Abstract
Bacterial chemotaxis influences the ability of bacteria to survive and thrive in most environments, including polluted ones. Despite numerous reports of the phenotypic characterization of chemotactic bacteria, only a few molecular details of chemoreceptors for aromatic pollutants have been described. In this study, the molecular basis of chemotaxis toward an environmentally toxic chlorinated aromatic pollutant, 4-chloroaniline (4CA), was evaluated. Among the three Pseudomonas spp. tested, Pseudomonas aeruginosa PAO1 exhibited positive chemotaxis both to the nonmetabolizable 4CA, where 4-chloroacetanilide was formed as a dead-end transformation product, and to the metabolizable catechol. Molecular analysis of all 26 mutants with a disrupted methyl-accepting chemotaxis gene revealed that CtpL, a chromosomally encoded chemoreceptor, was responsible for the positive chemotactic response toward 4CA. Since CtpL has previously been described to be a major chemoreceptor for inorganic phosphate at low concentrations in PAO1, this report describes a fortuitous ability of CtpL to function toward aromatic pollutants. In addition, its regulation not only was dependent on the presence of the chemoattractant inducer but also was regulated by conditions of phosphate starvation. These results expand the range of known chemotactic transducers and their function in the environmental bacterium PAO1.
INTRODUCTION
Chemotaxis is one of the most important behavioral adaptations that bacteria use to mediate a balance between the nutritious and toxic effects of surrounding chemicals. Bacterial chemotactic attraction to environmental pollutants can enhance biodegradation rates by increasing pollutant bioavailability, and this potentially leads to an improvement in bioremediation efficiency (1). Several studies have reported the isolation and characterization of bacteria with a chemotactic response toward a variety of hazardous aromatic pollutants, including pesticides (2, 3), petroleum-associated monoaromatic (4–6), and polyaromatic (7, 8) and aromatic (9, 10) derivatives. However, such information is relatively scarce for some of the recently recognized recalcitrant xenobiotics, such as chloronitroaromatics, furan, and chlorinated anilines (9, 11). Despite the numerous reports of the isolation and phenotypic characterization of chemotactic bacteria, the specificity of the bacterial chemotactic response to chemicals is actually determined by their chemoreceptors (12). However, so far only three chemoreceptors for aromatic pollutants have been described: the plasmid-encoded NahY in the naphthalene-degrading strain Pseudomonas putida G7 (13), the plasmid-encoded NbaY in the 2-nitrobenzoate-degrading strain P. fluorescens KU-7 (14), and the plasmid-encoded McpT in the toluene-, benzene-, and ethylbenzene-degrading strain P. putida DOT-T1E (5, 12).
4-Chloroaniline (4CA) is a chlorinated aromatic amine (i.e., a chlorinated aniline) that is now ubiquitous in the environment (15) not only because it has been intensively used in and released from chemical industries but also because it is a key intermediate in the natural transformation of pesticides (16). Due to its toxicity, it has been recognized as one of the priority hazardous substances and is subject to cleanup using biological treatment (17). Biodegradation of the pollutant can be enhanced using motile bacteria with suitable chemotactic capabilities to target the pollutant chemical(s), but for 4CA, chemotaxis has yet to be reported.
In this study, the potential chemotactic response to 4CA was initially examined in three well-characterized chemotactic Pseudomonas species (P. putida F1, P. fluorescens Pf01, and P. aeruginosa PAO1). The positive chemotactic response of PAO1 to 4CA led to further investigations consisting of the quantitative analysis of bacterial chemotaxis and analysis of the biotransformation of 4CA by this bacterium. Molecular analysis revealed that CtpL is the chemoreceptor responsible for the chemoattraction of PAO1 to the nonmetabolizable 4CA as well as to the metabolizable catechol. The highlight of the work is to report a chromosomally encoded chemoreceptor responsible for chemoattraction to chlorinated aromatic pollutants and to describe a fortuitous capability of CtpL, which has previously been reported to serve as a major chemoreceptor for inorganic phosphate (Pi) at low concentrations in PAO1 (18).
MATERIALS AND METHODS
Bacterial strains, plasmids, growth conditions, and culture media.
The bacterial strains, mutants, and plasmids used in this study are listed in Table 1 (19–26). Each strain of the three Pseudomonas species and the series of PAO1 mutants was initially cultured in 2× YT medium (1.6% [wt/vol] tryptone, 1.0% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl) at 28°C for 18 h. For the chemotaxis assay, a 2% (vol/vol) culture inoculum was then transferred to the Pi-free T0 medium (Tris-HCl [pH 7.6], 10 g/liter; glucose, 2 g/liter; NaCl, 2 g/liter; NH4Cl, 1 g/liter; KCl, 0.1 g/liter; Na2SO4, 0.1 g/liter; CaCl2·2H2O, 0.01 g/liter; MgCl2·6H2O, 0.01 g/liter; FeCl3, 0.001 g/liter) (18) or the 5 mM Pi-containing T5 medium (as indicated above for T0 medium but with 5 mM Pi) with or without the addition of the target chemical (either 4CA or catechol). After 6 h of growth, cells were harvested, washed twice at room temperature, and resuspended in chemotaxis buffer (10 mM HEPES buffer, pH 7.0) (18).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference(s) |
|---|---|---|
| Strains | ||
| Pseudomonas putida strain F1 | Wild-type strain | 26 |
| Pseudomonas fluorescens strain PfO1 | Wild-type strain | 19 |
| Pseudomonas aeruginosa | ||
| PAO1 | Wild-type strain | 23 |
| ΔCHE1 | PAO1 derivative, ΔChe cluster (cheY cheZ cheA cheB motA2 motB2 cheW) | 20, 21 |
| ΔCHE3 | PAO1 derivative, ΔPil-Chp cluster (pilJ pilK pill chpA chpB) | 20, 21 |
| ΔCHE4 | PAO1 derivative, ΔChe2 cluster (cheY2 cheA2 cheW2 aer-2 cheR2 PA0174 cheB2) | 20, 21 |
| ΔCHE5 | PAO1 derivative, ΔWsp cluster (cheW3 cheR3 cheW4 cheA3 cheB3) | 20, 21 |
| PC4 | NTG-derived mutant of PAO1 cheR in CheVR cluster | 20, 22 |
| mcp-disrupted PAO1 mutants | PAO1 derivatives | 18, 20, 27, 36 |
| ΔCTPL (previously known as PP2) | PAO1 derivative ctpL (PA4844)::kan | 18 |
| Escherichia coli JM109 | recA1 endA1 gyrA96 thi-1 hsdR17(rk− mk+), e14 negative (mcrA negative) supE44 relA1 Δ(lac-proAB) [F′ traD36 proAB+ lacIq lacZΔM15] | 24 |
| Plasmids | ||
| pUCP18 | Escherichia coli-Pseudomonas shuttle vector; Cbr | 25 |
| pCTPL | pUCP18 containing a 1,974-kb PCR fragment of ctpL (PA4844); Cbr | This study |
Quantitative chemotaxis assay.
Computer-assisted capillary assays were conducted as described previously (27). Cell movement was observed under an inverted microscope within an area of approximately 0.8 by 0.8 mm2 around the capillary orifice and was recorded as an MP4 file. Still digital images captured in each frame were used to count the number of bacterial cells at the initial time (N0) and at each given time interval (Nt) around the mouth of a capillary tube containing a known concentration of the test compound solidified with 1% (wt/vol) agarose. Casamino Acids at 2% (wt/vol) was used as a positive control. The strength of the chemoeffector was determined in terms of the normalized cell number per frame (Nt/N0) as a measure of the magnitude of the chemotactic response of the cell toward the test chemical.
DNA manipulation, plasmid construction, and electroporation.
Standard techniques for DNA preparation, plasmid construction, and cloning were used (24). The enzymes were all from Toyobo, Tokyo, Japan. PAO1 was transformed by electroporation as described previously (18).
RNA extraction and qrt-RT-PCR.
Cells grown overnight in 2× YT medium were used as an inoculum for cell growth in the following media: 11 mM glucose-containing T0, or T5, each with or without 4CA (1 mM). After 6 h of incubation, cells were harvested and the total RNA was extracted from them using a NucleoSpin RNA II kit (Macherey-Nagel, PA) according to the manufacturer's instructions. Two-stage quantitative real-time (qrt) reverse transcriptase PCR (RT-PCR) was performed by first using RT-PCR to generate the cDNA from the total RNA using a One-Step SYBR PrimeScript RT-PCR kit (TaKaRa Bio Inc., Japan) and then using this cDNA for the qrt-PCR stage on a LightCycler (version 1.5) thermocycler (Roche Diagnostics). Thermal cycling for the qrt-PCR was performed at 42°C (5 s), followed by 40 cycles of 95°C (5 s), 57°C (10 s), and 72°C (6 s), using primers for ctpL or gyrB. The gene expression data were normalized to the level of expression of the endogenous reference gyrB gene and are reported as the relative value. The primers for ctpL, which were designed between transmembrane regions 1 and 2 to give an expected product size of 119 bp, were 5′-GGAGTTGCGCGAATTCAG-3′ and 5′-CGGACCATTGTTCCAGGTT-3′. The primers for gyrB were 5′-TGCTGCTGACCTTCTTCTTCC-3′ and 5′-CTTGCTTGCCTTTCTTGACCTT-3′ and gave an expected product size of 98 bp. Subsequently, gene expression data were subjected to one-way analysis of variance with the Student-Newman-Keuls posttest using GraphPad InStat (version 3.00) software (GraphPad, San Diego, CA). Data were the means of at least three independent experiments with standard deviations. P values of <0.05 were considered significant.
4CA biotransformation.
4CA transformation was conducted using a resting-cell suspension of PAO1. Cells were grown at 28°C and 150 rpm for 14 h in mineral salt basal (MSB) medium [4.3 g of K2HPO4, 3.4 g of KH2PO4, 2.0 g of (NH4)2SO4, 0.34 g of MgCl2·6H2O, 1 mg of MnCl2·4H2O, 6 mg of FeSO4·7H2O, 26 mg of CaCl2·2H2O, 0.02 mg of Na2MoO4·2H2O, 0.01 mg of ZnCl2·7H2O, 0.01 mg of CoCl2·6H2O, 0.01 mg of CuSO4, 0.001 mg of NiSO4·6H2O, and 1 μg of Na2SeO4 per liter of deionized water] supplemented with 0.1 g/liter of yeast extract (MSBY). The cells were grown overnight and then harvested. The cell pellet was washed twice with MSB medium and resuspended in MSBY to an optical density at 600 nm of 5. Then, 4CA at 2 mM was added to the suspension culture and the culture was incubated at 28°C and 150 rpm for 30 h. At the specified time interval, samples were taken for chromatography analysis.
Chromatography analysis.
The transformation of 4CA was analyzed by high-performance liquid chromatography (HPLC; JASCO Co., Tokyo, Japan) with a reverse-phase column (TSKgel ODS-80; Tosoh, Yamaguchi, Japan) at a flow rate of 0.8 ml/min. UV absorption was measured at 240 nm. The compounds were eluted using a linear gradient of 10 to 70% (vol/vol) acetonitrile-water over 30 min. Qualitative and quantitative analyses were performed using a range of standard concentrations of 4CA (Nacalai Tesque, Japan) for comparative peak area evaluation. 4CA had a HPLC retention time of 19.18 min under these test conditions. The transformation intermediate formed during the reaction was further analyzed using a p-chloroacetanilide (4CD) standard (Sigma-Aldrich), which had two HPLC retention times of 14.50 and 14.75 min under these test conditions. The sample fraction collected from the transformation reaction was then extracted, concentrated with ethyl acetate, and dried over anhydrous sodium sulfate. The dried sample was redissolved with ethyl acetate prior to identification using liquid chromatography-mass spectroscopy (LC-MS) on an LTQ Orbitrap XL (J108) instrument (Thermo Fischer Scientific Inc., MA) in flow-injection mode. Methanol and ethyl acetate (Riedel-de Haen) were used as controls. The mass spectrum was recorded by use of the monoisotopic negative mode ([M− + H]−), and then the expected mass of each intermediate was determined with the Xcalibur program (Thermo Scientific).
RESULTS
Chemotactic response of Pseudomonas strains to 4CA.
Members of the genus Pseudomonas are ubiquitous in a large variety of natural environments, which reflects not only the fact that they are considered one of the most metabolically diverse microorganisms but also the fact that they have a range of physiological and genetic adaptabilities, such as chemotactic responses to surrounding chemicals, for their growth and survival (28). Several Pseudomonas spp. have been reported to chemotactically respond to aromatic hydrocarbons and their substituted derivatives, but their abilities to sense each of these chemoeffectors varies, presumably due to their chemoreceptor diversity (3, 5–8, 13, 14). In this study, the well-studied motile strains P. putida F1, P. fluorescens Pf01, and P. aeruginosa PAO1 (Table 1) were grown without chemical induction and analyzed for their chemotactic response to 4CA using a computer-assisted capillary assay. All strains showed normal motility and a positive chemotactic response to 2% (wt/vol) Casamino Acids, the positive control, but they responded differently to 4CA. Under the test conditions, F1 and Pf01 exhibited very weak chemotactic responses to 4CA, with the Nt60/Nt0 values ranging from 1 to 1.2 (data not shown), while PAO1 showed a stronger chemotactic attraction (Fig. 1A). This result is in agreement with the fact that the different Pseudomonas species/strains can respond differently to the same chemoattractant (11). Since PAO1, one of the bacteria with a well-characterized chemosensory system (21), positively responded to the environmental pollutant 4CA, PAO1 was selected for further studies.
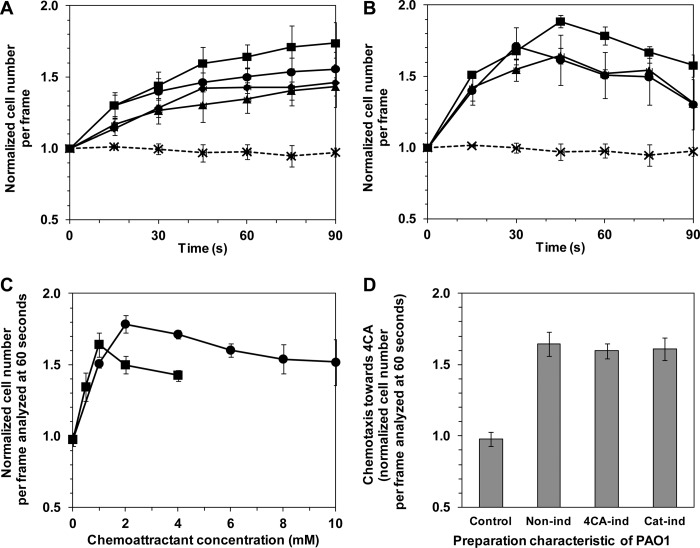
Fig 1.
Quantitative analysis and induction of the chemotactic response of PAO1 to 4CA and catechol. (A, B) Noninduced PAO1 cells were prepared in T0 medium (as described in Materials and Methods) prior to exposure to 4CA at 0.5 mM (▲), 1 mM (■), 2 mM (●), or 4 mM (◆) (A) or catechol at 1 mM (▲), 2 mM (■), 10 mM (●) (B) solidified with 1% (wt/vol) agarose in a capillary tube. The results were expressed as the normalized cell number (Nt/Nt0). The chemotaxis buffer (10 mM HEPES) in agarose (* and dashed line) was the negative control. Casamino Acids was the positive control and gave an Nt60/Nt0 value of approximately 4. (C) Concentration-response curve for the chemotaxis of noninduced PAO1 cells to 4CA (■) or catechol (●) measured at 60 s of exposure and expressed as Nt60/Nt0. (D) Induction of PAO1 chemotaxis to 4CA. Cells were cultured overnight in 2× YT medium and then in 11 mM glucose-containing T0 medium without induction (Non-ind) or with induction by either 1 mM 4CA (4CA-ind) or 1 mM catechol (Cat-ind). Chemotaxis was then performed using a computer-assisted capillary assay with 1 mM 4CA or catechol. The strength of chemotactic attraction is shown as the normalized Nt60/Nt0 cell ratio. In panels A to D, the data are shown as the mean ± SD and are derived from at least three independent experiments conducted in triplicate.
PAO1 chemotactic response and metabolic activity in response to 4CA and aromatic analogues.
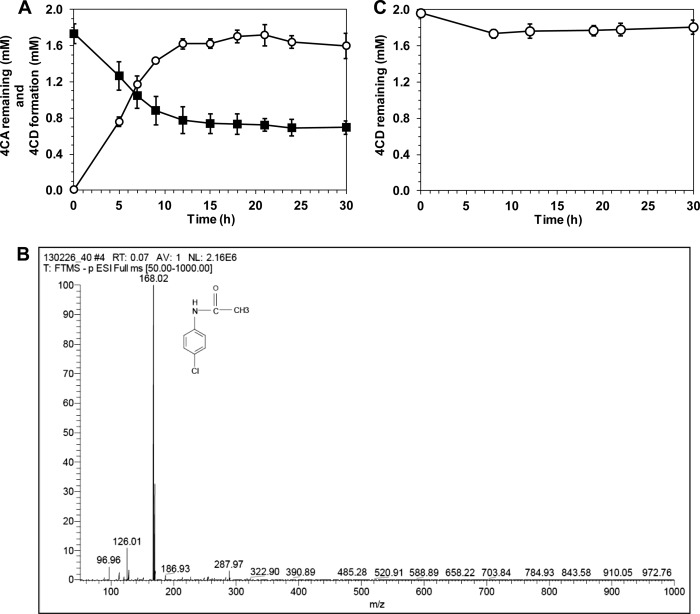
As the chemotactic responses of bacteria allow them to move toward a microenvironment that is optimal for their growth and survival (or away from detrimental ones), several bacterial growth-supporting chemicals generally act as their chemoattractants (29). To determine the metabolic activity and chemotactic behavioral responses of PAO1 to 4CA and other aromatic analogues, cell growth was determined in MSB medium supplemented with each chemical tested as a sole carbon source, while the chemotactic response of cells grown under a noninduced condition was examined. The results revealed that PAO1 was chemotactically attracted by almost all of the metabolizable, growth-supporting test chemicals, the exception being 4-hydroxybenzoic acid (Table 2). Of the four growth-supporting aromatics tested, catechol was the strongest attractant for PAO1. In addition, PAO1 showed positive chemotactic responses to some nonmetabolizable chemicals, such as 3-chloroaniline (3CA), 4CA, 3,4-dichloroaniline, and 4-aminobenzoic acid. Further investigation showed that although PAO1 was not able to utilize 4CA as a carbon source, it could stoichiometrically transform 4CA to 4CD (Fig. 2A and B), which appeared in PAO1 to be a dead-end product in this degradation pathway (Fig. 2C). Chemotaxis assays also revealed that 4CD was not a chemoeffector for PAO1 (Table 2). Thus, the motility behavior of PAO1 toward 4CA was solely a positive chemotactic response of cells to 4CA.
Table 2.
Chemotaxis and growth of PAO1 and the ΔCTPL on chloroanilines and aromatic analogues
| Compounda | Chemotactic responseb |
Cell growthc | |
|---|---|---|---|
| PAO1 | ΔCTPL | ||
| Aniline | − | − | − |
| 2-Chloroaniline (2CA) | − | − | − |
| 3-Chloroaniline (3CA) | + | − | − |
| 4-Chloroaniline (4CA) | + | − | − |
| 2,4-Dichloroaniline (24DCA) | − | ND | − |
| 3,4-Dichloroaniline (34DCA) | + | − | − |
| 4-Chloroacetanilide | − | ND | − |
| Catechol | ++ | − | + |
| 4-Chlorocatechol | + | − | ND |
| Sodium salicylate | − | ND | ND |
| Anthranilic acid | + | − | + |
| 4-Aminobenzoic acid | + | − | − |
| Sodium benzoate | + | − | + |
| 4-Hydroxybenzoic acid | − | ND | + |
| Nitrobenzene | − | ND | ND |
| 4-Chloronitrobenzene | + | − | ND |
| Benzene | − | ND | ND |
| Toluene | − | ND | ND |
| Casamino Acids (positive control) | +++ | ++ | + |
| HEPES (10 mM, negative control) | − | − | − |
| H2O (negative control) | − | − | − |
The concentration of the test compound was 1 mM for the chemotaxis test and 10 mM for growth determination.
The chemotactic response was measured by a computer-assisted capillary assay as described in Materials and Methods. Cell numbers (N) in the analysis frame area were captured at a starting time (t0) and a 60-s chemical exposure time (t60). The strength of chemotactic attraction is shown by the increase in cell number in the analysis frame area at t60 after normalization with that at t0 (Nt60/Nt0). The value of the normalized cell number is represented by the symbols, as follows: 4 ≥ +++ > 3; 3 ≥ ++ > 2; 2 ≥ + > 1.2; 1.2 ≥ − > 0; and ND, not determined.
Cell growth in MSB medium was determined as the optical density of the cells at 600 nm (OD600) after 24 h of incubation. The cell optical density value is represented by the symbols, as follows: ++, >0.6; 0.6 ≥ + > 0.1; 0.1 ≥ − > 0 (no growth); and ND, not determined.
Fig 2.
Biotransformation of 4CA and the formation of a transformation product in PAO1. (A) Biotransformation of 4CA (■; initial concentration, 2 mM) by PAO1 using a resting-cell technique and the formation of 4-chloroacetanilide (4CD; ○) as a transformation product. (B) LC-MS spectra of the transformation product obtained from the PAO1 culture supernatant of medium containing 4CA after 15 h of incubation. The m/z value was 168.02, which indicates that 4CD (C8H7ONCl) is the transformation product. (C) Biotransformation of 4CD (○; initial concentration, 2 mM) by PAO1 using a resting-cell technique.
The results of the quantitative analysis of PAO1 chemotaxis with various concentrations of the nonmetabolizable 4CA and the metabolizable catechol were expressed as bell-shaped concentration-response curves, which indicated that the cell chemotactic response was dependent on the chemoeffector concentration (Fig. 1A to C). The strength of the chemotactic response of PAO1 to both 4CA and catechol increased as the concentration increased up to the optimal concentration of 1 mM for 4CA and 2 mM for catechol and then decreased at higher concentrations (Fig. 1C). The difference in the cell chemotactic behavior toward the nonmetabolizable 4CA and the metabolizable catechol was that the cell chemoattraction to catechol rapidly declined after 45 s of exposure (Fig. 1B), whereas it tended to reach a plateau for 4CA (Fig. 1A).
The results presented above indicate that the chemotactic response of PAO1 to 4CA is constitutive under the conditions tested. Nevertheless, this natural ability can generally be enhanced if it is inducible (9). Therefore, an induction test was conducted by growing cells in the presence and absence of its chemoeffector as an inducer (1 mM either 4CA or catechol) for 6 h prior to chemotaxis assay with each corresponding chemoeffector. The results indicated that there was no noticeable difference in the apparent chemotactic strength toward 4CA among noninduced cells, 4CA-induced cells, and catechol-induced cells (Fig. 1D).
Identification of the che gene involved in the chemotactic response of PAO1 to 4CA.
PAO1 has a complex chemosensory system with several known chemotaxis (che) genes (21, 30, 31). To identify the che gene(s) potentially involved in the chemoattraction of PAO1 to 4CA, five Che cluster-defective mutants (Table 1) were examined for their responses to 4CA. All of these mutants were confirmed to be fully motile. Upon testing with 4CA (1 mM) using the computer-assisted capillary assay, the ΔCHE1 mutant, which has a deletion of che cluster 1 (cheY cheZ cheA cheB motA2 motB2 cheW), and PC4, a cheR mutant, completely lost chemoattraction to 4CA, while the chemotactic behavior of the ΔCHE3 (ΔPil-Chp cluster), ΔCHE4 (ΔChe2 cluster), and ΔCHE5 (ΔWsp cluster) mutants remained normal and similar to the response of the PAO1 wild type (WT) (Fig. 3A). The results indicate that the chemoattraction of PAO1 to 4CA appears to essentially involve the action of che cluster 1 and cheR. This indicates that PAO1 chemotaxis to 4CA is, in fact, methyl-accepting chemotaxis protein (MCP) dependent.
Fig 3.
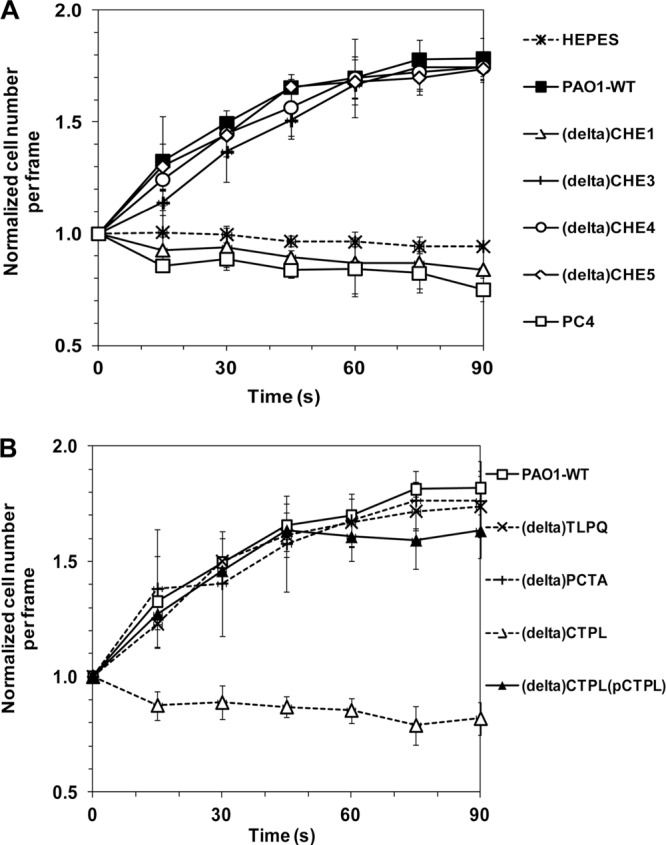
Chemotactic response of PAO1 mutants to 4CA. Noninduced PAO1 mutants and the WT were prepared prior to exposure to 4CA (1 mM) in a computer-assisted capillary assay. The chemotactic response of PAO1 WT to 4CA was used as the reference positive control. The results are expressed as the normalized cell number (Nt/Nt0). (A) Chemotactic behavior of che mutants ΔCHE1, ΔCHE3, ΔCHE4, ΔCHE5, and PC4. The response of the PAO1 WT to chemotaxis buffer (HEPES)-containing agar was the negative control. (B) Chemotactic behavior of the single Δmcp mutants. The responses of the tlpQ-deficient mutant (ΔTLPQ) and pctA-deficient mutant (ΔPCTA) are shown as representative examples of the responses of the mcp mutants, which exhibited chemotaxis similar to that of the PAO1 WT, while no chemotactic response to 4CA was found in the ctpL-deficient mutant (ΔCTPL), but the response was restored by ctpL gene complementation with the ΔCTPL(pCTPL) strain. In panels A and B, the data are shown as the mean ± SD and are derived from at least three independent experiments conducted in triplicate.
CtpL was identified as a chemotactic transducer for 4CA and catechol in PAO1.
Bacterial cells detect chemoeffectors using cell surface MCP chemoreceptors. PAO1 contains 26 known mcp-like genes in its genome (21, 30, 31). To identify which mcp gene(s) is potentially involved in the 4CA chemoattraction, each of a series of 26 mcp mutants which had previously been generated by either deletion or insertion of a kanamycin resistance cassette (kan) of an individual mcp-like gene in the PAO1 genome (18, 32, 33) was examined for its chemotactic behavior toward 4CA. Twenty-five of these mcp mutants exhibited a normal chemoattractive behavior toward 4CA that was similar to that of the PAO1 WT. The responses of the tlpQ (PA2573)- and pctA (PA4309)-deficient mutants are shown as examples in Fig. 3B. In contrast, the ctpL-deficient mutant (ΔCTPL) showed no detectable chemotactic response to 4CA (Fig. 3B).
To elucidate the potential role of CtpL in the chemoattraction of PAO1 to 4CA, gene complementation was conducted prior to assaying the transformant for its chemotactic ability. A plasmid harboring a functional copy of ctpL (pCTPL) was constructed (Table 1) and introduced into the corresponding ΔctpL single mutant. Positive chemotaxis to 4CA was fully restored in the transformed ΔCTPL(pCTPL) strain (Fig. 3B). Furthermore, the chemotactic response of the ctpL-deficient mutant with and without ctpL complementation to catechol (2 mM) revealed similar results; the ΔCTPL strain showed no positive response to catechol, while the ΔCTPL(pCTPL) transformant fully regained normal chemoattraction (data not shown). This result suggests that CtpL is responsible for the positive chemotaxis toward 4CA and catechol in PAO1.
In addition, to further examine the gene dosage dependency of the chemotactic response, the chemotaxis assay with 4CA was conducted with the ctpL-overexpressing PAO1 transformant PAO1(pCTPL). Chemoattraction to 4CA in the ctpL-overexpressing cells was not significantly increased (data not shown), suggesting that additional ctpL gene copies did not markedly enhance the positive chemotactic response in PAO1.
Expression of ctpL and the cell response toward 4CA and catechol in the presence of Pi.
CtpL has previously been identified as a chemoreceptor in PAO1 for the chemotaxis to Pi at low Pi concentrations (18). The synthesis of CtpL is transcriptionally regulated by the phosphate starvation regulon (Pho regulon) under conditions of Pi limitation, but not under conditions of excess Pi (18, 34). In this study, the effect of the chemoeffectors (4CA and catechol) on ctpL expression and on the cell chemotactic response under condition of Pi starvation (T0 medium) or excess Pi (T5 medium) was investigated using qrt-RT-PCR analysis. Cultures were initially grown in 2× YT medium with excess Pi to suppress expression of ctpL, and then cells were reinoculated and grown for 6 h in 11 mM glucose-containing T0 medium to induce ctpL expression or in T5 medium to keep the ctpL levels suppressed. In both media, 1 mM 4CA or 2 mM catechol was added at the concentration previously determined to be optimal for cell chemoattraction. In the Pi-deficient T0 medium, the ctpL transcript level was relatively high, as expected, and also detectable in the presence of catechol or 4CA (although the expression levels were 3.5- and 2.5-fold lower than the level in the absence of the test chemoeffector) (Fig. 4). In the Pi-rich T5 medium, the ctpL transcript level was very low, as expected (6-fold less than that in T0 medium), but was upregulated 1.3- and 2.4-fold in the presence of catechol or 4CA, respectively (Fig. 4).
Fig 4.
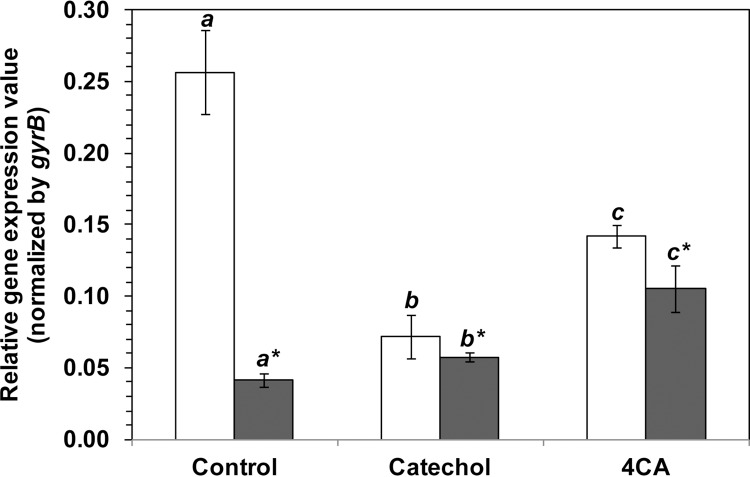
qrt-RT-PCR analysis of ctpL mRNA expression in PAO1. Cells grown overnight in 2× YT medium were used as an inoculum for cell growth in 11 mM glucose-containing T0 (open bars) or T5 (solid bars) medium, each in the presence of the solvent only (Control), catechol (2 mM), or 4CA (1 mM). After 6 h of incubation, total RNA was isolated and qrt-RT-PCR analysis of the ctpL and gyrB transcript levels was performed. The results are expressed as the relative ctpL gene expression value after normalization to the gyrB transcript level. Data are shown as the mean ± 1 SD and are derived from at least three independent experiments. Italic letters (with and without an asterisk) indicate a significant difference (P < 0.05) from the control within the same group, according to the Student-Newman-Keuls posttest.
Analysis of the chemotactic responses of PAO1 toward 4CA and catechol in the presence of Pi at 0, 1, 10, and 100 μM in chemotaxis buffer revealed a positive chemoattraction of cells toward 4CA and catechol at low Pi concentrations (0 and 1 μM) (Fig. 5). However, essentially no chemoattraction to the chemoeffector was noted at the higher Pi concentrations of 10 μM and 100 μM (Fig. 5).
Fig 5.
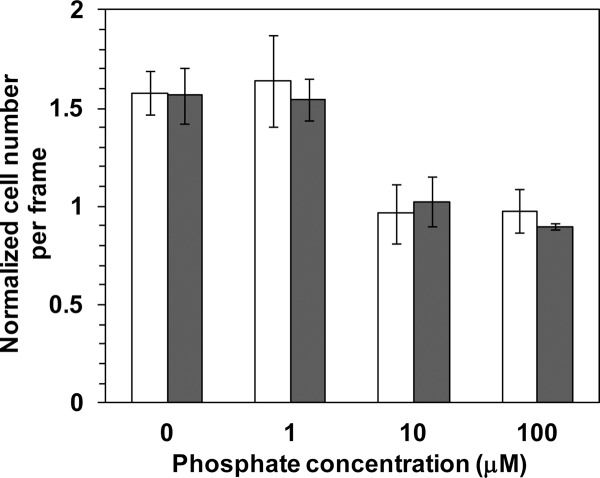
Quantitative analysis of the chemotactic response of PAO1 to 2 mM catechol (open bars) and 1 mM 4CA (solid bars) in the presence of different Pi concentrations in the chemotaxis buffer using a computer-assisted capillary assay. Cells grown overnight in 2× YT medium were cultured in T0 or T5 medium, as indicated in the text, and then resuspended in the chemotaxis buffer with various concentrations of Pi (KH2PO4). The strength of the chemotactic attraction was determined by the increase in the cell number in the analysis frame area at t60 (60-s exposure) normalized to that at t0 (Nt60/Nt0). Data are shown as the mean ± SD and are derived from at least three independent experiments conducted in triplicate.
DISCUSSION
Chemotaxis is one of the most important bacterial mechanisms for sensing and responding to chemical changes in ecologically diverse environments, including toxic environments. The genus Pseudomonas has been shown to have a capability for broad chemotactic responses to a wide range of compounds, which in part allows its physiological and metabolic diversity (28, 35). Previous studies have shown that several Pseudomonas spp. are capable of chemotactic responses to a wide range of aromatic pollutants, but with diverse sensing specificities (3, 5–8, 13, 14). In this study, among the three well-studied, motile Pseudomonas spp. evaluated, only PAO1 showed a positive chemotactic response to 4CA, the target pollutant.
The difference in bacterial chemosensing ability lies in the type and number of chemoreceptors. PAO1 has a complex chemosensory system with more than 20 chemotaxis (che) genes and 26 chemoreceptor genes (21), which results in its ability to thrive in various environmental niches. Chemotactic behavior is based on the recognition of environmental signals through cell surface methyl-accepting chemotaxis proteins (MCPs). So far the following MCPs have been identified in PAO1 for several stimuli: PctA, PctB, and PctC for amino acids (36), CtpL and CtpH for inorganic phosphate (37), Aer and Aer-2 for oxygen (32), PA2652 for malate (38), TlpQ for ethylene (39), and PctA for volatile chlorinated aliphatic hydrocarbons (40). In this study, a role of CtpL (PA4844), a chromosomally encoded, transmembrane protein in PAO1, as a chemotactic transducer for aromatic compounds, including 4CA and catechol, was revealed. Although the precedent of a chromosomally encoded chemoreceptor(s) for aromatic hydrocarbons was briefly discussed for strain DOT-T1E (5), all previously reported chemoreceptor genes for aromatic pollutants so far have been plasmid borne (5, 13, 14). Moreover, chemoreceptor gene products in bacteria with similar chemotactic phenotypes have been reported to be close homologues. For instance, homologues of the McpT receptor with 99% sequence identity were found in a self-transmissible plasmid in DOT-T1E and the pMAQU02 plasmid of Marinobacter aquaeolei VT8, both of which are responsible for the phenotype of chemotaxis toward aromatic hydrocarbons (41). In contrast, the distinct phenotype of chemotaxis toward 4CA in P. aeruginosa PAO1, P. putida F1, and P. fluorescens Pf01 is perhaps the result of a low sequence identity (65 to 66%) between CtpL in PAO1 and the Blast-matched MCP in F1 (NCBI reference sequence YP_001265953.1) and Pf01 (NCBI reference sequence YP_346355.1).
In general, compounds capable of promoting and/or inducing a positive chemotactic response serve as metabolizable nutrients. In accord with this, PAO1 exhibited a relatively strong attraction to the metabolizable catechol, which it can utilize as a sole carbon source. The rapid decline in cell chemoattraction, which was observed after 45 s of catechol exposure, likely reflected the decreased local concentration of catechol due to its uptake and metabolism. In contrast, PAO1 could transform 4CA only to 4-chloroacetanilide, which then accumulated as a dead-end product, indicating that the chemotactic response of PAO1 is independent of the cell metabolic activity to 4CA. Although the physiological sensing of bacterial chemotaxis to nonmetabolizable compounds is not yet thoroughly understood, the results indicate that CtpL in PAO1 can recognize and mediate a positive chemotactic response toward metabolizable and nonmetabolizable aromatic pollutants.
The capability of CtpL to be a chemoreceptor for the test aromatic pollutants is proposed to be a fortuitous activity in PAO1, since CtpL has been established to be a major chemoreceptor for Pi taxis at low Pi concentrations, while CtpH functions for Pi taxis at high Pi concentrations (18). The expression of the ctpL gene is induced under Pi-limiting conditions under the regulation of the PhoB and PhoU proteins (18). In this study, because cells were initially cultured under Pi starvation conditions (in T0 medium) prior to the assay, a relatively high transcript expression level was detected. This high level of expression correlates with the positive chemotactic response of the noninduced cells to 4CA or catechol. When cells were cultured in the presence of 5 mM Pi in T5 medium, the ctpL transcript expression level was suppressed but could be significantly increased by the addition of 4CA or catechol to the T5 medium, indicating the positive inducibility of ctpL by these chemoeffectors. Nevertheless, the physiological chemotactic response of PAO1 cells toward these chemoeffectors was reduced to a much greater extent in the presence of high concentrations of Pi, probably due to the toxicity effect of the pollutant. These results suggest that both Pi limitation and the presence of an aromatic chemoeffector (catechol or 4CA) are the inducers for ctpL transcript expression, but the response is not cumulative. In addition, this may suggest that these chemoeffectors and Pi share a common binding site on CtpL. Further investigation by a direct binding assay is required to clarify this point. Despite the fact that the increase in ctpL expression was conducted by either gene induction or an increase in the gene dosage in PAO1(pCTPL), the physiological chemosensing activity of PAO1 toward 4CA was not markedly enhanced. This could reflect the fact that CtpL does not function independently like other chemoreceptors for aromatic pollutants. To acquire full function, it may require complex formation with other proteins in the Pst system under the regulation of the Pho regulon (18).
In summary, the results of this study reveal that CtpL acts as a chemoreceptor for the positive chemotactic response of PAO1 to the nonmetabolizable 4CA as well as the metabolizable catechol. The discovery of a chromosomally encoded chemoreceptor with broader fortuitous activity toward aromatic pollutants in PAO1 expands the range of the known chemotactic transducers and their function. Future comprehensive investigation of CtpL-protein complex formation and its regulation will be necessary to elicit or enhance the positive chemotactic response of PAO1 or other environmental bacteria toward aromatic pollutants, including 4CA.
ACKNOWLEDGMENTS
This work was supported by the Bio-Oriented Technology Research Advancement Institution (BRAIN) in the National Agricultural and Food Research Organization, Japan (NARO), and by the Thai Government Stimulus Package 2 (TKK2555) under the Project for Establishment of a Comprehensive Center for Innovative Food, Health Products, and Agriculture (PERFECTA).
Footnotes
Published ahead of print 13 September 2013
REFERENCES
- 1.Pandey J, Chauhan A, Jain RK. 2009. Integrative approaches for assessing the ecological sustainability of in situ bioremediation. FEMS Microbiol. Rev. 33:324–375 [DOI] [PubMed] [Google Scholar]
- 2.Hawkins AC, Harwood CS. 2002. Chemotaxis of Ralstonia eutropha JMP134(pJP4) to the herbicide 2,4-dichlorophenoxyacetate. Appl. Environ. Microbiol. 68:968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Parales RE. 2009. Bacterial chemotaxis to atrazine and related s-triazines. Appl. Environ. Microbiol. 75:5481–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwood CS, Rivelli M, Ornston LN. 1984. Aromatic acids are chemoattractants for Pseudomonas putida. J. Bacteriol. 160:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacal J, Munoz-Martinez F, Reyes-Darias JA, Duque E, Matilla M, Segura A, Calvo JJ, Jimenez-Sanchez C, Krell T, Ramos JL. 2011. Bacterial chemotaxis towards aromatic hydrocarbons in Pseudomonas. Environ. Microbiol. 13:1733–1744 [DOI] [PubMed] [Google Scholar]
- 6.Parales RE, Ditty JL, Harwood CS. 2000. Toluene-degrading bacteria are chemotactic towards the environmental pollutants benzene, toluene, and trichloroethylene. Appl. Environ. Microbiol. 66:4098–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordillo F, Chavez FP, Jerez CA. 2007. Motility and chemotaxis of Pseudomonas sp. B4 towards polychlorobiphenyls and chlorobenzoates. FEMS Microbiol. Ecol. 60:322–328 [DOI] [PubMed] [Google Scholar]
- 8.Grimm AC, Harwood CS. 1997. Chemotaxis of Pseudomonas spp. to the polyaromatic hydrocarbon naphthalene. Appl. Environ. Microbiol. 63:4111–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey J, Sharma NK, Khan F, Ghosh A, Oakeshott JG, Jain RK, Pandey G. 2012. Chemotaxis of Burkholderia sp. strain SJ98 towards chloronitroaromatic compounds that it can metabolise. BMC Microbiol. 12:19. 10.1186/1471-2180-12-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parales RE. 2004. Nitrobenzoates and aminobenzoates are chemoattractants for Pseudomonas strains. Appl. Environ. Microbiol. 70:285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols NN, Lunde TA, Graden KC, Hallock KA, Kowalchyk CK, Southern RM, Soskin EJ, Ditty JL. 2012. Chemotaxis to furan compounds by furan-degrading Pseudomonas strains. Appl. Environ. Microbiol. 78:6365–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacal J, Reyes-Darias JA, Garcia-Fontana C, Ramos JL, Krell T. 2013. Tactic responses to pollutants and their potential to increase biodegradation efficiency. J. Appl. Microbiol. 114:923–933 [DOI] [PubMed] [Google Scholar]
- 13.Grimm AC, Harwood CS. 1999. NahY, a catabolic plasmid-encoded receptor required for chemotaxis of Pseudomonas putida to the aromatic hydrocarbon naphthalene. J. Bacteriol. 181:3310–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwaki H, Muraki T, Ishihara S, Hasegawa Y, Rankin KN, Sulea T, Boyd J, Lau PC. 2007. Characterization of a pseudomonad 2-nitrobenzoate nitroreductase and its catabolic pathway-associated 2-hydroxylaminobenzoate mutase and a chemoreceptor involved in 2-nitrobenzoate chemotaxis. J. Bacteriol. 189:3502–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Toxic Substances and Disease Registry 2011. The Agency for Toxic Substances and Disease Registry (ATSDR) 2011 priority list of hazardous substances, p 20 Agency for Toxic Substances and Disease Registry, Atlanta, GA [Google Scholar]
- 16.Dom N, Knapen D, Benoot D, Nobels I, Blust R. 2010. Aquatic multi-species acute toxicity of (chlorinated) anilines: experimental versus predicted data. Chemosphere 81:177–186 [DOI] [PubMed] [Google Scholar]
- 17.Hongsawat P, Vangnai AS. 2011. Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J. Hazard. Mater. 186:1300–1307 [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Kato J, Kuroda A, Ikeda T, Takiguchi N, Ohtake H. 2000. Identification and characterization of two chemotactic transducers for inorganic phosphate in Pseudomonas aeruginosa. J. Bacteriol. 182:3400–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compeau G, Al-Achi BJ, Platsouka E, Levy SB. 1988. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl. Environ. Microbiol. 54:2432–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong CS, Shitashiro M, Kuroda A, Ikeda T, Takiguchi N, Ohtake H, Kato J. 2004. Chemotaxis proteins and transducers for aerotaxis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 231:247–252 [DOI] [PubMed] [Google Scholar]
- 21.Kato J, Kim HE, Takiguchi N, Kuroda A, Ohtake H. 2008. Pseudomonas aeruginosa as a model microorganism for investigation of chemotactic behaviors in ecosystem. J. Biosci. Bioeng. 106:1–7 [DOI] [PubMed] [Google Scholar]
- 22.Kato J, Nakamura T, Kuroda A, Ohtake H. 1999. Cloning and characterization of chemotaxis genes in Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 63:155–161 [DOI] [PubMed] [Google Scholar]
- 23.Royle PL, Matsumoto H, Holloway BW. 1981. Genetic circularity of the Pseudomonas aeruginosa PAO1 chromosome. J. Bacteriol. 145:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 25.Schweizer HP. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109–121 [DOI] [PubMed] [Google Scholar]
- 26.Spain JC, Gibson DT. 1988. Oxidation of substituted phenols by Pseudomonas putida F1 and Pseudomonas sp. strain JS6. Appl. Environ. Microbiol. 54:1399–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikata T, Sumida K, Kato J, Ohtake H. 1992. Rapid method for analyzing bacterial behavioral responses to chemical stimuli. Appl. Environ. Microbiol. 58:2250–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiers AJ, Buckling A, Rainey PB. 2000. The causes of Pseudomonas diversity. Microbiology 146(Pt 10):2345–2350 [DOI] [PubMed] [Google Scholar]
- 29.Alexandre G, Zhulin IB. 2001. More than one way to sense chemicals. J. Bacteriol. 183:4681–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croft L, Beatson SA, Whitchurch CB, Huang B, Blakeley RL, Mattick JS. 2000. An interactive web-based Pseudomonas aeruginosa genome database: discovery of new genes, pathways and structures. Microbiology 146(Pt 10):2351–2364 [DOI] [PubMed] [Google Scholar]
- 31.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964 [DOI] [PubMed] [Google Scholar]
- 32.Hong CS, Kuroda A, Ikeda T, Takiguchi N, Ohtake H, Kato J. 2004. The aerotaxis transducer gene aer, but not aer-2, is transcriptionally regulated by the anaerobic regulator ANR in Pseudomonas aeruginosa. J. Biosci. Bioeng. 97:184–190 [DOI] [PubMed] [Google Scholar]
- 33.Taguchi K, Fukutomi H, Kuroda A, Kato J, Ohtake H. 1997. Genetic identification of chemotactic transducers for amino acids in Pseudomonas aeruginosa. Microbiology 143(Pt 10):3223–3229 [DOI] [PubMed] [Google Scholar]
- 34.Vershinina OA, Znamenskaia LV. 2002. The Pho regulons of bacteria. Mikrobiologiia 71:581–595 [PubMed] [Google Scholar]
- 35.Miller LD, Russell MH, Alexandre G. 2009. Diversity in bacterial chemotactic responses and niche adaptation. Adv. Appl. Microbiol. 66:53–75 [DOI] [PubMed] [Google Scholar]
- 36.Kuroda A, Kumano T, Taguchi K, Nikata T, Kato J, Ohtake H. 1995. Molecular cloning and characterization of a chemotactic transducer gene in Pseudomonas aeruginosa. J. Bacteriol. 177:7019–7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato J, Ito A, Nikata T, Ohtake H. 1992. Phosphate taxis in Pseudomonas aeruginosa. J. Bacteriol. 174:5149–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Ortega C, Harwood CS. 2007. Identification of a malate chemoreceptor in Pseudomonas aeruginosa by screening for chemotaxis defects in an energy taxis-deficient mutant. Appl. Environ. Microbiol. 73:7793–7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HE, Shitashiro M, Kuroda A, Takiguchi N, Kato J. 2007. Ethylene chemotaxis in Pseudomonas aeruginosa and other Pseudomonas species. Microbes Environ. 22:186–189 [Google Scholar]
- 40.Shitashiro M, Tanaka H, Hong CS, Kuroda A, Takiguchi N, Ohtake H, Kato J. 2005. Identification of chemosensory proteins for trichloroethylene in Pseudomonas aeruginosa. J. Biosci. Bioeng. 99:396–402 [DOI] [PubMed] [Google Scholar]
- 41.Krell T, Lacal J, Guazzaroni ME, Busch A, Silva-Jimenez H, Fillet S, Reyes-Darias JA, Munoz-Martinez F, Rico-Jimenez M, Garcia-Fontana C, Duque E, Segura A, Ramos JL. 2012. Responses of Pseudomonas putida to toxic aromatic carbon sources. J. Biotechnol. 160:25–32 [DOI] [PubMed] [Google Scholar]