Abstract
Mixed infections have important consequences for the ecology and evolution of host-parasite interactions. In vector-borne diseases, interactions between pathogens occur in both the vertebrate host and the arthropod vector. Spirochete bacteria belonging to the Borrelia burgdorferi sensu lato genospecies complex are transmitted by Ixodes ticks and cause Lyme borreliosis in humans. In Europe, there is a high diversity of Borrelia pathogens, and the main tick vector, Ixodes ricinus, is often infected with multiple Borrelia genospecies. In the present study, we characterized the pairwise interactions between five B. burgdorferi sensu lato genospecies in a large data set of I. ricinus ticks collected from the same field site in Switzerland. We measured two types of pairwise interactions: (i) co-occurrence, whether double infections occurred more or less often than expected, and (ii) spirochete load additivity, whether the total spirochete load in double infections was greater or less than the sum of the single infections. Mixed infections of Borrelia genospecies specialized on different vertebrate reservoir hosts occurred less frequently than expected (negative co-occurrence) and had joint spirochete loads that were lower than the additive expectation (inhibition). In contrast, mixed infections of genospecies that share the same reservoir hosts were more common than expected (positive co-occurrence) and had joint spirochete loads that were similar to or greater than the additive expectation (facilitation). Our study suggests that the vertebrate host plays an important role in structuring the community of B. burgdorferi sensu lato genospecies inside the tick vector.
INTRODUCTION
Most hosts are infected with multiple parasite species or parasite strains (1–3). Interactions among parasite taxa infecting the same host can take a variety of forms (4). Competition for limited host resources can result in the elimination of the less competitive parasite; for example, Wolbachia bacteria prevent dengue viruses, chikungunya viruses, and malaria parasites from infecting Aedes aegypti mosquitoes (5). Alternatively, the presence of one pathogen may facilitate opportunistic infection by another pathogen; for example, HIV and fungal/bacterial infections (6–8), intestinal helminth and malaria infections (9), or influenza virus and pneumococcal infections (10). Multiple infections involving genetically distinct clones of the same parasite species are of particular interest, because their interactions are important in shaping the evolution of parasite virulence and disease severity (11–13), as shown in malaria (14, 15) and Pasteuria infections (16). Characterizing the interactions among parasite taxa in multiply infected hosts represents a major challenge for understanding the epidemiology of infectious diseases. In the case of vector-borne diseases, this task is further complicated because the parasites interact in both the arthropod vector and the vertebrate host.
The Borrelia burgdorferi sensu lato complex is a group of tick-borne spirochete bacteria that cause Lyme borreliosis, the most common tick-borne disease in the Northern Hemisphere (17). In Europe, there are at least 10 different B. burgdorferi sensu lato genospecies (18–21). All of these spirochetes are vectored by the hard-bodied tick Ixodes ricinus and maintained in a variety of vertebrate reservoir hosts (mostly birds and small mammals) (22). Previous field surveys have repeatedly shown that questing I. ricinus ticks often carry multiple spirochete infections (21, 23), providing ample opportunity for interactions among Borrelia genospecies. While many studies report the incidence of single and multiple Borrelia infections in I. ricinus (reviewed in reference 21), to our knowledge only one study (24) has tested whether the incidence of multiple infections deviates from the random expectation. To better understand patterns of co-occurrence and abundance of these tick-borne pathogens, it is crucial that we compare their observed distributions to the random or neutral expectation (25, 26).
The specificity of Borrelia genospecies for their vertebrate reservoir hosts plays a key role in shaping the ecology of mixed Borrelia infections in both the host and the tick vector. Previous work has shown that B. burgdorferi sensu stricto, B. afzelii, and B. bavariensis are specific for rodents (27–30), whereas other genospecies, such as B. garinii and B. valaisiana, are specific for birds (30, 31). This host specificity appears to be mediated by the complement system of the vertebrate host (32, 33). Thus, the vertebrate complement system reduces the probability of encounter between rodent- and bird-adapted Borrelia genospecies. When encounters between maladapted coinfection partners do occur, the vertebrate immune system likely plays a key role in shaping the joint spirochete load in the tick vector. Spirochete growth rates may also influence the outcome of the mixed infection inside the tick vector. For example, the total spirochete population first expands following the larval blood meal and then declines during the larval premolting period (34), providing further opportunities for competitive interactions among Borrelia genospecies.
The purpose of this paper was to characterize the pattern of interactions among B. burgdorferi sensu lato genospecies in I. ricinus ticks. We sampled ticks from the same sampling site in Switzerland multiple times during a 3-year period and used this data set (7,400 nymphal ticks) to test whether double infections occurred more or less often than the random expectation. We limited our study to genospecies interactions in questing I. ricinus nymphs, because this stage is responsible for infecting the next generation of reservoir hosts and is therefore the most important stage from an epidemiological point of view. We predicted that mixed infections of rodent- and bird-adapted Borrelia genospecies (e.g., B. afzelii-B. garinii and B. afzelii-B. valaisiana) in I. ricinus nymphs would be relatively rare compared to the random expectation. We also predicted that the joint spirochete load of such mixed infections would be lower than the additive expectation, because we expected the host complement system to suppress the density of the maladapted coinfection partner. We predicted that mixed infections of B. burgdorferi sensu lato genospecies that use the same vertebrate reservoir hosts (e.g., B. garinii-B. valaisiana) would occur more often than expected by chance. For these mixed infections, we did not have a clear prediction for the joint spirochete load. However, as the tick vector presumably sets some upper limit on the spirochete load, we expected negative interactions between spirochete loads to be the norm. Detecting positive and negative patterns of species co-occurrence is an important first step toward understanding species interactions in general and competitive and facilitative interactions in particular. Although such comparative data cannot decipher the underlying causal mechanisms, they are critical for generating new hypotheses that can be tested further experimentally.
MATERIALS AND METHODS
Meta-analysis of Borrelia infection data from the same population of I. ricinus ticks.
The present study is a meta-analysis of seven independent sampling occasions that were conducted by Coralie Herrmann over the course of her Ph.D. thesis (35–38). The ticks collected on these sampling occasions were used in studies to quantify fat content in ticks and to test how temperature, humidity, and Borrelia infection influenced the physiology, behavior, and survival of I. ricinus nymphs (Table 1). Over a period of 3 years (2009 to 2011), 7,400 questing I. ricinus nymphs were collected from the same sampling site in Switzerland, and all of these ticks were processed in the same way with respect to quantification of spirochete load and Borrelia genospecies identification. Therefore, this data set presented a unique opportunity to test how B. burgdorferi sensu lato genospecies interact in the tick vector. After pooling the data from the sampling occasions, the large sample size gave us sufficient statistical power to test whether the frequency and spirochete load of mixed infections was different from the random expectation.
Table 1.
Description of the seven sampling occasions (A to G) which provided the data on Borrelia genospecies infections in I. ricinus nymphs
| Study | Season of collection | Yr of collection | Sample size | Treatment | Publication |
|---|---|---|---|---|---|
| A | Spring | 2009 | 500 | Survival under hot conditions | 35 |
| B | Spring | 2010 | 1,500 | Humidity attraction | 36 |
| C | Spring | 2010 | 2,250 | Survival under hot conditions | Unpublished |
| D | Fall | 2010 | 450 | Survival under cold conditions | Unpublished |
| E | Spring | 2011 | 900 | Fat content quantification | 38 |
| F | Spring | 2011 | 800 | Survival under cold conditions | 37 |
| G | Fall | 2011 | 1,000 | Survival under cold conditions | 37 |
Tick collection, spirochete load, and Borrelia genospecies identification.
The sampling site was a mixed forest situated 600 m above sea level on the south-facing slope of Chaumont Mountain, Neuchâtel, Switzerland (47°00′ N, 6°57′ E). Field sampling of questing ticks and subsequent molecular methods have been described in detail elsewhere (35, 36–38). Briefly, our molecular protocol consisted of two successive steps: (i) quantitative PCR (qPCR) to identify Borrelia-infected nymphs and to estimate the spirochete load, and (ii) reverse line blot (RLB) of the Borrelia-infected nymphs to identify the Borrelia genospecies. Thus, the sensitivity of our protocol depended on the ability of the qPCR to identify the Borrelia-infected ticks, whereas the ability to discriminate among the various Borrelia genospecies depended on the RLB. As the qPCR protocol estimated the total spirochete load, we do not have separate spirochete loads for each genospecies in the case of doubly infected nymphs.
We used the qPCR protocol from Schwaiger et al. (39), which targets the flagellin gene. For the RLB, we used the primers of Alekseev et al. (40) to amplify the variable spacer region between 2 repeated copies of the 23S and 5S ribosomal genes. The RLB protocol contains three general probes for B. burgdorferi sensu lato in addition to specific probes that allow us to detect each of the five B. burgdorferi sensu lato genospecies present at our study site: B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. valaisiana, and B. bavariensis (23). Our RLB protocol also contains a probe that allows us to detect the relapsing fever-like spirochete B. miyamotoi (23). However, in the present paper, we only consider the pairwise interactions between Borrelia genospecies belonging to the B. burgdorferi sensu lato complex.
Statistical methods. (i) Positive and negative co-occurrence of B. burgdorferi sensu lato genospecies.
We focused on interactions between pairs of B. burgdorferi sensu lato genospecies because higher-order interactions (i.e., triple infections) were exceedingly rare. Our study area contained five different B. burgdorferi sensu lato genospecies (B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. valaisiana, and B. bavariensis), resulting in 10 possible genospecies pairs. We used log-linear analysis to characterize the co-occurrence patterns between pairs of B. burgdorferi sensu lato genospecies in the tick vector. For each genospecies pair, the data consist of the counts of four groups of ticks: (i) uninfected, (ii) infected with genospecies A, (iii) infected with genospecies B, and (iv) infected with genospecies A and B. A log-linear analysis essentially consists of modeling these count data as a function of genospecies A (presence/absence), genospecies B (presence/absence), and the genospecies A-genospecies B interaction using a generalized linear model with a Poisson error distribution. The sign (positive or negative) and statistical significance of the interaction term test whether the two genospecies co-occurred more or less often than expected by chance. The advantage of log-linear analyses over chi-square tests is that the sign and magnitude of the interaction term provide a quantitative estimate of the genospecies co-occurrence pattern.
We used a two-step approach to hypothesis testing. In the first step, we used three-way log-linear analysis to test whether we were justified in pooling the data from the seven sampling occasions for each of the 10 B. burgdorferi sensu lato genospecies pairs. Specifically, we tested whether the genospecies co-occurrence pattern was the same among the seven sampling occasions by evaluating the three-way interaction term for sampling occasion, genospecies A, and genospecies B. In the second step, we used two-way log-linear analysis to test the co-occurrence pattern for each of the 10 genospecies pairs depending on the results of the first step. If the three-way interaction (sampling occasion-genospecies A-genospecies B) was not statistically significant, we pooled the data from the different sampling occasions and estimated the global two-way interaction. If the three-way interaction was statistically significant, we did not pool the data and analyzed the two-way interaction separately for each of the seven sampling occasions. We used the glm() function in R with a Poisson error distribution to run the log-linear analyses. All statistics were calculated with R for Mac OS X (41).
(ii) Spirochete load in doubly infected ticks (inhibition and facilitation).
We analyzed the subset of B. burgdorferi sensu lato-infected ticks (n = 1,731 infected nymphs − 35 B. miyamotoi-infected nymphs = 1,696 B. burgdorfer sensu lato-infected nymphs [Table 2]) to test whether the spirochete load of mixed infections deviated from the neutral (additive) expectation. For this analysis, we pooled the results of the seven sampling occasions to maximize our sample size and statistical power. The naive or null hypothesis of additivity assumes that the expected spirochete load in a doubly infected tick (X̄A∩B) is simply the sum of the mean spirochete loads of genospecies A (X̄A) and genospecies B (X̄B) in singly infected ticks (i.e., X̄A∩B = X̄A + X̄B). The two alternative hypotheses are inhibition and facilitation where the spirochete load of doubly infected ticks is less than (X̄A∩B < X̄A + X̄B) or greater than (X̄A∩B > X̄A + X̄B) the additive expectation.
Table 2.
Data on single and double infections
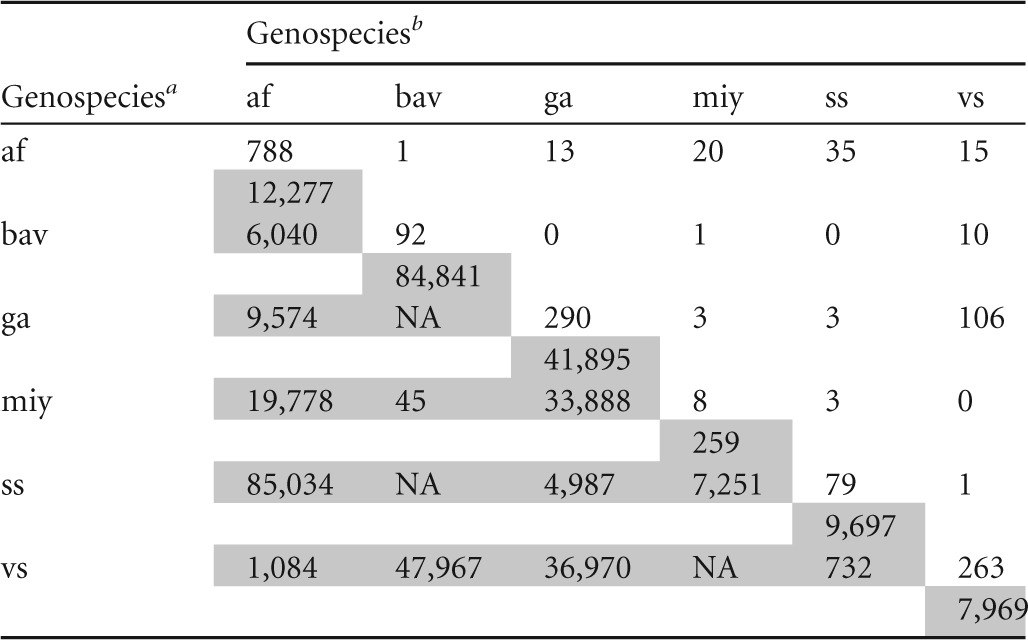
af, B. afzelii; bav, B. bavariensis; ga, B. garinii; miy, B. miyamotoi; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
Number of single and double infections (upper right, unshaded area) and the corresponding mean spirochete load (lower left, shaded area) for the six Borrelia genospecies (on the diagonal) and the 15 genospecies pairs (off the diagonal) in questing I. ricinus nymphs (n = 7,400) sampled in Neuchâtel, Switzerland. Some genospecies associations did not occur (n = 0), so the mean spirochete load was not available (NA).
For each of the 10 B. burgdorferi sensu lato genospecies pairs, we calculated the observed mean spirochete load (X̄A∩B) for the sample of coinfected ticks (nAB) (Table 2). To generate the null distribution of additivity, we randomly sampled (with replacement) nAB pairs of spirochete loads from the set of singly infected ticks for each genospecies in the pair. We summed each randomly sampled pair of spirochete loads to form the sample of joint spirochete loads and then calculated the expected mean spirochete load. We repeated this random sampling protocol 100,000 times to create a null distribution with sufficient precision for calculating P values. Thus, for each genospecies pair, the mean and variance of the null distribution were X̄A + X̄B and σA2 + σB2, respectively. We used the 2.5th and 97.5th percentiles from the null distribution as the 95% confidence limits (CL) of the mean expected spirochete load. To facilitate comparison among genospecies pairs, we divided the mean expected spirochete load and the 95% CL by the corresponding observed mean spirochete load. Genospecies pairs where the 95% CL of the expected mean/observed mean ratio overlap 1.0 exhibit additivity. Genospecies pairs where the 95% CL of the ratio are above or below 1.0 exhibit inhibition and facilitation, respectively.
RESULTS
Prevalence of Borrelia genospecies and spirochete loads.
Among the 7,400 questing nymphs sampled, there were 1,520 single, 211 double, and 10 triple infections. The triple infections were excluded from our analyses. B. afzelii was the most common genospecies, representing 50.4% of single and double infections (872/1,731) (Table 2), followed by B. garinii (24.0%; 415/1,731) and B. valaisiana (22.8%; 395/1,731). B. burgdorferi sensu stricto (7.0%; 121/1,731), B. bavariensis (5.4%; 94/1,731), and the relapsing fever-like spirochete B. miyamotoi (2.0%; 35/1,731) were less common. Among nymphs infected with a single Borrelia genospecies, the rank order of median spirochete load per tick was B. bavariensis (23,050), B. garinii (5,080), B. burgdorferi sensu stricto (3,410), B. afzelii (3,140), B. valaisiana (1,830), and B. miyamotoi (1,160).
Positive and negative co-occurrence of B. burgdorferi sensu lato genospecies.
The three-way log-linear analysis showed that the co-occurrence between B. burgdorferi sensu lato genospecies was the same across the seven sampling occasions for 9 of the 10 genospecies pairs (Table 3). This result means that we were justified in pooling the data of the seven sampling occasions and interpreting the global two-way interaction for these genospecies pairs. Of the nine genospecies pairs where pooling was justified, three showed positive co-occurrence and six showed negative co-occurrence (Table 4). Two of the three positive co-occurrences (B. afzelii-B. burgdorferi sensu stricto and B. garinii-B. valaisiana) and three of the six negative co-occurrences (B. afzelii-B. bavariensis, B. afzelii-B. garinii, and B. bavariensis-B. garinii) were statistically significant. Pooling was not justified for the B. afzelii-B. valaisiana genospecies pair (degrees of freedom [df] = 6; deviance = 20.232; P = 0.0025) (Table 3), and examination of the seven sampling occasions found six cases of negative co-occurrence (four were statistically significant) and one case of positive co-occurrence (not statistically significant) (data not shown).
Table 3.
Three-way log-linear analyses testing whether the pairwise co-occurrence between B. burgdorferi sensu lato genospecies differed among the seven sampling occasions for each of the 10 genospecies pairsc
| Genospeciesa |
df | Deviance | P value | |
|---|---|---|---|---|
| A | B | |||
| af | bav | 6 | 4.938 | 0.5518 |
| af | ga | 6 | 5.486 | 0.4832 |
| af | ss | 6 | 9.125 | 0.1667 |
| af | vs | 6 | 20.232 | 0.0025b |
| bav | ga | 6 | <0.001 | 1.0000 |
| bav | ss | 6 | <0.001 | 1.0000 |
| bav | vs | 6 | 1.849 | 0.9330 |
| ga | ss | 6 | 9.444 | 0.1501 |
| ga | vs | 6 | 7.750 | 0.2570 |
| ss | vs | 6 | 2.847 | 0.8278 |
af, B. afzelii; bav, B. bavariensis; ga, B. garinii; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
Only the af-vs genospecies pair had a significant three-way interaction after Bonferroni correction (P = 0.05/10 = 0.005).
Shown are the degrees of freedom, the deviance, and the P value testing whether the three-way interaction between sampling occasion, genospecies A, and genospecies B was statistically significant. After Bonferroni correction, α/n = 0.05/10 = 0.005 (where n is the number of pairwise comparisons).
Table 4.
Two-way log-linear analyses testing whether the pairwise associations between B. burgdorferi sensu lato genospecies are positive or negative when the seven independent sampling occasions are combined into a single data setd
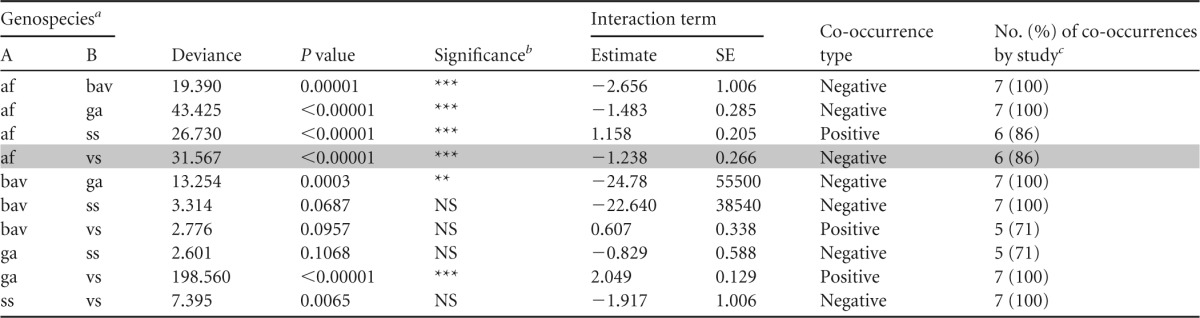
af, B. afzelii; bav, B. bavariensis; ga, B. garinii; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
Level of significance after Bonferroni correction: no significance (NS), P > 0.05/10 = 0.005; low significance (*), P = 0.05/10 = 0.005; intermediate significance (**), P = 0.01/10 = 0.001; high significance (***), P = 0.001/10 = 0.0001.
Number of times the co-occurrence type was observed out of the seven sampling occasions.
Shown are the deviance, the P value, and the estimate and standard errors (SE) of the two-way interaction term describing the pairwise associations between B. burgdorferi sensu lato genospecies. The co-occurrence type column indicates whether the genospecies pair exhibits positive co-occurrence (two-way interaction is positive) or negative co-occurrence (two-way interaction is negative). The by-study column indicates the number of studies where the sign of the two-way interaction was in the same direction (positive or negative) as the overall estimate (estimate column). The three-way log-linear analysis (Table 3) found a significant effect of study on the pairwise association for the B. afzelii-B. valaisiana genospecies pair (shaded gray).
Spirochete load in doubly infected ticks (inhibition and facilitation).
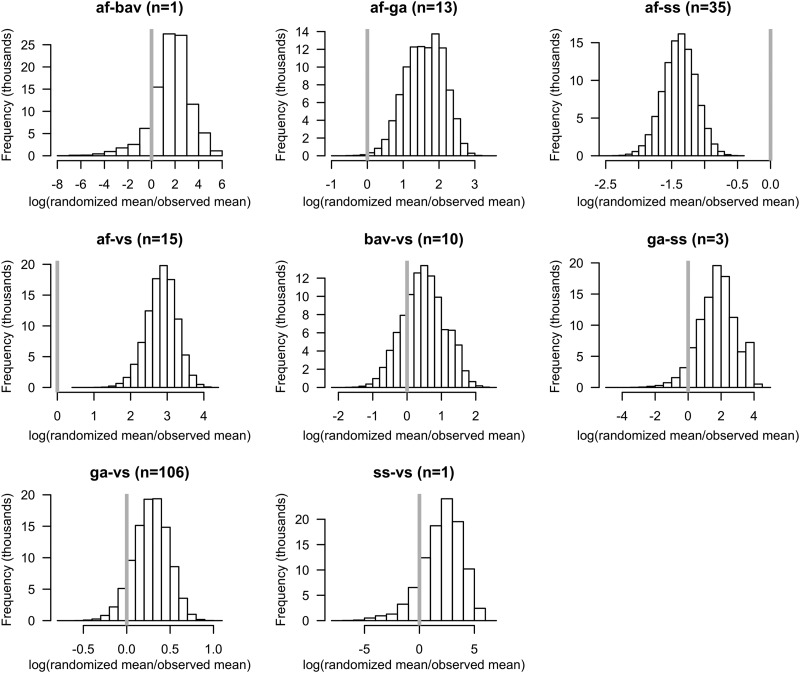
We used simulations to describe the spirochete load of nymphal ticks infected with two B. burgdorferi sensu lato genospecies. Specifically, for each genospecies pair we set out to test whether the joint spirochete load observed in doubly infected ticks was unusual relative to null distribution. We generated the null distribution by randomly sampling and then summing the spirochete loads of the singly infected ticks belonging to each member of the genospecies pair. We calculated a null distribution for 8 of the 10 genospecies pairs (Fig. 1). Two genospecies pairs were excluded (B. bavariensis-B. burgdorferi sensu stricto and B. bavariensis-B. garinii) because they had no doubly infected ticks. Of the eight remaining genospecies pairs, two pairs (B. afzelii-B. garinii and B. afzelii-B. valaisiana) showed inhibition (95% CL of the ratio > 1.0), one pair (B. afzelii-B. burgdorferi sensu stricto) showed facilitation (95% CL of the ratio < 1.0), and the remaining five pairs showed additivity (Fig. 1 and Table 5). After Bonferroni correction, three of the eight spirochete load interactions remained statistically significant (Table 5). Spirochete loads in nymphs with double infections of B. afzelii-B. garinii or B. afzelii-B. valaisiana were 6 to 19 times lower than the additive expectation. In contrast, spirochete loads of double infections of B. afzelii-B. burgdorferi sensu stricto were four times greater than the additive expectation.
Fig 1.
Distribution of the expected B. burgdorferi sensu lato spirochete load under the null hypothesis of additivity for eight genospecies pairs. To facilitate visualization of the null distribution, expected values were divided by the observed value and subsequently log transformed. Applying this transformation sets the observed value to zero (vertical gray line). Null distributions centered on zero indicate additivity. Null distributions centered on positive values (right-shifted) indicate inhibition among genospecies pairs. Null distributions centered on negative values (left-shifted) indicate facilitation. Abbreviations: af, B. afzelii; bav, B. bavariensis; ga, B. garinii; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
Table 5.
Results from the randomization protocol testing the null hypothesis of additivity of B. burgdorferi sensu lato spirochete loadb
| Genospeciesa | Genospecies |
||||
|---|---|---|---|---|---|
| af | bav | ga | ss | vs | |
| af | 16.137 (0.08–82.59) | 5.668 (1.71–12.47) | 0.259 (0.15–0.40) | 18.732 (7.39–36.25) | |
| bav | 0.2428 | NA | NA | 1.938 (0.50–4.92) | |
| ga | 0.0040 | NA | 10.350 (0.55–42.63) | 1.349 (0.88–1.90) | |
| ss | <0.0002 | NA | 0.1206 | 24.117 (0.10–134.43) | |
| vs | <0.0002 | 0.4444 | 0.1660 | 0.2512 | |
af, B. afzelii; bav, B. bavariensis; ga, B. garinii; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
Entries above the diagonal show the ratio of the mean expected spirochete load to the mean observed spirochete load; the 95% confidence limits are in parentheses (the 2.5th and the 97.5th quantiles from the null distribution of expected spirochete loads divided by the observed spirochete load). Ratios with 95% confidence limits that overlap 1.0 indicate additivity, ratios with 95% confidence limits of less than 1.0 indicate facilitation, and ratios with 95% confidence limits greater than 1.0 indicate inhibition. Entries below the diagonal show the proportion of randomized values that were more extreme than the observed mean spirochete load multiplied by 2.0 (two-tailed test). The randomization protocol was not possible for two genospecies pairs, and these are described as not available (NA). For the remaining eight genospecies pairs, we used a Bonferroni-corrected α level of 0.05/8 = 0.00625 to determine the statistical significance (shown in boldface).
Relationship between co-occurrence and spirochete load inhibition in doubly infected ticks.
B. burgdorferi sensu lato genospecies pairs fell into two broad categories when jointly considering the two types of interactions measured in this study (Fig. 2). Genospecies pairs that had negative co-occurrence had combined spirochete loads that were lower than expected (stronger inhibition), whereas genospecies pairs with positive co-occurrence had spirochete loads that met or surpassed the additive expectation (weaker inhibition or facilitation). Thus, broadly speaking, B. burgdorferi sensu lato genospecies pairs either fell in the negative co-occurrence/inhibition or the positive co-occurrence/facilitation quadrant (Fig. 2). Across the set of eight genospecies pairs, there was a significant negative correlation between the two types of interactions (Pearson r = −0.851; t = −3.97; df = 6; P = 0.0074).
Fig 2.
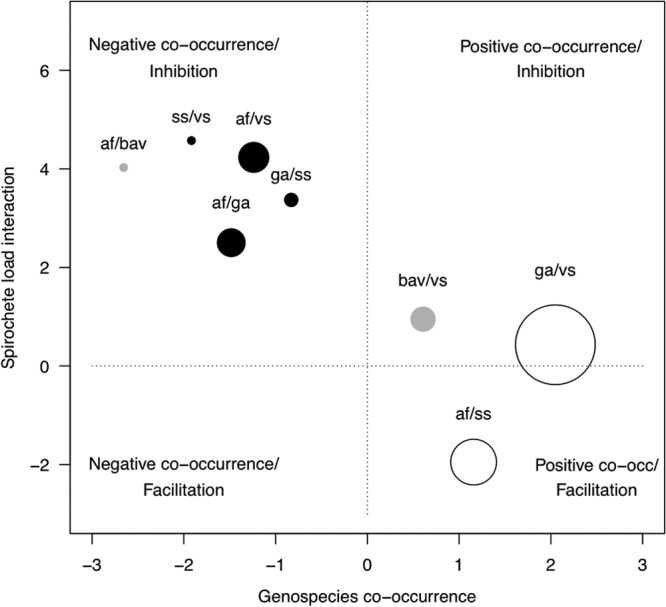
Relationship between our two measures of interaction for the eight pairs of B. burgdorferi sensu lato genospecies. Genospecies co-occurrence refers to whether double infections occurred more or less often than expected (positive or negative). The spirochete load interaction refers to whether the total spirochete load in the doubly infected ticks was greater or less than the sum of the single infections (facilitation or inhibition). The circle size refers to the sample size of double infections for each genospecies pair (Table 2). Solid black dots and open dots refer to genospecies pairs for which we had a priori predictions of negative and positive co-occurrence, respectively. Solid gray dots refer to genospecies pairs for which we had no a priori predictions for the co-occurrence pattern. The dotted lines (0 on x and y axes) refer to the null hypotheses of independence (i.e., the frequency of double infections equals the product of the frequencies of single infections) and additivity (i.e., spirochete load of double infection equals the sum of the single infections). To facilitate the graphing of the spirochete interaction, we log transformed the ratios shown in Table 5 (negative values indicate ratios of <1 [representing facilitation], whereas positive values indicate ratios of >1 [inhibition]). Abbreviations: af, B. afzelii; bav, B. bavariensis; ga, B. garinii; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
DISCUSSION
To our knowledge, this is the first study to test the frequency and spirochete load of mixed B. burgdorferi sensu lato infections in the epidemiologically relevant nymphal stage of I. ricinus against the background null hypotheses of random species co-occurrence patterns and additive spirochete loads in double infections. The value of our statistical approach was that it allowed us to quantify the degree of co-occurrence and the degree of spirochete load inhibition between co-infection partners. Plotting these measures of co-occurrence and spirochete load inhibition revealed broad patterns of interactions between B. burgdorferi sensu lato genospecies pairs (Fig. 2). Common coinfections have higher-than-expected joint spirochete loads inside the nymphal tick (Fig. 2). B. burgdorferi sensu lato genospecies that share the same vertebrate reservoir hosts (B. afzelii-B. burgdorferi sensu stricto and B. garinii-B. valaisiana) frequently occur together and exhibit weak inhibition and even facilitation with respect to the spirochete load inside the nymphal tick. Conversely, B. burgdorferi sensu lato genospecies pairs that are specialized on different vertebrate reservoir hosts (B. afzelii-B. garinii, B. afzelii-B. valaisiana, B. garinii-B. burgdorferi sensu stricto, and B. burgdorferi sensu stricto-B. valaisiana) rarely occur together and exhibit strong inhibition with respect to spirochete load. This negative association between occurrence and spirochete load inhibition is likely driven by the vertebrate immune system. Specifically, the vertebrate complement system, which is present in the tick midgut (42), is capable of lysing B. burgdorferi sensu lato spirochetes that are not adapted to that particular vertebrate host (32, 33). In cases where the complement system fails to eliminate the maladapted coinfection partner, the spirochete load of the latter likely would be much reduced, resulting in the observed pattern of inhibition in the nymphal tick. For example, when the same larva takes multiple blood meals from different vertebrate hosts after interrupted attachments (43, 44), the complement system of the second host could reduce the spirochete load of the Borrelia pathogen from the first host. Thus, the vertebrate immune system is the most likely explanation as to why coinfections involving rodent- and bird-adapted B. burgdorferi sensu lato genospecies exhibit lower-than-expected spirochete loads. Our results for the more common genospecies associations correspond to what has previously been reported in the literature (17, 21, 24). Rauter and Hartung (21) reported that B. garinii-B. valaisiana was the most common mixed infection of I. ricinus ticks, but they did not test whether mixed infections occurred more or less often than the random expectation. Kurtenbach et al. (24) used a simple chi-square test to show that B. garinii-B. valaisiana and B. afzelii-B. garinii mixed infections were more and less common than the random expectation in a variety of European countries. However, their study focused on adult ticks, which mostly feed on incompetent hosts such as deer and do not contribute significantly to the epidemiology of Lyme borreliosis.
The specificity of rodent and avian-adapted Borrelia genospecies for their respective reservoir hosts is not perfect. Our study found that double infections between rodent and avian-adapted Borrelia genospecies do occur (Table 2), and we suggest four mechanisms. First, some B. burgdorferi sensu lato strains might be generalists that are capable of infecting both rodent and bird reservoir hosts. For example, a recent field study on the Siberian chipmunk found one double infection with B. afzelii and B. garinii (45). Second, larvae may acquire a double infection via a combination of systemic and cofeeding transmission (46). Systemic transmission refers to the standard mode where ticks acquire spirochetes that have established a widespread and long-term infection in the host. Cofeeding transmission refers to the process where hosts are not systemically infected but instead function as a temporary bridge (47) that facilitates transmission between infected and uninfected ticks feeding in close proximity to each other (48). Third, double infections may result from a combination of vertical (transovarial) and horizontal (blood meal) transmission of Borrelia genospecies, although the former is believed to be rare or nonexistent in Ixodes ticks (49–51). Fourth, larval ticks taking multiple blood meals from different hosts (interrupted blood meals) (44) could also produce double infections of rodent- and bird-specific genospecies (43). These four mechanisms illustrate the diversity of transmission pathways that can produce ticks doubly infected with rodent- and bird-adapted Borrelia genospecies. These double infections are of interest because they connect the avian and rodent Lyme borreliosis systems and provide opportunities for genetic exchange between Borrelia genospecies.
Importantly, while the prevalence of B. burgdorferi sensu lato genospecies often fluctuates through time and space, the nature of the pairwise interaction appeared to be robust. For 6 of 10 genospecies pairs, the nature of the pairwise interaction was always in the same direction for each of the seven sampling occasions (Table 4), despite the fact that the questing nymphs had been collected in different years and seasons and had been exposed to different abiotic conditions prior to B. burgdorferi sensu lato genospecies identification (Table 1). Similarly, the fact that the three-way interaction was not statistically significant for 9 of the 10 genospecies pairs (Table 3) indicates that the different experimental conditions of the seven sampling occasions did not bias the co-occurrence patterns of the B. burgdorferi sensu lato genospecies. Thus, an important aspect of this study is our demonstration that the genospecies associations appear to be relatively constant over time at our site.
Most of the double infections had spirochete loads that were considerably lower than the additive expectation, suggesting that genospecies compete for limited resources in the tick (i.e., for seven of the eight genospecies pairs shown in Table 5, the average ratio of the mean expected spirochete load to the mean observed spirochete load is greater than 1, suggesting inhibition). The two statistically significant inhibition interactions include the B. afzelii-B. garinii and the B. afzelii-B. valaisiana genospecies pairs. As previously discussed, this inhibition was likely caused by the host complement system suppressing the spirochete load of the maladapted coinfection partner. In contrast, the only statistically significant facilitation interaction involved two genospecies, B. afzelii-B. burgdorferi sensu stricto, that are both adapted to rodent reservoir hosts (28–30, 52). One possible explanation for facilitation is that one genospecies suppresses the host immune system and thereby enhances the infection and transmission success of its coinfection partner.
Our approach of using qPCR to detect Borrelia infections followed by RLB to determine the community of B. burgdorferi sensu lato genospecies had its advantages and disadvantages. Advantages of our approach include low cost, relative simplicity, and time efficiency. Our qPCR protocol gives reliable estimates of the spirochete load, because the median spirochete load in the present study (3,200 spirochetes/tick across all Borrelia genospecies) was similar to that in another study (4,000 spirochetes/tick) on B. burgdorferi sensu lato infections in I. ricinus ticks (53). Our RLB protocol gives reliable identification of the B. burgdorferi sensu lato genospecies and B. miyamotoi, because there were only 3 out of 1,731 Borrelia-infected ticks (as detected by qPCR) where the PCR product failed to hybridize with a genospecies-specific probe (subsequent sequencing revealed two B. afzelii and one B. garinii infection). The reverse situation, where the RLB protocol identifies infections in ticks but the qPCR fails to detect them, may also occur (unpublished data). One major advantage of RLB is that the technique allows identification of mixed infections in samples. A disadvantage of our approach using qPCR is that we could not estimate separate spirochete loads for each partner in the mixed infections. Thus, in the case of inhibition or facilitation, we do not know which of the two B. burgdorferi sensu lato genospecies reduced or increased their spirochete load in the mixed infection relative to the single infection. Future studies should use next-generation sequencing approaches that can identify all possible B. burgdorferi sensu lato genospecies and estimate genospecies-specific spirochete loads. In addition, experimental infections would greatly clarify the underlying mechanisms of the pairwise interactions observed in the present study.
In addition to B. burgdorferi sensu lato genospecies, the RLB identified a low prevalence of the relapsing fever-like spirochete B. miyamotoi (2.0%; 35/1,731) in our I. ricinus population (Table 2). The identity of this relapsing fever-like spirochete has been confirmed with DNA sequencing of the flagellin gene and the 16S rRNA genes of samples hybridizing with the B. miyamotoi RLB probe (23). Screening ticks for Borrelia infection using only the RLB protocol (i.e., no upstream qPCR identification of infected ticks) found that between 0.5% (7/1,324) (unpublished data) and 5.3% (5/94) (23) of nymphs collected in the same forest were infected with B. miyamotoi, showing that the prevalence rates of B. miyamotoi are similar regardless of the protocol. In the present study, double infections with B. miyamotoi and rodent-specialized genospecies such as B. afzelii (n = 20) were more common than double infections with bird-specialized genospecies such as B. garinii (n = 3) (Table 2). A recent study conducted on field-captured rodents in Switzerland (C. Burri, O. Schumann, C. Schumann, and L. Gern, unpublished data) showed that rodents are relevant reservoir hosts for B. miyamotoi and B. afzelii, which explains the high number of double infections involving these two genospecies. Alternatively, the co-occurrence of these two genospecies in nymphs may be due to a combination of transovarial transmission of B. miyamotoi (54) and horizontal transmission of B. afzelii.
In conclusion, the present study found that the co-occurrence and joint spirochete load of rodent- and bird-adapted B. burgdorferi sensu lato genospecies in I. ricinus nymphs both were lower than the neutral expectation. This observation is consistent with the theory that the vertebrate immune system plays an important role in structuring the B. burgdorferi sensu lato genospecies community in the I. ricinus tick vector. Conversely, B. burgdorferi sensu lato genospecies that are specialized on the same reservoir host co-occurred more often than expected from chance, and their joint spirochete loads followed or exceeded the additive expectation, suggesting facilitation. Future experimental infections will further elucidate the mechanisms shaping the community ecology of B. burgdorferi sensu lato genospecies inside the tick vector.
Supplementary Material
ACKNOWLEDGMENTS
This study was part of the Ph.D. thesis of C. Herrmann and was supported by the Swiss National Science Foundation grant (FN 320030_113936 and FN 310030_127064) and the Canton de Neuchâtel, Switzerland.
We thank Blake Matthews, Jacob Koella, Caroline Burri, and two anonymous reviewers for comments on the manuscript.
Footnotes
Published ahead of print 13 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02158-13.
REFERENCES
- 1.Petney TN, Andrews RH. 1998. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 28:377–393 [DOI] [PubMed] [Google Scholar]
- 2.Read AF, Taylor LH. 2001. The ecology of genetically diverse infections. Science 292:1099–1102 [DOI] [PubMed] [Google Scholar]
- 3.Rigaud T, Perrot-Minnot M-J, Brown MJF. 2010. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. R. Soc. B Biol. Sci. 277:3693–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330:243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139:1268–1278 [DOI] [PubMed] [Google Scholar]
- 6.Diedrich CR, Flynn JL. 2011. HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect. Immun. 79:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egusa H, Soysa NS, Ellepola AN, Yatani H, Samaranayake LP. 2008. Oral candidosis in HIV-infected patients. Curr. HIV Res. 6:485–499 [DOI] [PubMed] [Google Scholar]
- 8.Graham SM. 2005. Non-tuberculosis opportunistic infections and other lung diseases in HIV-infected infants and children. Int. J. Tuber. Lung Dis. 9:592–602 [PubMed] [Google Scholar]
- 9.Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen P, Looareesuwan S. 2002. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J. Parasitol. 88:55–58 [DOI] [PubMed] [Google Scholar]
- 10.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Normark BH. 2010. Coinfection and the evolution of influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 202:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank SA. 1996. Models of parasite virulence. Q. Rev. Biol. 71:37–78 [DOI] [PubMed] [Google Scholar]
- 12.May RM, Nowak MA. 1995. Coinfection and the evolution of parasite virulence. Proc. R. Soc. B Biol. Sci. 261:209–215 [DOI] [PubMed] [Google Scholar]
- 13.Nowak MA, May RM. 1994. Superinfection and the evolution of parasite virulence. Proc. R. Soc. B Biol. Sci. 255:81–89 [DOI] [PubMed] [Google Scholar]
- 14.Bell AS, De Roode JC, Sim D, Read AF. 2006. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution 60:1358–1371 [PubMed] [Google Scholar]
- 15.de Roode JC, Pansini R, Cheesman SJ, Helinski MEH, Huijben S, Wargo AR, Bell AS, Chan BHK, Walliker D, Read AF. 2005. Virulence and competitive ability in genetically diverse malaria infections. Proc. Natl. Acad. Sci. U. S. A. 102:7624–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Ami F, Mouton L, Ebert D. 2008. The effects of multiple infections on the expression and evolution of virulence in a Daphnia-endoparasite system. Evolution 62:1700–1711 [DOI] [PubMed] [Google Scholar]
- 17.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129:S191–S220 [DOI] [PubMed] [Google Scholar]
- 18.Casjens SR, Fraser-Liggett CM, Mongodin EF, Qiu W-G, Dunn JJ, Luft BJ, Schutzer SE. 2011. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J. Bacteriol. 193:1489–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotte V, Bonnet S, Cote M, Vayssier-Taussat M. 2010. Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis. 10:723–730 [DOI] [PubMed] [Google Scholar]
- 20.Margos G, Vollmer SA, Cornet M, Garnier M, Fingerle V, Wilske B, Bormane A, Vitorino L, Collares-Pereira M, Drancourt M, Kurtenbach K. 2009. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 75:5410–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauter C, Hartung T. 2005. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl. Environ. Microbiol. 71:7203–7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gern L, Humair P-F. 2002. Ecology of Borrelia burgdorferi sensu lato in Europe, p 149–174 Lyme borreliosis: biology, epidemiology, and control. CAB International, Wallingford, United Kingdom [Google Scholar]
- 23.Gern L, Douet V, Lopez Z, Rais O, Moran Cadenas F. 2010. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick Borne Dis. 1:23–29 [DOI] [PubMed] [Google Scholar]
- 24.Kurtenbach K, De Michelis S, Sewell HS, Etti S, Schafer SM, Hails R, Collares-Pereira M, Santos-Reis M, Hanincova K, Labuda M, Bormane A, Donaghy M. 2001. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl. Environ. Microbiol. 67:4926–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clay K, Klyachko O, Grindle N, Civitello D, Oleske D, Fuqua C. 2008. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol. Ecol. 17:4371–4381 [DOI] [PubMed] [Google Scholar]
- 26.Gotelli NJ. 2000. Null model analysis of species co-occurrence patterns. Ecology 81:2606–2621 [Google Scholar]
- 27.Huegli D, Hu CM, Humair P-F, Wilske B, Gern L. 2002. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J. Clin. Microbiol. 40:4735–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humair P-F, Gern L. 1998. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 69:213–227 [DOI] [PubMed] [Google Scholar]
- 29.Humair P-F, Péter O, Wallich R, Gern L. 1995. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J. Med. Entomol. 32:433–438 [DOI] [PubMed] [Google Scholar]
- 30.Kurtenbach K, Peacey M, Rijpkema SGT, Hoodless AN, Nuttall PA, Randolph SE. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humair PF, Postic D, Wallich R, Gern L. 1998. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentralbl. Bakteriol. 287:521–538 [PubMed] [Google Scholar]
- 32.Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74–79 [DOI] [PubMed] [Google Scholar]
- 33.Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, Nuttall PA. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piesman J, Oliver JR, Sinsky RJ. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 42:352–357 [DOI] [PubMed] [Google Scholar]
- 35.Herrmann C, Gern L. 2010. Survival of Ixodes ricinus (Acari: Ixodidae) under challenging conditions of temperature and humidity is influenced by Borrelia burgdorferi sensu lato infection. J. Med. Entomol. 47:1196–1204 [DOI] [PubMed] [Google Scholar]
- 36.Herrmann C, Gern L. 2012. Do the level of energy reserves, hydration status and Borrelia infection influence walking by Ixodes ricinus (Acari: Ixodidae) ticks? Parasitology 139:330–337 [DOI] [PubMed] [Google Scholar]
- 37.Herrmann C, Gern L. 2013. Survival of Ixodes ricinus (Acari: Ixodidae) nymphs under cold conditions is negatively influenced by frequent temperature variations. Ticks Tick Borne Dis. 4:445–451 [DOI] [PubMed] [Google Scholar]
- 38.Herrmann C, Voordouw MJ, Gern L. 2013. Ixodes ricinus ticks infected with the causative agent of Lyme disease, Borrelia burgdorferi sensu lato, have higher energy reserves. Int. J. Parasitol. 43:477–483 [DOI] [PubMed] [Google Scholar]
- 39.Schwaiger M, Péter O, Cassinotti P. 2001. Routine diagnosis of Borrelia burgdorferi (sensu lato) infections using a real-time PCR assay. Clin. Microbiol. Infect. 7:461–469 [DOI] [PubMed] [Google Scholar]
- 40.Alekseev AN, Dubinina HV, Van De Pol I, Schouls LM. 2001. Identification of Ehrlichia spp, and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 39:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Development Core Team 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 42.Papatheodorou V, Brossard M. 1987. C-3 levels in the sera of rabbits infested and reinfested with Ixodes ricinus L and in midguts of fed ticks. Exp. Appl. Acarol. 3:53–59 [DOI] [PubMed] [Google Scholar]
- 43.Moran Cadenas F, Rais O, Humair P-F, Douet V, Moret J, Gern L. 2007. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J. Med. Entomol. 44:1109–1117 [DOI] [PubMed] [Google Scholar]
- 44.Humair P-F, Douet V, Moran Cadenas F, Schouls LM, Van de Pol I, Gern L. 2007. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J. Med. Entomol. 44:869–880 [DOI] [PubMed] [Google Scholar]
- 45.Marsot M, Sigaud M, Chapuis JL, Ferquel E, Cornet M, Vourc'h G. 2011. Introduced Siberian chipmunks (Tamias sibiricus barberi) harbor more-diverse Borrelia burgdorferi sensu lato genospecies than native bank voles (Myodes glareolus). Appl. Environ. Microbiol. 77:5716–5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu CM, Cheminade Y, Perret J-L, Weynants V, Lobet Y, Gern L. 2003. Early detection of Borrelia burgdorferi sensu lato infection in Balb/c mice by co-feeding Ixodes ricinus ticks. Int. J. Med. Microbiol. 293:421–426 [DOI] [PubMed] [Google Scholar]
- 47.Randolph SE. 2011. Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda's enduring paradigm. Ticks Tick Borne Dis. 2:179–182 [DOI] [PubMed] [Google Scholar]
- 48.Gern L, Rais O. 1996. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae). J. Med. Entomol. 33:189–192 [DOI] [PubMed] [Google Scholar]
- 49.Bellet-Edimo R, Betschart B, Gern L. 2005. Frequency and efficiency of transovarial and subsequent transstadial transmission of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks. Bull. Soc. Neuchât. Sci. Nat. 128:117–125 [Google Scholar]
- 50.Nefedova VV, Korenberg EI, Gorelova NB, Kovalevskii YV. 2004. Studies on the transovarial transmission of Borrelia burgdorferi sensu lato in the taiga tick Ixodes persulcatus. Folia Parasitol. 51:67–71 [DOI] [PubMed] [Google Scholar]
- 51.Rollend L, Fish D, Childs JE. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 4:46–51 [DOI] [PubMed] [Google Scholar]
- 52.Humair P-F, Rais O, Gern L. 1999. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118:33–42 [DOI] [PubMed] [Google Scholar]
- 53.Rauter C, Oehme R, Diterich I, Engele M, Hartung T. 2002. Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. J. Clin. Microbiol. 40:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scoles GA, Papero M, Beati L, Fish D. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1:21–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.