Abstract
The principal mode of avian influenza A virus (AIV) transmission among wild birds is thought to occur via an indirect fecal-oral route, whereby individuals are exposed to virus from the environment through contact with virus-contaminated water. AIV can remain viable for an extended time in water; however, little is known regarding the influence of the biotic community (i.e., aquatic invertebrates) on virus persistence and infectivity in aquatic environments. We conducted laboratory experiments to investigate the ability of an aquatic filter-feeding invertebrate, Daphnia magna, to accumulate virus from AIV-dosed water under the hypothesis that they represent a potential vector of AIV to waterfowl hosts. We placed live daphnids in test tubes dosed with low-pathogenicity AIV (H3N8 subtype isolated from a wild duck) and sampled Daphnia tissue and the surrounding water using reverse transcription-quantitative PCR (RT-qPCR) at 3- to 120-min intervals for up to 960 min following dosing. Concentrations of viral RNA averaged 3 times higher in Daphnia tissue than the surrounding water shortly after viral exposure, but concentrations decreased exponentially through time for both. Extracts from Daphnia tissue were negative for AIV by cell culture, whereas AIV remained viable in water without Daphnia present. Our results suggest daphnids can accumulate AIV RNA and effectively remove virus particles from water. Although concentrations of viral RNA were consistently higher in Daphnia tissue than the water, additional research is needed on the time scale of AIV inactivation after Daphnia ingestion to fully elucidate Daphnia's role as a potential vector of AIV infection to aquatic birds.
INTRODUCTION
Wild birds that inhabit wetlands and aquatic environments, especially dabbling ducks of the genus Anas, form the major natural reservoir for avian influenza A virus (AIV) (1, 2). Circulation of AIV in wild duck populations is characterized by seasonal peaks of infection affecting 15 to 30% of the population, which requires an efficient and widespread mode of transmission (3). Considerable evidence suggests that maintenance of the AIV reservoir is intricately linked to aquatic environments inhabited by waterfowl (4). High concentrations of AIV excreted in feces of infected birds, combined with the ability of the virus to remain viable in water for extended periods, are thought to drive transmission via an indirect fecal-oral route (5, 6). That is, most infections in wild aquatic birds are thought to occur as a result of contact with virus-contaminated water, as opposed to direct bird-to-bird transmission, or direct fecal-oral transmission (4).
AIV can remain viable for extended periods in water (7–9); however, detection of AIV in water from natural aquatic systems generally occurs in less than 2% of samples (10–12). Among studies pairing fecal sampling with water sampling in areas of high waterbird densities, detection of AIV tends to be considerably lower in water than fecal samples (11, 12, but see reference 13), likely as a result of dilution and uneven distribution of AIV in the water. Compared to detection of AIV via molecular methods, isolation of viable virus from natural lake water has occurred even less frequently (4, 11). Low rates of AIV detection and isolation from natural surface water, combined with recent advances to concentrate large volumes of water for detection of AIV (14–16), demonstrate that even amid favorable conditions (i.e., high concentrations of aquatic birds and active viral shedding), AIV likely exists at low concentrations in water. As such, any factor that acts to increase virus concentrations and facilitate ingestion by birds could play an important role in transmission.
Filter-feeding bivalves are able to accumulate a number of viruses and are known vectors of enteric viruses to humans (17). Therefore, it is plausible that aquatic filter-feeding invertebrates, an important source of food to a number of waterfowl species, may play a role as an intermediate vector and facilitate transmission of AIV. In the first test of this hypothesis, Faust et al. (18) exposed Asiatic clams (Corbicula fluminea) to AIV-dosed water and documented dramatic reductions in AIV concentrations in the water and reduced viral infectivity associated with the filtering behavior of the bivalves. In contrast, zebra mussels (Dreissena polymorpha) maintained detectable amounts of viable AIV in their tissue for up to 16 days postexposure (19), and freshwater clams acted as bioconcentrators for AIV, highlighting their potential use as sentinels in AIV surveillance (20). Based on a study of H5N1 persistence in simulated complex aquatic habitats, Horm et al. (21) detected infectious virus in bivalve tissue only shortly after exposure and suggested that bivalves and other aquatic fauna were passive carriers of AIV. In the first assessment of the effects of filter-feeding zooplankton on AIV persistence, Abbas et al. (22) exposed Daphnia magna to AIV-dosed water and found detectable levels of virus associated with Daphnia tissue for up to 6 days postexposure. Concentrations of AIV were higher per volume of Daphnia than the water; however, filtration by Daphnia may have inactivated the virus. These studies documented uptake and the potential for accumulation of AIV by aquatic invertebrates, but further research is needed to fully understand the role of aquatic invertebrates in AIV persistence and transmission.
Daphnia spp. are small planktonic crustaceans of the suborder Cladocera that range between 0.2 and 3.0 mm in length and are ubiquitous in freshwater aquatic environments (23). Daphnia spp. play an integral role in aquatic food webs and are consumed by numerous avian taxa, including important AIV reservoir species such as the northern shoveler (Anas clypeata) and mallard (Anas platyrhynchous) (24–26). Daphnids feed by filtering algae, bacteria, and organic detritus (e.g., duck feces) from the water column (27). Filtration rates of individual daphnids are influenced by a number of factors (e.g., species, size, water temperature, and photoperiod) but generally range between 0.1 and 3.0 ml h−1 (27, 28). Life expectancies of Daphnia range from weeks to months, and the daphnids are capable of quickly reaching large population densities through asexual reproduction (23). In situ examinations of Daphnia filtering rates demonstrate that in some cases, natural zooplankton communities are capable of filtering all water in a given lake within a 24-h period (29).
A solid understanding of AIV ecology and transmission requires detailed assessment of both abiotic and biotic factors potentially influencing AIV persistence and transmission. The ubiquitous presence of Daphnia in waterfowl habitats and the significance of daphnids as a food source to waterfowl and other aquatic birds make them a prime candidate for studies of AIV persistence. The objective of this study was to assess the potential role of filter-feeding invertebrates as a vector facilitating AIV infection. Specifically, we conducted laboratory experiments to investigate the ability of live Daphnia magna to accumulate viable virus from AIV-dosed water and assessed temporal persistence of AIV within these organisms and the associated water column.
MATERIALS AND METHODS
Daphnia magna culture.
We began culturing approximately 500 daphnids of the species Daphnia magna (DaphniaForSale.com, Merced, CA) 3 months prior to conducting the experiments in aerated, 40-liter glass aquaria filled with groundwater kept at 20°C under 24 h of light. Daphnids were fed a mixture of spirulina powder, soy protein powder, and active dry yeast daily. At the time of the experiments, the culture numbered greater than 10,000 individuals; the daphnids used in the experimental trials were sampled randomly from the culture.
Virus source and propagation.
For all experiments, we used the wild-bird-origin low-pathogenicity AIV strain A/northern pintail/Alaska/44340-268/2007(H3N8) that was isolated from a cloacal swab of a northern pintail duck (Anas acuta) captured on the Yukon-Kuskokwim Delta, AK, in 2007 and initially cultured in allantoic fluid at the USGS National Wildlife Health Center, Madison, WI (30). AIV was propagated in MDCK cells (American Type Culture Collection, Manassas, VA) in 75-cm2 flasks containing Eagle's minimal essential medium, 10% fetal bovine serum, and tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (1 μg ml−1). We harvested AIV when the cell monolayer exhibited 75% to 100% cytopathic effect (CPE) by centrifuging the culture at 900 × g for 10 min; the supernatant was subdivided into aliquots stored at −80°C (AIV concentration of 1 × 107 genomic copies ml−1).
Experimental trials.
We added 0.05 g of live Daphnia magna organisms (∼30 individuals) and 1 μl of Daphnia food to 5 ml of ground water (pH 8.1) in 50-ml polypropylene test tubes. We then added 100 μl AIV stock to all test tubes to achieve a final concentration of 2 × 105 genomic copies AIV ml−1. Test tubes were incubated without aeration at 20°C in a temperature-controlled room and maintained out of direct light. We conducted 3 experimental trials in which daphnids were allowed to incubate at intervals ranging from 3 min early in the trial up to 120-min intervals later, for a maximum duration of 960 min. In each trial, there were 2 experimental replicates at each time interval and one control replicate (i.e., virus, water, and Daphnia food without daphnids) at a subset of time intervals for a total of 52 experimental test tubes and 16 control test tubes.
At the specified time interval, selected tubes were destructively sampled by transferring the entire 5 ml of water to a new 15-ml polypropylene tube containing 2 ml viral lysing buffer (AVL buffer) (Qiagen, Valencia, CA) and then vortexed. To the 30 Daphnia individuals remaining in an experimental tube bottom, we added 2 ml of AVL buffer, immediately killing the daphnids to cease filtration. Daphnids were poured into a clean mortar, pulverized with a pestle, rinsed with 5 ml clean well water back into the experimental tube, and vortexed. Daphnids and water tubes were incubated for 60 min at room temperature, after which the entire 5-ml volumes were extracted with the DNA blood Midi kit (Qiagen) (31). The final extracted eluate volume was 300 μl.
Dead Daphnia experiment.
To differentiate between the effects of active filtration by Daphnia and the effects of Daphnia tissue on virus persistence, we conducted a post hoc experiment in which we replicated our primary experiment with freshly killed daphnids. Water, Daphnia food, and live daphnids were added to test tubes as previously described, but prior to dosing the water with AIV, we placed the test tubes in a freezer at −80°C for approximately 15 min. When the water was frozen solid, the test tubes were removed, allowed to equilibrate at 20°C, inspected to ensure Daphnia death, and then spiked with AIV (2 × 105 genomic copies ml−1). We conducted this experiment with 2 replicates per 6 time intervals ranging from 6 to 360 min. Other than the use of dead daphnids, we followed the same methods and procedures as in the primary experiment.
RT-qPCR procedure.
Two-step reverse transcription-quantitative PCR (RT-qPCR) was performed. To 8.6 μl of the extracted RNA, we added 8.6 μl nuclease-free water and 0.7 μl (0.007 μg μl−1) random hexamers (Promega, Madison, WI). This mixture was heated for 4 min at 99°C followed by addition of 32.1 μl RT master mix, yielding final concentrations of the following components: 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 70 μM each deoxynucleoside triphosphate (Promega), 30 U of RNasin (Promega), and 100 U of SuperScript II reverse transcriptase (Invitrogen Life Technologies, Rockville, MD). The thermal conditions for reverse transcription were 25°C for 15 min, 42°C for 60 min, 99°C for 5 min, and then 4°C for no longer than 1 h.
Six microliters of the RT reaction mixture was combined with the LightCycler DNA master probe kit reagents (Roche Diagnostics, Mannheim, Germany) for a final reaction volume of 20 μl. qPCR targeted the AIV matrix gene using previously published primers and probe (32). The final concentrations of the primers (Integrated DNA Technologies, Coralville, IA) and probe (TIB Molbiol, Berlin, Germany) were 500 nM and 100 nM, respectively. Amplification was conducted with the LightCycler 480 machine (Roche Diagnostics) under the following thermal conditions: 10 min at 95°C, followed by 45 cycles of 15 s at 94°C and 1 min at 60°C.
The qPCR standard curve was generated by treating AIV stock solution with Benzonase (Novagen, Madison, WI) following the methods described by Borchardt et al. (31) to produce intact virions free of all extraneous nucleic acid. Treated virion RNA was extracted with the QIAamp DNA blood minikit (Qiagen), and RNA mass was measured with RiboGreen (Molecular Probes, Eugene, OR) and the GloMax-Multi Jr. fluorometer (Promega). RNA mass was converted to genomic copies based on the AIV nucleic acid molecular weight. Standards were created from serial 1:10 dilutions of the treated virions followed by nucleic acid extraction as described above. The crossing points of the standards were regressed against the logarithm of genomic copy number using LightCycler 480 software.
RT-qPCR controls.
All water and Daphnia samples were tested for RT-qPCR inhibition following the methods described by Gibson et al. (33), except the inhibition control was bovine viral diarrhea virus (BVDV) type 1 using the primers and probe described by Brooks et al. (34). None of the analyses were inhibited. To test for potential differences in qPCR efficiencies of water extraction versus Daphnia extraction, we compared cycle quantification (Cq) values of the BVDV inhibition controls added to every extraction. Additional controls included AIV positive controls, no-template controls for RT and PCR master mixes, negative extraction controls consisting of 5 ml well water and 2 ml AVL buffer, and positive extraction controls consisting of 30 Daphnia individuals ground with mortar and pestle and spiked with 2 × 105 AIV. All controls were in compliance.
AIV viability.
In one experimental trial, an additional replicate was performed to assess AIV viability in feeding Daphnia and in water without Daphnia. Daphnids were collected at 6 and 12 min and hourly from 1 h to 16 h after AIV addition, and from the controls (AIV and food only), 5 ml well water was collected at 5, 6, and 10 h after AIV addition. Daphnids were disrupted by freezing without water at −80°C, adding 5 ml sterile phosphate-buffered saline (PBS), vortexing, and filtering the Daphnia tissue solution through a 0.22-μm-pore filter. Control water was prepared for culture by adding 4.5 ml to 0.5 ml 10× PBS followed by 0.22-μm filtration. AIV in Daphnia and control water inocula (both 2.5 ml) were cultured on primary rhesus monkey kidney cells in 15-ml culture tubes (ViroMed Laboratories, Minnetonka, MN) as described by Szretter et al. (35). Following inoculation, cells were incubated for 90 min at room temperature. We then removed the inoculum, added maintenance medium (Eagle's minimal essential medium [MEM] with 2% fetal bovine serum [FBS]), and incubated the medium at 37°C. Cultures were refed with maintenance medium every 7 days and passaged at 2 and 4 weeks. Controls included a positive control of 0.2 ml AIV stock in 2.5 ml maintenance medium and a negative control of 2.5 ml PBS. After 6 weeks, the maintenance medium was removed, 150 μl nuclease-free water was added, and the monolayer was removed by scraping with a rubber spatula. The entire cell suspension was extracted with the QIAamp DNA blood minikit, and AIV was quantified by RT-qPCR as described above. Cultures negative for CPE but after 6 weeks containing 10-times more AIV genomic copies than measured in the initial inoculum were considered positive by integrated cell culture-qPCR (ICC-qPCR), similar to the approach used by Borchardt et al. (31).
Statistical analysis.
To quantify the effect of Daphnia on AIV persistence, we assessed variation of AIV concentrations in water from control test tubes (AIV and Daphnia food only) and experimental test tubes (AIV, Daphnia food, and daphnids) through time using combined data from the 3 experimental trials. We considered 8 candidate models: the models included linear, 2- and 3-parameter exponential decay and modified 3-parameter exponential decay applied to (i) all data combined (i.e., one function fitted to all data) and (ii) data split by type (i.e., separate functions fitted to control water and experimental water). To assess differences in AIV concentrations between Daphnia tissue and the associated water through time, we conducted a separate analysis in which the same models were applied to (i) all data combined and (ii) data split by Daphnia tissue versus water separated from Daphnia. The response variable in both analyses was the number of genomic copies of AIV as determined by RT-qPCR ml−1 of water or g−1 of Daphnia. We conducted statistical analyses using nonlinear models computed with Proc NLIN in SAS 9.1 and selected the best approximating model from each analysis using Akaike's information criterion corrected for small sample size (AICc) and model weights (wi) (36).
RESULTS
Experimental trials.
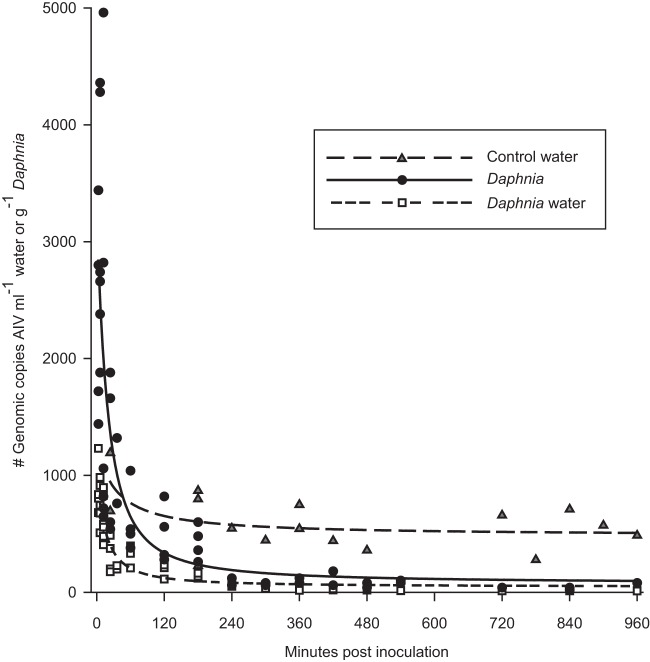
Concentrations of AIV in water within test tubes containing Daphnia differed substantially from those in control test tubes lacking Daphnia. The best approximating model (wi = 0.93) supported a modified 3-parameter exponential decay in which AIV concentrations in Daphnia water declined through time to an estimated asymptote of 44.6 genomic copies of AIV ml−1 (standard error [SE] = 23.78), while AIV concentrations in control water remained approximately 10-fold higher, declining to an estimated asymptote of 482.7 genomic copies of AIV ml−1 (SE = 135.48) (Table 1 and Fig. 1).
Table 1.
Models of AIV persistence associated with water incubated with live Daphnia and water without Daphnia through timea
| Model | Functional form | Kb | Adjusted R2 | Log σ̂2d | ΔAICc | wi |
|---|---|---|---|---|---|---|
| By treatment | ||||||
| Modified exponential decay | AIV = {aCW × exp[(bCW/(X + cCW)]} + {aDW × exp[bDW/(X + cDW)]} | 6 | 0.76 | 9.91 | 0.00c | 0.93 |
| Exponential decay | AIV = [aCW + bCW × exp(−cCW × X)] + [aDW + bDW × exp(−cDW × X)] | 6 | 0.75 | 9.99 | 5.26 | 0.07 |
| AIV = [aCW × exp(−bCW × X)] + [aDW × exp(−bDW × X)] | 4 | 0.70 | 10.17 | 13.11 | 0.00 | |
| Linear | AIV = (aCW + bCW × X) + (aDW + bDW × X) | 4 | 0.37 | 10.92 | 64.08 | 0.00 |
| Pooled | ||||||
| Modified exponential decay | AIV = a × exp[b/(X + c)] | 3 | 0.42 | 10.99 | 66.51 | 0.00 |
| Exponential decay | AIV = a + b × exp(−c × X) | 3 | 0.42 | 10.99 | 66.66 | 0.00 |
| AIV = a × exp(−b × X) | 2 | 0.26 | 11.26 | 82.83 | 0.00 | |
| Linear | AIV = a + b × X | 2 | 0.16 | 11.38 | 90.72 | 0.00 |
Shown are models of AIV persistence associated with water incubated with live Daphnia (DW) and water without Daphnia (CW) through time (X, in minutes). Strength of support for each model is inferred by AICc model weight (wi). We considered linear (2-parameter) relationships, exponential decay functions (2 and 3 parameters), and a modified exponential decay function (3 parameters) fitted to pooled data and data separated by treatment.
Number of parameters.
AICc = 687.17.
Log of the estimated variance.
Fig 1.
AIV concentration (genomic copies ml−1) in water incubated with live Daphnia organisms and in water without daphnids (control water) and AIV concentration associated with live Daphnia tissue (genomic copies g−1) as a function of time since virus addition (X, in minutes; range, 3 to 960 min). Points represent raw data, and lines depict predicted [AIV] from the top approximating model: AIV = aeb/(X + c). The model coefficients are control water (a = 482.67, SE = 135.48; b = 52.27, SE = 130.25; c = 53.81, SE = 166.7), Daphnia water (a = 44.63, SE = 23.78; b = 177.40, SE = 89.44; c = 56.46, SE = 20.88), and Daphnia tissue (a = 72.38, SE = 123.01; b = 310.18, SE = 372.66; c = 81.55, SE = 65.67).
Concentrations of AIV associated with Daphnia tissue differed from the AIV concentrations in water; the best approximating model (wi = 0.48) supported a modified 3-parameter exponential decay in which AIV concentrations associated with Daphnia tissue were consistently higher than that of the water, especially during the first 120 min of the experiment (Table 2 and Fig. 1). This difference of AIV concentrations between Daphnia tissue and water cannot be attributed to the qPCR efficiencies of the Daphnia and water nucleic acid extracts. Comparing cycle quantification (Cq) values of the BVDV inhibition controls added to every extraction, the qPCR efficiencies of Daphnia and water extracts did not differ (paired t test, P = 0.48; n = 52 per extract type). Predicted viral RNA concentrations in Daphnia tissue ranged from more than 3 times greater (during the first hour postinoculation) to 1.8 times greater (at 940 min postinoculation) than those in the surrounding water. In contrast to live daphnids, dead daphnids did not accumulate AIV to concentrations greater than that of the water, and the AIV concentrations in the water incubated with dead daphnids remained near the initial levels through time (Fig. 2).
Table 2.
Models of AIV persistence associated with water incubated with live Daphnia and Daphnia tissue through timea
| Model | Functional form | Kb | Adjusted R2 | Log σ̂2d | ΔAICc | wi |
|---|---|---|---|---|---|---|
| By treatment | ||||||
| Modified exponential decay | AIV = {aDW × exp[bDW/(X + cDW)]} + {aDT × exp[bDT/(X + cDT)]} | 6 | 0.64 | 12.55 | 0.00c | 0.48 |
| Exponential decay | AIV = [aDW × exp(−bDW × X)] + [aDT × exp(−bDT × X)] | 4 | 0.63 | 12.60 | 0.85 | 0.32 |
| AIV = [aDW + bDW × exp(−cDW × X)] + [aDT + bDT × exp(−cDT × X)] | 6 | 0.64 | 12.56 | 1.78 | 0.20 | |
| Linear | AIV = (aDW + bDW × X) + (aDT + bDT × X) | 4 | 0.30 | 13.24 | 67.85 | 0.00 |
| Pooled | ||||||
| Modified exponential decay | AIV = a × exp[b/(X + c)] | 3 | 0.43 | 13.17 | 58.59 | 0.00 |
| Exponential decay | AIV = a × exp(−b × X) | 2 | 0.42 | 13.20 | 59.02 | 0.00 |
| AIV = a + b × exp(−c × X) | 3 | 0.43 | 13.18 | 59.52 | 0.00 | |
| Linear | AIV = a + b × X | 2 | 0.21 | 13.52 | 92.20 | 0.00 |
Shown are models of AIV persistence associated with water incubated with live Daphnia (DW) and Daphnia tissue (DT) through time (X, in minutes). Strength of support for each model is inferred by AICc model weight (wi). We considered linear (2-parameter) relationships, exponential decay functions (2 and 3 parameters), and a modified exponential decay function (3 parameters) fitted to pooled data and data separated by treatment.
Number of parameters.
AICc = 1,317.64.
Log of the estimated variance.
Fig 2.
AIV concentration (genomic copies ml−1) in water incubated with dead Daphnia organisms (squares) and AIV concentration (genomic copies g−1) associated with dead Daphnia tissue (circles) as a function of time since virus addition (X, in minutes; range, 6 to 360 min) for each trial.
AIV viability.
All Daphnia samples from all time intervals ranging from 6 min to 16 h (samples from 16 time intervals were analyzed) were negative for viable AIV, as measured by CPE and by ICC-qPCR. In contrast, in water without Daphnia, viable AIV was not detected by CPE, but it was detected at all time intervals (5, 6, and 10 h) by ICC-qPCR. ICC-qPCR concentrations of AIV after 6 weeks of culture were 1,070 to 2,580 times greater than AIV concentrations in the initial inocula, indicating AIV from the water without Daphnia was viable and multiplied on the cell line but without producing CPE. The AIV positive control exhibited a similar pattern; it did not produce CPE, but it was positive by ICC-qPCR.
DISCUSSION
Efficient transmission of AIV is undoubtedly linked to its ability to remain viable in aquatic habitats. Aquatic environments inhabited by AIV reservoir species (i.e., waterfowl) are complex, and persistence of AIV is influenced by a combination of abiotic factors, including pH, temperature, and salinity (7, 37), and biotic factors, including zooplankton, bivalves, fish, amphibians, and vegetation (21). Daphnia spp. are an important component of natural aquatic ecosystems, and their widespread occurrence in aquatic habitats aligns with the widespread abundance of waterfowl and other aquatic birds. During seasonal peaks in freshwater systems, Daphnia densities can exceed 400 individuals liter−1 (23), and at an important waterfowl breeding area in Manitoba, Canada, Collias and Collias (24) documented Daphnia densities of greater than 1,000 individuals liter−1. At these densities, daphnids are capable of filtering all water in a water body within a 24-h period (29). In addition to being ubiquitous in waterfowl habitat, daphnids are consumed opportunistically by a variety of waterfowl species (24, 25) and comprise a large proportion of the diet of the zooplankton specialist northern shoveler (26), a duck species known to have high rates of AIV prevalence (38; B. W. Meixell, unpublished data). Therefore, models of maintenance and transmission of AIV in the wild should take into consideration the effect of invertebrates, such as Daphnia, on viral persistence.
We assessed the potential of Daphnia magna to bioconcentrate AIV under the hypothesis that the daphnids may act as a vector of infection to aquatic birds that consume zooplankton in the wild. Concentrations of viral RNA in water containing live daphnids decreased exponentially through time, while remaining near initial levels throughout the experiment in control test tubes without Daphnia. We detected the highest concentrations of AIV associated with Daphnia within 12 min post-viral exposure, and viral concentrations declined exponentially thereafter. At a filtration rate on the order of 2 ml h−1 (39), the time for our sample of approximately 30 Daphnia individuals to filter all water within a given test tube once was about 5 min, corresponding with the observed peaks in AIV concentration associated with Daphnia tissue. The observed peak in AIV was followed by an apparent destruction of viral RNA such that overall AIV in the experimental test tubes was reduced drastically within 5 h postinoculation.
In our post hoc experiment, AIV concentrations associated with dead, nonfeeding daphnids did not exceed AIV concentrations in the water. Furthermore, AIV concentrations in the water remained near initial levels through the duration of the experiment, suggesting that filtration by Daphnia was responsible for depletion of AIV in the primary experiment. Gentry et al. (40) found very high concentrations of human norovirus associated with the >200-μm size fraction of zooplankton in a Georgia, USA, estuary and hypothesized the noroviruses were electrostatically adsorbed to zooplankton surfaces. Differences in water chemistries and microbe surface properties can have large effects on adsorption (41), which might explain why we did not observe high levels of AIV adsorption to the exoskeletons of dead daphnids.
Our results are consistent with previous studies that showed Daphnia spp. effectively reduced abundance of some waterborne pathogens (e.g., Campylobacter jejuni, Giardia lamblia, and Cryptosporidium parvum) (42, 43). Recently, Abbas et al. (22) exposed Daphnia magna to AIV-dosed water for 24 h, rinsed the daphnids, and transferred them to clean water, where they were sampled over a 6-day period. In contrast to our results, concentrations of AIV associated with Daphnia tissue and AIV concentrations in clean water remained mostly stable through time (22). In 2 of their 4 experiments, the AIV concentrations associated with Daphnia declined during the first 24 h after being transferred to clean water but thereafter remained stable.
At early time intervals in our experiment, concentrations of viral RNA associated with Daphnia tissue were more than 3 times greater than concentrations in water. However, the negative cell culture results for AIV associated with Daphnia suggested the AIV was inactivated. AIV inactivation could not be attributed to factors associated with the groundwater or Daphnia food used in this study because culture-positive AIV was detected at 5, 6, and 10 h after AIV and Daphnia food was added to water without Daphnia. It appears Daphnia quickly inactivated AIV after ingestion. Abbas et al. (22) also suggested Daphnia filtration was responsible for AIV inactivation after observing accumulated viral RNA in Daphnia exposed to AIV and finding the Daphnia-associated AIV was cell culture negative. Their observations, however, occurred over a period of days, whereas we are suggesting Daphnia ingestion and inactivation of AIV can be rapid. Alternatively, negative culture results may be a consequence of method sensitivity (22, 44). Inoculation of AIV into embryonated chicken eggs may lead to higher rates of successful virus isolation than cell lines (45), and future use of this method may be warranted.
The hypothesis that aquatic, filter-feeding invertebrates may act as a vector facilitating AIV infection in aquatic birds requires additional study. Results from laboratory studies that exposed filter-feeding invertebrates to AIV range from complete depletion of the virus by clams (18) to accumulation of viable virus for more than 2 weeks in zebra mussels (19). Daphnia in our study rapidly reduced AIV concentrations in the water. The observed depletion of AIV, combined with negative results of virus viability, may indicate that the presence of Daphnia in aquatic habitats acts to impede transmission. Indeed, our results demonstrate that daphnids impose a negative effect on viral persistence. However, we postulate this does not preclude their potential as a vector. During early time intervals in our experiment, AIV concentrations associated with Daphnia tissue averaged more than 3 times that of the water, and even during the later time intervals when AIV concentrations were reduced substantially, concentrations of AIV associated with Daphnia tissue remained higher than that of the water. Hence, an individual bird would be expected to encounter more AIV by volume in Daphnia than in water. Furthermore, in the wild, especially during peaks of AIV infection in aquatic bird populations, input of AIV to water would occur not as a single, finite input (as in this study) but nearly continuously as birds actively feed and defecate. Under this scenario, the relative concentrations of AIV in the wild might be maintained at levels that resemble results from the early time intervals of our experiment (i.e., substantially higher AIV concentrations associated with Daphnia than water), without the subsequent drastic reduction in AIV concentrations. The time scale for AIV inactivation associated with Daphnia tissue remains uncertain; however, based on documented accumulation of viral RNA, we cannot rule out the potential for Daphnia to act as a vector facilitating AIV infection.
ACKNOWLEDGMENTS
We thank J. Gonnering, T. Spivey, and J. Altmann for Daphnia husbandry and laboratory support. P. Flint provided valuable analytical advice and assistance with project design. H. Ip with the USGS National Wildlife Health Center supplied the AIV. We thank M. Whalen for assistance with figures. T. Arnold, C. Amundson, J. Pearce, P. Flint, and D. Andersen provided helpful comments on earlier versions of the manuscript.
The use of trade or product names is for descriptive purposes only and does not constitute endorsement by the U.S. Government.
Footnotes
Published ahead of print 13 September 2013
REFERENCES
- 1.Munster VJ, Baas C, Lexmond P, Waldenstrom J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WE, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 3:e61. 10.1371/journal.ppat.0030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. 2006. Global patterns of influenza A virus in wild birds. Science 312:384–388 [DOI] [PubMed] [Google Scholar]
- 3.Clark L, Hall J. 2006. Avian influenza in wild birds: status as reservoirs, and risks to humans and agriculture. Ornithol. Monogr. 60:3–29 [Google Scholar]
- 4.Franklin AB, Van Dalen KK, Huyvaert KP. 2011. Avian influenza virus in aquatic environments: an ecological perspective, p 59–72 In Majumdar SK, Brenner FJ, Huffman JE, McLean RG, Panah AI, Pietrobon PJ. (ed), Pandemic influenza viruses: science, surveillance, and public health. Pennsylvania Academy of Science, Easton, PA [Google Scholar]
- 5.Hinshaw VS, Webster RG, Turner B. 1979. Water-borne transmission of influenza A viruses? Intervirology 11:66–68 [DOI] [PubMed] [Google Scholar]
- 6.VanDalen KK, Franklin AB, Mooers NL, Sullivan HJ, Shriner SA. 2010. Shedding light on avian influenza H4N6 infection in mallards: modes of transmission and implications for surveillance. PLoS One 5:e12851. 10.1371/journal.pone.0012851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. 2009. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 136:20–26 [DOI] [PubMed] [Google Scholar]
- 8.Nazir J, Haumacher R, Ike A, Stumpf P, Böhm R, Marschang RE. 2010. Long-term study on tenacity of avian influenza viruses in water (distilled water, normal saline, and surface water) at different temperatures. Avian Dis. 54:720–724 [DOI] [PubMed] [Google Scholar]
- 9.Lebarbenchon C, Yang M, Keeler SP, Ramakrishnan MA, Brown JD, Stallknecht DE, Sreevatsan S. 2011. Viral replication, persistence in water and genetic characterization of two influenza A viruses isolated from surface lake water. PLoS One 6:e26566. 10.1371/journal.pone.0026566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stallknecht DE, Goekjian VH, Wilcox BR, Poulson RL, Brown JD. 2010. Avian influenza virus in aquatic habitats: what do we need to learn? Avian Dis. 54:461–465 [DOI] [PubMed] [Google Scholar]
- 11.Henaux V, Samuel MD, Dusek RJ, Fleskes JP, Ip HS. 2012. Presence of avian influenza viruses in waterfowl and wetlands during summer 2010 in California: are resident birds a potential reservoir. PLoS One 7:e31471. 10.1371/journal.pone.0031471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Ramírez E, Acevedo P, Allepuz A, Gerrikagoitia X, Alba A, Busquets N, Díaz-Sánchez S, Alvarez V, Abad FX, Barral M, Majó N, Höfle U. 2012. Ecological factors driving avian influenza virus dynamics in Spanish wetland ecosystems. PLoS One 7:e46418. 10.1371/journal.pone.0046418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Okazaki K, Kawaoka Y, Takada A, Webster RG, Kida H. 1995. Perpetuation of influenza-A viruses in Alaskan waterfowl reservoirs. Arch. Virol. 140:1163–1172 [DOI] [PubMed] [Google Scholar]
- 14.Khalenkov A, Laver WG, Webster RG. 2008. Detection and isolation of H5N1 influenza virus from large volumes of natural water. J. Virol. Methods 149:180–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deboosere N, Horm SV, Pinon A, Gachet J, Coldefy C, Buchy P, Vialette M. 2011. Development and validation of a concentration method for the detection of influenza a viruses from large volumes of surface water. Appl. Environ. Microbiol. 77:3802–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millen HT, Gonnering JC, Berg RK, Spencer SK, Jokela WE, Pearce JM, Borchardt JS, Borchardt MA. 2012. Glass wool filters for concentrating waterborne viruses and agricultural zoonotic pathogens. J. Vis. Exp. 61:e3930. 10.3791/3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees D. 2000. Viruses and bivalve shellfish. Int. J. Food Microbiol. 59:81–116 [DOI] [PubMed] [Google Scholar]
- 18.Faust C, Stallknecht D, Swayne D, Brown J. 2009. Filter-feeding bivalves can remove avian influenza viruses from water and reduce infectivity. Proc. Biol. Sci. 276:3727–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stumpf P, Failing K, Papp T, Nazir J, Bohm R, Marschang RE. 2010. Accumulation of a low pathogenic avian influenza virus in zebra mussels (Dreissena polymorpha). Avian Dis. 54:1183–1190 [DOI] [PubMed] [Google Scholar]
- 20.Huyvaert KP, Carlson JS, Bentler KT, Cobble KR, Nolte DL, Franklin AB. 2012. Freshwater clams as bioconcentrators of avian influenza virus in water. Vector Borne Zoonotic Dis. 12:904–906 [DOI] [PubMed] [Google Scholar]
- 21.Horm VS, Gutierrez RA, Nicholls JM, Buchy P. 2012. Highly pathogenic influenza A (H5N1) virus survival in complex artificial aquatic biotopes. PLoS One 7:e34160. 10.1371/journal.pone.0034160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas MD, Nazir J, Stumpf P, Marschang RE. 2012. Role of water fleas (Daphnia magna) in the accumulation of avian influenza viruses from the surrounding water. Intervirology 55:365–371 [DOI] [PubMed] [Google Scholar]
- 23.Smith DG. 2001. Pennak's freshwater invertebrates of the United States: Porifera to Crustacea, 4th ed. John Wiley & Sons, New York, NY [Google Scholar]
- 24.Collias NE, Collias EC. 1963. Selective feeding by wild ducklings of different species. Wilson Bull. 75:6–14 [Google Scholar]
- 25.Swanson GA. 1977. Diel food selection by Anatinae on a waste-stabilization system. J. Wildl. Manag. 41:226–231 [Google Scholar]
- 26.Dubowy PJ. 1985. Feeding ecology and behavior of postbreeding male blue-winged teal and northern shovelers. Can. J. Zool. 63:1292–1297 [Google Scholar]
- 27.Wetzel RG. 2001. Limnology: lake and river ecosystems, 3rd ed. Academic Press, San Diego, CA [Google Scholar]
- 28.Jürgens K. 1994. Impact of Daphnia on planktonic microbial food webs—a review. Mar. Microb. Food Webs 8:295–324 [Google Scholar]
- 29.Haney JF. 1973. An in situ examination of the grazing activities of natural zooplankton communities. Arch. Hydrobiol. 72:87–132 [Google Scholar]
- 30.Ramey AM, Pearce JM, Flint PL, Ip HS, Derksen DV, Franson JC, Petrula MJ, Scotton BD, Sowl KM, Wege ML, Trust KA. 2010. Intercontinental reassortment and genomic variation of low pathogenic avian influenza viruses isolated from northern pintails (Anas acuta) in Alaska: examining the evidence through space and time. Virology 401:179–189 [DOI] [PubMed] [Google Scholar]
- 31.Borchardt MA, Spencer SK, Kieke BA, Lambertini E, Loge FJ. 2012. Viruses in nondisinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ. Health Perspect. 120:1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. 2002. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40:3256–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson KE, Schwab KJ, Spencer SK, Borchardt MA. 2012. Measuring and mitigating inhibition during quantitative real time PCR analysis of viral nucleic acid extracts from large-volume environmental water samples. Water Res. 46:4281–4291 [DOI] [PubMed] [Google Scholar]
- 34.Brooks JW, Key DW, Hattel AL, Hovingh EP, Peterson R, Shaw DP, Fisher JS. 2007. Failure to detect bovine viral diarrhea virus in necropsied farm-raised white-tailed deer (Odocoileus virginianus) in Pennsylvania. J. Vet. Diagn. Invest. 19:298–300 [DOI] [PubMed] [Google Scholar]
- 35.Szretter KJ, Balish AL, Katz JM. 2005. Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. Chapter 15:Unit 15G.1. 10.1002/0471729256.mc15g01s3 [DOI] [PubMed] [Google Scholar]
- 36.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd ed. Springer-Verlag, New York, NY [Google Scholar]
- 37.Keeler SP, Berghaus RD, Stallknecht DE. 2012. Persistence of low pathogenic avian influenza viruses in filtered surface water from waterfowl habitats in Georgia, U. S. A. J. Wildl. Dis. 48:999–1009 [DOI] [PubMed] [Google Scholar]
- 38.Hill NJ, Takekawa JY, Cardona CJ, Ackerman JT, Schultz AK, Spragens KA, Boyce WM. 2010. Waterfowl ecology and avian influenza in California: do host traits inform us about viral occurrence? Avian Dis. 54:426–432 [DOI] [PubMed] [Google Scholar]
- 39.Porter KG, Feig YS, Vetter EF. 1983. Morphology, flow regimes, and filtering rates of Daphnia, Ceriodaphnia, and Bosmina fed natural bacteria. Oecologia 58:156–163 [DOI] [PubMed] [Google Scholar]
- 40.Gentry J, Vinje J, Guadagnoli D, Lipp EK. 2009. Norovirus distribution within an estuarine environment. Appl. Environ. Microbiol. 75:5474–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bos R, van der Mei HC, Busscher HJ. 1999. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol. Rev. 23:179–230 [DOI] [PubMed] [Google Scholar]
- 42.Schallenberg M, Bremer PJ, Henkel S, Launhardt A, Burns CW. 2005. Survival of Campylobacter jejuni in water: effect of grazing by the freshwater crustacean Daphnia carinata (Cladocera). Appl. Environ. Microbiol. 71:5085–5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bichai F, Payment P, Barbeau B. 2008. Protection of waterborne pathogens by higher organisms in drinking water: a review. Can. J. Microbiol. 54:509–524 [DOI] [PubMed] [Google Scholar]
- 44.Moresco KA, Stallknecht DE, Swayne DE. 2010. Evaluation and attempted optimization of avian embryos and cell culture methods for efficient isolation and propagation of low pathogenicity avian influenza viruses. Avian Dis. 54:622–626 [DOI] [PubMed] [Google Scholar]
- 45.Lombardo T, Dotti S, Renzi S, Ferrari M. 2012. Susceptibility of different cell lines to avian and swine influenza viruses. J. Virol. Methods 185:82–88 [DOI] [PubMed] [Google Scholar]