, Hefting MM, Bodrossy L, von Bergen M, Seifert J. 20 June 2013. Microbial minorities modulate methane consumption through niche partitioning. ISME J. [Epub ahead of print.] 10.1038/ismej.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knief C, Lipski A, Dunfield P. 2003. Diversity and activity of methanotrophic bacteria in different upland soils. Appl. Environ. Microbiol. 69:6703–6714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh BK, Tate K. 2007. Biochemical and molecular characterization of methanotrophs in soil from a pristine New Zealand beech forest. FEMS Microbiol. Lett. 275:89–97 [DOI] [PubMed] [Google Scholar]
- 58.Tchawa Yimga M, Dunfield P, Ricke P, Heyer J, Liesack W. 2003. Wide distribution of a novel pmoA-like gene copy among type II methanotrophs, and its expression in Methylocystis strain SC2. Appl. Environ. Microbiol. 69:5593–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henckel T, Roslev P, Conrad R. 2000. Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil. Environ. Microbiol. 2:666–679 [DOI] [PubMed] [Google Scholar]
- 60.Ho A, Lüke C, Reim A, Frenzel P. 2013. Selective stimulation in a natural community of methane oxidizing bacteria: effects of copper on pmoA transcription and activity. Soil Biol. Biochem. 65:211–216 [Google Scholar]
- 61.Murase J, Frenzel P. 2008. Selective grazing of methanotrophs by protozoa in a rice field soil. FEMS Microbiol. Ecol. 65:408–414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.