Abstract
Brucella abortus, a smooth strain of the genus Brucella, is the causative agent of bovine brucellosis. To support the ongoing development of diagnostic tests for bovine brucellosis, the use of Protein Saver cards (Whatman) for bovine blood serum and plasma sample collection has been evaluated. These cards offer significant logistical and safety alternatives to transporting and storing liquid samples and may aid in diagnostic programs and validation studies. To evaluate the utility of these cards, 204 bovine blood serum samples from Brucella-infected and noninfected animals were stored on and eluted from the Protein Saver cards. Anti-Brucella smooth lipopolysaccharide (sLPS) antibody titers for the serum eluates were compared to those of the unprocessed original serum samples by indirect enzyme-linked immunosorbent assay (ELISA). The results showed a highly significant correlation between titers from the serum eluates and the unprocessed sera. Therefore, under these circumstances, serum eluates and unprocessed serum samples may be used interchangeably. Blood plasma from 113 mitogen-stimulated whole-blood samples was added to and eluted from the Protein Saver cards. The gamma interferon (IFN-γ) titers in the plasma eluates were compared to those of the unprocessed plasma samples obtained by IFN-γ ELISA. The results showed a significant correlation between the plasma eluates and the unprocessed plasma samples. To derive a signal in the plasma eluate, it was necessary to develop a novel and highly sensitive ELISA for the detection of IFN-γ. The serum samples stored on cards at room temperature over a 10-day period showed little variation in antibody titers. However, the plasma eluates showed a progressive loss of IFN-γ recovery over 10 days when stored at room temperature.
INTRODUCTION
Brucella abortus is a Gram-negative facultative intracellular bacterium that causes disease in both humans and animals. In humans, brucellosis causes undulant fever, arthritis, endocarditis, and meningitis (1). In domestic and wild animals, the disease causes abortion and infertility (2). Brucellosis is prevalent worldwide and is widely distributed (3). It is therefore a disease of significant economic and social importance.
Bacteriological isolation of B. abortus is considered to be the gold standard for diagnosis; however, culture methods are time-consuming, require considerable expertise, and can result in low frequency of isolation (4). There is a lack of clinical signs of infection other than abortions; therefore, serological tests are used for surveillance, as they are cost-effective, rapid, and useful for diagnosis (5). Serological techniques are therefore considered to be the mainstay of diagnostic and mass testing programs for screening, trade, and eradication schemes (6). However, classical and contemporary serological tests are prone to problems with false-positive serological reactors (FPSRs) caused by cross-reactions with other Gram-negative bacteria that possess a similar O polysaccharide, such as Yersinia enterocolitica (Ye 0:9), in areas of low or zero brucellosis prevalence (7). Such FPSRs may result in the unnecessary isolation of animals, trade restrictions, animal slaughter, loss of value, and compensation costs.
The impact of cross-reactivity in serological assays leading to FPSRs may be reduced by additionally measuring the cellular immune response to Brucella antigens. The Brucella-specific production of gamma interferon (IFN-γ) in blood plasma produced by lymphocytes in antigen-stimulated whole-blood samples may be detected by indirect enzyme-linked immunosorbent assay (iELISA) (8, 9). Therefore, further refinements of these serological and cellular methods and their subsequent validation with large sample numbers are of interest.
Laboratories in countries with an official brucellosis-free status rely on archived collections and collaborations with other countries to establish panels of samples for use in assay development and validation (10). The import of samples can prove to be a challenge due to the legal restrictions on importing material from infected animals and the requirement for cold transportation, as the latter is not always available or is too expensive in some countries where brucellosis is endemic. The transportation of liquid samples is also considered to be a safety risk due to the potential infection hazard that would occur in the event of leakage. Samples on cards are still considered infectious material; however, the volumes of the samples are reduced and the samples are not in a liquid format, thus reducing the risk of leakage. Protein Saver cards (Whatman) may therefore be a valuable tool for aiding sample transportation and establishing a panel of samples.
Protein Saver cards (Whatman), often known as Guthrie cards, have been used as a means of sample storage for neonatal screening since 1963 (11). Since the development of these cards, they have been used in hospital laboratories for testing card eluates by ELISA and PCR for the clinical diagnosis of multiple diseases (12–18). These cards have also been used in veterinary diagnostics, most recently for the detection of anti-Brucella antibodies in caribou (19–21). To our knowledge, the use of these cards to directly store and elute bovine blood serum and plasma samples for the detection of anti-Brucella lipopolysaccharide (LPS) antibodies and IFN-γ is a novel use for a well-established method.
In this study, the blood serum and plasma supernatants were absorbed onto and subsequently eluted off Protein Saver cards (Whatman). In order to detect the IFN-γ content in the plasma eluates, it was necessary to develop a more sensitive ELISA, since the volume of sample eluted from each spot was 1.875 μl (when calculating the volume of sample within the area of a circle) and we could not replicate the 1:2 dilution (50 μl plasma and 50 μl buffer) required for the Bovigam IFN-γ ELISA. IFN-γ was undetectable at such small volumes, so the IFN-γ ELISA was modified with a streptavidin–poly-horseradish peroxidase (HRP) amplification step to increase sensitivity and derive a signal from the plasma eluate. The Protein Saver card eluates were tested in parallel with the unprocessed original samples by ELISAs in order to detect both Brucella-specific antibodies and cytokines raised in response to Staphylococcus enterotoxin B (SEB) mitogens. The aim of this investigation was to determine whether samples stored on these cards can be used interchangeably with unprocessed liquid samples. The interchangeable use of cards and liquid samples may ultimately lead to an increased number of samples being available for validation, test development, and assistance for diagnostic efforts in other countries.
MATERIALS AND METHODS
Sample collection.
A panel of 204 bovine blood serum samples was selected from a frozen serum archive. The panel contained 28 samples from cattle confirmed by bacterial culture to be infected with B. abortus and 16 serologically positive serum samples from cattle within areas of Turkey where brucellosis is endemic, all subsequently referred to as “Brucella-infected.” The panel also included 160 serum samples from cattle from a Brucella-free area (Great Britain), subsequently referred to as “noninfected.” Bovine whole-blood samples were also collected for IFN-γ detection from 113 animals in areas of Turkey where Brucella spp. are endemic. The serum sample for the time study was from an experimentally infected Brucella culture-positive animal. The SEB-stimulated whole-blood sample for the time study was from a Brucella-negative animal from Great Britain.
Standard indirect ELISA (iELISA) for the detection of anti-Brucella smooth LPS antibodies in serum.
The iELISA smooth lipopolysaccharide (sLPS) antigen was prepared by hot phenol extraction (22, 23). The iELISA was performed as described previously (24), with some modifications. Briefly, Nunc 96-well polystyrene plates were coated with B. abortus strain S99 sLPS antigen, incubated for 18 to 24 h between 2 and 8°C, and then washed five times with distilled water.
Bovine serum samples were diluted 1:200 in phosphate-buffered saline (PBS)-Tween 20 and added to the B. abortus S99 sLPS antigen-coated ELISA plate. The plate was incubated for 1 h at room temperature on a rotary plate shaker at 120 rpm. Next, the plate was washed five times with PBS-Tween 20. Sheep anti-bovine IgG-horseradish peroxidase conjugate (Serotec) was diluted to a working strength of 0.1 μg/ml in PBS-Tween 20 and added to the plate. The plate was incubated as described above.
The plate was washed again, as described before. Next, substrate buffer (Fluka) with H2O2 (VWR) and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) chromogen (Sigma) were added to the plate and incubated at room temperature for 15 min. The reaction was then slowed with 0.01 M sodium azide stopping solution and the plate was read at an absorbance of 405 nm using a Beckman Coulter plate reader. The optical density (OD) was indicative of the amount of antibody binding, and the results were calculated as the mean ODs of the samples, tested in duplicate.
Whole-blood antigenic stimulation IFN-γ assay.
Bovine whole blood was collected from the jugular vein in heparinized Vacutainers and stimulated within 18 to 24 h after collection, following a method based upon the method developed by Weynants et al. (8). Briefly, SEB (Sigma) was diluted on the plate in sterile PBS at 8 μg/ml, for a final dilution of 4 μg/ml. SEB was included as a mitogen to generate IFN-γ for the measurement of the amount of IFN-γ recovered from the card compared to that recovered from the unprocessed blood plasma sample. A negative stimulatory control containing no antigen, only sterile PBS, was also included. Each whole-blood sample was tested with the SEB and PBS in duplicate.
The SEB and PBS were added to a Nunclon Delta 96-well MicroWell cell culture plate (Thermo Scientific Nunc) (100 μl per well). Next, 100 μl of whole-blood sample was added to each well and mixed. The samples were incubated at 37°C with 5% CO2 for 18 to 24 h.
After stimulation, the red blood cells had settled and the plasma supernatant was harvested from each well and stored in nonbinding storage plates at −20°C until further analysis using the Bovigam IFN-γ test kit (Prionics AG, Switzerland) or an adaptation of this method (as described below).
Bovigam IFN-γ test kit ELISA.
IFN-γ content was measured using an ELISA kit, the Bovigam gamma interferon test kit for cattle (Prionics AG, Switzerland). The test was performed according to the manufacturer's instructions (25, 26). The optical densities of the assay wells containing no sample were subtracted from the optical densities of the antigen-stimulated blood plasma samples and controls in order to calculate the results. The results were then expressed as the OD as a percentage of the Bovigam IFN-γ test kit positive control OD.
Protein Saver cards smooth LPS iELISA for serum.
Blood serum (30 μl) was placed on premarked 1.2-cm-diameter sample circles on the Whatman Protein Saver 903 cards and dried overnight at room temperature.
A 3-mm-diameter disposable biopsy punch with a plunger (Miltex) was used to punch out spots from the cards impregnated with the serum samples.
The spots (1 spot per well) were added to a Nunclon Delta 96-well MicroWell plate (Thermo Scientific Nunc) containing 300 μl PBS-Tween. The plate was incubated at room temperature for 18 to 24 h on a rotary shaker at 120 rpm to elute analytes from the spots.
Blood serum-impregnated spot eluates were added without further dilution (100 μl per well) to wells of a B. abortus S99 LPS antigen-coated ELISA plate. The unprocessed original serum samples were tested alongside on the same plate following the serum iELISA method described above.
Modified IFN-γ ELISA.
An amplification step using streptavidin–poly-horseradish peroxidase (poly-HRP; Fisher) was investigated (27).
(i) Development of the modified IFN-γ ELISA.
The Bovigam IFN-γ test kit (Prionics AG, Switzerland) was modified for enhanced sensitivity by the inclusion of an amplification step using streptavidin-poly-HRP. No Protein Saver cards were used for this initial development.
The Bovigam IFN-γ test kit positive control was titrated in doubling dilutions in the kit sample diluent and added to the kit microplate in duplicate wells (100 μl per well). The sample was tested following the Bovigam IFN-γ test kit method as described above.
The same dilutions of the Bovigam IFN-γ test kit positive control were also tested using an amplification step. The sample dilutions were added to a test kit microplate, incubated on a rotary shaker at 600 rpm for 1 h, and washed 6 times with wash buffer. Mouse anti-bovine IFN-γ:biotin (Serotec) was diluted to 0.5 μg/ml in Bovigam IFN-γ test kit conjugate diluent buffer instead of the kit's mouse anti-bovine HRP-labeled conjugate; 100 μl per well was added to the plate and incubated as described above. After incubation, the plate was washed as described above.
Streptavidin-poly-HRP (Fisher) was diluted to 0.04 μg/ml in the Bovigam IFN-γ test kit conjugate diluent buffer and was added to the wells being tested at the incubation step. The plate was incubated as described above. Next, the plate was developed according to the manufacturer's instructions (25, 26).
(ii) Application of stimulated whole-blood plasma to Protein Saver card eluates.
The plasma from the whole-blood stimulation was added to 1.2-cm-diameter sample circles already marked on Whatman Protein Saver 903 cards (30 μl per sample) and allowed to dry at room temperature for 18 to 24 h. The 3-mm-diameter spots (1 spot per well) were then punched from the plasma-impregnated cards using a biopsy punch with a plunger (Miltex) and added to a Nunclon Delta 96-well MicroWell plate (Thermo Scientific Nunc) containing 100 μl PBS-Tween 20 (duplicate wells per sample). The plate was incubated at room temperature for 18 to 24 h on a rotary shaker at 120 rpm to elute the analytes from the cards.
The blood plasma eluates were transferred to a Bovigam IFN-γ test kit microplate (Prionics AG, Switzerland) in duplicate without further dilution and incubated at room temperature for 1 h on a rotary shaker at 600 rpm. Next, the plate was washed six times with kit wash buffer. The method then followed the modified IFN-γ ELISA method at the amplification step. The optical densities of the assay wells containing no sample were subtracted from the optical densities of the antigen-stimulated plasma samples and controls in order to calculate the results. The results were then expressed as the OD as a percentage of the Bovigam IFN-γ test kit positive control.
Evaluation of sample intra- and intercard uniformity.
The inter- and intracard variations for the serum eluates were assessed by taking spots from 5 different positions on 5 cards and testing in different wells of an ELISA plate. The results were compared by two-way analysis of variance (ANOVA).
Evaluating analyte stability over time.
The samples were added to Whatman Protein Saver 903 cards and left at room temperature for up to 10 days prior to elution and evaluation to determine whether there would be any decrease in titers. Blood serum from a Brucella culture-positive animal was added to the cards at 10, 7, 5, 4, 3, 2, and 1 day before elution and stored at room temperature in sealed foil multibarrier pouches (Whatman) containing silica gel sachets. The analytes were tested following the Protein Saver cards sLPS iELISA method for serum.
Bovine whole blood from one animal was stimulated with SEB as described above. The plasma from this sample was added to Whatman Protein Saver 903 cards at 10, 9, 8, 7, 6, 5, 4, 3, 2, and 1 day before elution and stored between 1 and 10 days at room temperature in sealed foil multibarrier pouches (Whatman) containing silica gel sachets. The analytes were then tested following the modified IFN-γ ELISA method.
Sodium azide (2%) was also added to aliquots of the serum and plasma samples, and the effects of treatment with the bactericidal agent were evaluated over 10 days according to the above-described methods.
Statistical analysis.
The intra- and interassay variations were compared by two-way ANOVA and the coefficient of variation (CV). The sLPS ELISA results, expressed as the mean OD for the serum eluates and the unprocessed original samples (n = 204), were compared by Pearson's coefficients of correlation and regression coefficients. The results of the plasma eluates and unprocessed original plasma samples (n = 113) tested by the Bovigam IFN-γ test kit (Prionics AG, Switzerland) and modified IFN-γ ELISA were also compared by Pearson's coefficients of correlation and regression coefficients. The influence of the time of storage on the cards was compared based on the CV and regression analysis for the OD as a percentage of the positive control, for both the serum and plasma samples.
RESULTS
Sample intra- and intercard uniformity.
Figure 1 compares the replicate eluates with the unprocessed original serum sample in the standard iELISA by a vertical scatter plot. A two-way ANOVA was performed and the coefficient of variation was calculated. There was no significant difference in the ODs between the replicate card eluates and the unprocessed original serum samples by two-way ANOVA (P = 0.5194) and no significant difference between the card eluate replicates (P = 0.8168). The CV between the cards and spots was very low (1.49%). These data demonstrate that the analytes are uniformly distributed and that the position of the spot does not influence outcome.
Fig 1.
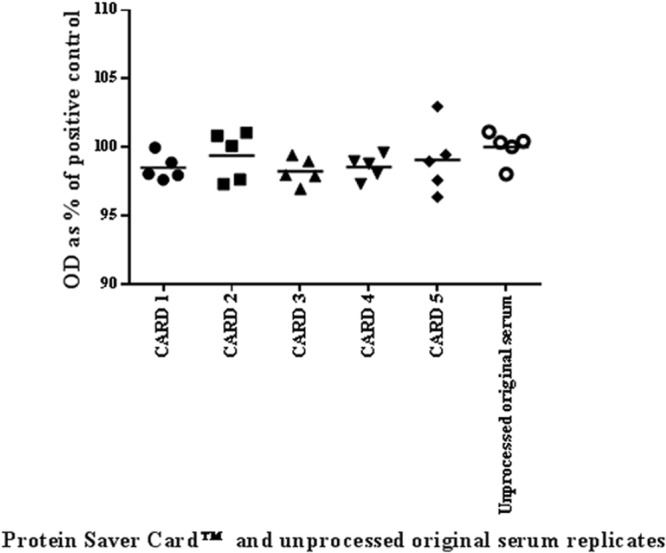
Vertical scatter plot to compare replicates of a serum sample eluted from Protein Saver cards and replicates of the unprocessed original serum sample tested by smooth LPS iELISA. The black horizontal lines represent the mean values of 5 replicate eluates taken from each of 5 Protein Saver cards and the means of the unprocessed original serum replicates.
Serum smooth LPS iELISA.
Figure 2 shows a significant positive correlation between the eluates and unprocessed original serum samples when tested by sLPS ELISA. The Pearson's correlation coefficient (r) for the 44 serum samples from infected animals was 0.9753 (P < 0.0001), with a 95% confidence interval of 0.9548 to 0.9865. The data for the serum samples from 160 uninfected animals gave a Pearson's correlation coefficient (r) of 0.7578 (P < 0.0001). The 95% confidence interval for these data was 0.6830 to 0.8169. The regression coefficient y was 1.0033x.
Fig 2.
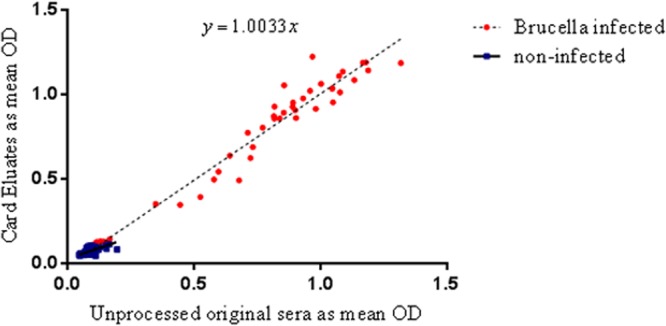
Scatter plot to compare serum samples tested as card eluates and as unprocessed original sera tested by smooth LPS iELISA. The mean results of two replicates are shown as the OD. Red circles indicate samples from Brucella-infected animals, and the blue squares indicate samples from noninfected animals.
Development of the modified IFN-γ ELISA.
Figure 3 demonstrates the effect of an amplification step added to the standard Bovigam ELISA. The use of streptavidin-poly-HRP produced a 32-fold increase in the analytical sensitivity of the assay. The lowest limit of detection of the standard Bovigam ELISA was at a 1:256 sample dilution and it had an upper limit of detection of 1:2. The lowest limit of detection for the amplified IFN-γ assay was at a dilution of 1:8,192 and it had an upper limit of detection of 1:256. Therefore, the modified IFN-γ ELISA has a narrower dynamic range but greater analytical sensitivity than the standard Bovigam ELISA.
Fig 3.
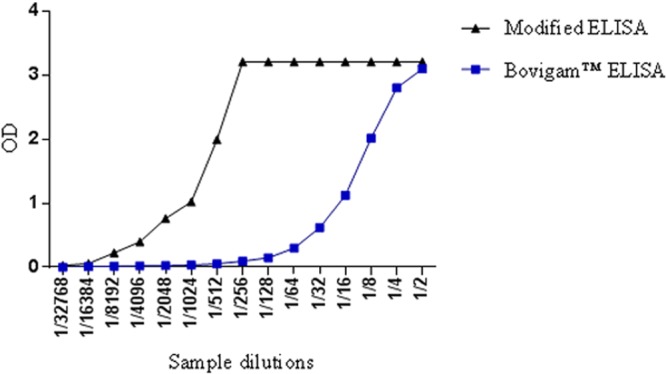
The optical density (OD) of the Bovigam ELISA compared with the modified IFN-γ ELISA. Blue squares represent the ODs of the samples tested by the Bovigam ELISA, and the black triangles represent the ODs of the samples tested by the modified IFN-γ ELISA with the amplification step.
Modified IFN-γ ELISA compared to Bovigam ELISA.
Figure 4 compares the data from 113 plasma samples taken from mitogenically stimulated whole blood. The data for both the eluates and the unprocessed original plasma samples were calculated as a percentage of the IFN-γ positive-control wells. The graph shows a positive correlation between both methods. The Pearson's correlation coefficient (r) for SEB was 0.7947 (P < 0.0001), with a 95% confidence interval of 0.7149 to 0.8541. The regression coefficient was y = 0.8608x.
Fig 4.
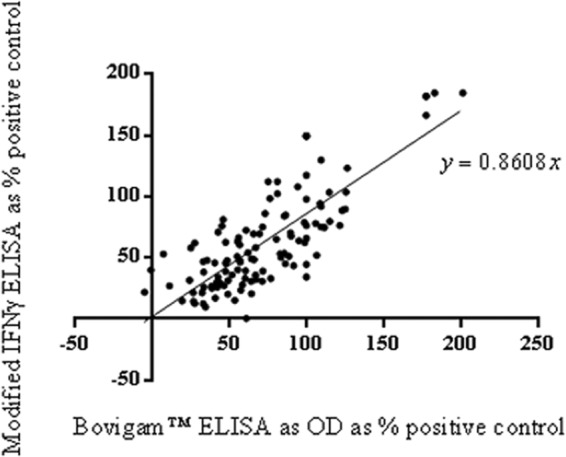
Scatter plot of plasma samples tested as Protein Saver card eluates by the modified IFN-γ ELISA compared to the unprocessed original plasma samples tested by the Bovigam ELISA.
Analyte stability over time.
Figure 5 demonstrates that there was very little difference in the antibody titers recovered from the blood serum added to the cards over a period of 10 days, with a CV of 2.4% between days. The slope of the regression line for the serum eluates did not differ significantly from 0 (P > 0.1) (regression line not shown). There was a gradual decrease in detectable levels of IFN-γ in the plasma stored on the cards over 10 days at room temperature. The CV for the IFN-γ titers for each of the days (as the OD as a percentage of the plasma positive control) from the plasma eluates was 22.9%. The slope of the regression line for the plasma eluates differed significantly from 0 (P > 0.05) over a period of 10 days (regression line not shown).
Fig 5.
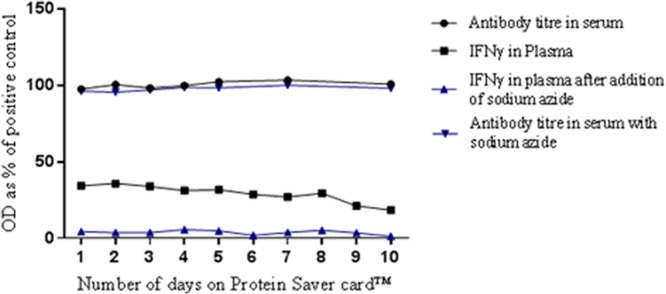
Antibody titers in serum eluates and IFN-γ in plasma eluates, as a percentage of positive controls, for samples stored on sample collection cards over 10 days. Data points represent the means of 4 replicates. Circles, serum eluates; squares, plasma eluates; blue inverted triangles, serum eluates with sodium azide; blue triangles, plasma eluates with sodium azide.
The addition of 2% sodium azide to the serum samples resulted in a CV of 2.2% between days, and the slope of the regression line did not differ significantly from 0 (P > 0.05) (regression line not shown). The addition of 2% sodium azide to the plasma samples had a significant impact on the IFN-γ titers, and they decreased significantly on all days. The addition of 2% sodium azide to the plasma samples showed a CV of 54.3% between days. The slope of the regression line for the plasma eluates differed significantly from 0 (P > 0.05) over a period of 10 days (regression line not shown).
DISCUSSION
This study evaluated the use of Protein Saver cards (Whatman) to store and elute bovine blood serum and plasma samples for the detection of anti-Brucella LPS antibodies and mitogen-stimulated IFN-γ by ELISA. The aim of this project was to determine the utility of these cards to be used interchangeably with unprocessed liquid samples to enable their future use as an aid in bovine brucellosis diagnosis.
It was determined that there was no significant inter- or intracard variation, demonstrating that the serum elution method was repeatable. This is in keeping with the findings of other studies, such as neonatal screening programs, where all spots are considered equal, therefore giving equivalent analyte recovery results when using the same-sized punch (11, 28, 29).
The utility of the Protein Saver cards was successfully validated with a small study of 204 bovine serum samples from known provenances. The detection parameters of the card eluates and the unprocessed original serum samples tested by the sLPS iELISA showed very good agreement. The slope of the line was y = 1.0033x, and it may be concluded that the eluates and the unprocessed serum samples tested by the sLPS iELISA can be used interchangeably.
Plasma from mitogen-stimulated whole-blood samples was used to assess a method for detecting IFN-γ content in plasma that was added to and eluted from Protein Saver cards. Initially, the detection of IFN-γ in the eluates was unsuccessful, as the ELISA was not sufficiently sensitive. The Bovigam IFN-γ ELISA (Prionics) required 50 μl of sample, whereas the eluate from the card gave a volume of 1.875 μl per spot (when calculating the volume of sample within the area of a circle) and could not replicate the 1:2 dilution required for the Bovigam IFN-γ ELISA. Therefore, an additional conjugate was included to increase the analytical sensitivity of the assay through amplification of the signal. The Bovigam ELISA was adapted to include a biotinylated conjugate instead of the anti-IFN-γ–horseradish peroxidase conjugate and was paired with streptavidin–poly-HRP. This amplification step was successful in generating a 32-fold increase in analytical sensitivity, and this enabled a comparison to be made.
Plasma samples (n = 113) were added to and eluted from the Protein Saver cards and tested in a modified IFN-γ ELISA. The unprocessed original plasma samples were tested in parallel with the Bovigam ELISA. The IFN-γ titers of the plasma eluates showed good correlation with those of the unprocessed original plasma samples (r = 0.7947), and the regression coefficient was y = 0.8608x. However, this correlation was not as strong as for the serum samples; therefore, the plasma eluates and unprocessed original plasma samples as tested with the Bovigam ELISA may not be used interchangeably.
The Protein Saver cards (Whatman) were investigated to determine whether samples degrade over time if kept at room temperature, which is a measure of the cards' utility. The degradation of samples impacts the length of time that samples on cards can remain in transit. Serum and plasma samples were added to the cards for up to 10 days and stored at room temperature in Whatman multibarrier pouches to replicate the conditions of mailing the samples without refrigeration. It was demonstrated that the antibody titers of the serum eluates remained stable over 10 days, with very low coefficients of variation between the days, and regression analysis demonstrated that the antibody titers were not significantly different between days. The addition of sodium azide resulted in no decrease of antibody titers and it is useful as a bactericidal agent; its inclusion would minimize the risks usually associated with transporting infectious agents. Therefore, appropriately stored serum samples on Protein Saver cards may be stored at ambient temperature and transported through the post with a reduction in the risk of exposure to live agents in serum, thus enabling greater access to samples from countries where cold transport is unavailable. This is in keeping with the findings of other studies that determined the longevity of titers from samples stored on cards (14, 30, 31).
The IFN-γ time study compared plasma eluates from an SEB mitogenically stimulated whole-blood sample for 10 days on the cards. These data demonstrate that IFN-γ titers decreased over time, suggesting possible degradation of the protein at room temperature, since cytokines are small fragile proteins (32, 33). Another possibility is that there may be a lower extraction rate of analyte from the older drier spots (34). This makes the recovery of IFN-γ from plasma unsuitable for long-term storage on Protein Saver cards that are kept at room temperature during transit. It may therefore be useful to test long-term storage conditions at higher and lower temperatures to replicate the ability to recover IFN-γ from the cards in countries without cold storage and fluctuations in temperature. It would also be of interest to determine if the samples can be stored on cards for longer periods under refrigerated conditions.
Previous studies have demonstrated that the storage conditions of Protein Saver cards play a significant role in the antibody titers recovered from the eluates. Initially, antibodies are relatively robust and remain unaffected by fluctuations in temperature during transportation and storage; however, humidity and desiccation do play important roles, and long-term storage conditions must be carefully considered (29, 30, 35–38).
In conclusion, this proof-of-concept study has successfully demonstrated that Whatman Protein Saver 903 cards may be used for sample storage and elution in order to detect anti-Brucella LPS antibodies and mitogen-stimulated gamma interferon. However, these cards were not suitable for long-term storage prior to detection of IFN-γ, and it may be of future interest to investigate the recovery of analytes from the cards when they are stored at higher and lower temperatures than room temperature. This may be of interest in order to reflect the conditions and temperatures in countries without cold storage and/or fluctuating temperatures.
These cards are a very useful tool for establishing panels of samples for assay development and validation, assisting other laboratories with diagnosis, and harmonization studies. The use of these cards in bovine brucellosis diagnosis is novel and offers much potential for greater access in testing previously inaccessible samples and for supporting the ongoing challenge of controlling bovine brucellosis.
ACKNOWLEDGMENTS
We thank our colleagues at the Pendik Veterinary Control and Research Institute, Turkey, for all their assistance with this collaboration. In particular, we thank E. Ayhan Baklan, A. Murat Saytekin, and M. Sencer Karagul for their help with sample collection.
This work was supported financially by the Department for Environment, Food, and Rural Affairs (United Kingdom).
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1.Franco MP, Mulder M, Gilman RH, Smits HL. 2007. Human brucellosis. Lancet Infect. Dis. 7:775–786 [DOI] [PubMed] [Google Scholar]
- 2.Carvalho Neta AV, Mol JPS, Xavier MN, Paixão TA, Lage AP, Santos RL. 2010. Pathogenesis of bovine brucellosis. Vet. J. 184:146–155 [DOI] [PubMed] [Google Scholar]
- 3.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99 [DOI] [PubMed] [Google Scholar]
- 4.Rolán HG, den Hartigh AB, Kahl-McDonagh M, Ficht T, Adams LG, Tsolis RM. 2008. VirB12 is a serological marker of Brucella infection in experimental and natural hosts. Clin. Vaccine Immunol. 15:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicoletti P. 2002. A short history of brucellosis. Vet. Microbiol. 90:5–9 [DOI] [PubMed] [Google Scholar]
- 6.McGiven JA, Tucker JD, Perrett LL, Stack JA, Brew SD, MacMillan AP. 2003. Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT, CFT, and iELISA. J. Immunol. Methods 278:171–178 [DOI] [PubMed] [Google Scholar]
- 7.Godfroid J, Saegerman C, Wellemans V, Walravens K, Letesson JJ, Tibor A, Mc Millan A, Spencer S, Sanna M, Bakker D, Pouillot R, Garin-Bastuji B. 2002. How to substantiate eradication of bovine brucellosis when aspecific serological reactions occur in the course of brucellosis testing. Vet. Microbiol. 90:461–477 [DOI] [PubMed] [Google Scholar]
- 8.Weynants V, Godfroid J, Limbourg B, Saegerman C, Letesson JJ. 1995. Specific bovine brucellosis diagnosis based on in vitro antigen-specific gamma interferon production. J. Clin. Microbiol. 33:706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirlwall R, Commander N, Brew S, Cutler S, McGiven J, Stack JA. 2008. Improving the specificity of immunodiagnosis for porcine brucellosis. Vet. Res. Commun. 32:209–213 [DOI] [PubMed] [Google Scholar]
- 10.Jacobson RH, Wright P. 2008. Principles and methods of validation of diagnostic assays for infectious diseases, 2008 ed, vol 1 OIE Terrestrial Manual. Office International des Epizooties, Paris, France [Google Scholar]
- 11.Guthrie R, Susi A. 1963. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32:338–343 [PubMed] [Google Scholar]
- 12.Parker SP, Cubitt WD, Ades AE. 1997. A method for the detection and confirmation of antibodies to hepatitis C virus in dried blood spots. J. Virol. Methods 68:199–205 [DOI] [PubMed] [Google Scholar]
- 13.Eaton RB, Petersen E, Seppänen H, Tuuminen T. 1996. Multicenter evaluation of a fluorometric enzyme immunocapture assay to detect toxoplasma-specific immunoglobulin M in dried blood filter paper specimens from newborns. J. Clin. Microbiol. 34:3147–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Mubarak HS, Yüksel S, Mustafa OM, Ibrahim SA, Osterhaus AD, de Swart RL. 2004. Surveillance of measles in the Sudan using filter paper blood samples. J. Med. Virol. 73:624–630 [DOI] [PubMed] [Google Scholar]
- 15.Tan HK, Petersen E, Møller LN, Phillips P, Neto EC, Gilbert RE. 2009. Recovery of anti-Toxoplasma gondii immunoglobulin M in stored Guthrie card blood spots. J. Clin. Microbiol. 47:2626–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd RM, Jr, Burns DA, Huong JT, Mathis RL, Winters MA, Tanner M, De La Rosa A, Yen-Lieberman B, Armstrong W, Taege A, McClernon DR, Wetshtein JL, Friedrich BM, Ferguson MR, O'Brien W, Feorino PM, Holodniy M. 2009. Dried-plasma transport using a novel matrix and collection system for human immunodeficiency virus and hepatitis C virus virologic testing. J. Clin. Microbiol. 47:1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterboer T, Dondog B, Michael KM, Michel A, Schmitt M, Vaccarella S, Franceschi S, Clifford G, Pawlita M. 2012. Dried blood spot samples for seroepidemiology of infections with human papillomaviruses, Helicobacter pylori, hepatitis C virus, and JC virus. Cancer Epidemiol. Biomarkers Prev. 21:287–293 [DOI] [PubMed] [Google Scholar]
- 18.Takkouche B, Iglesias J, Alonso-Fernandez JR, Fernandez-Gonzalez C, Gestal-Otero JJ. 1995. Detection of Brucella antibodies in eluted dried blood: a validation study. Immunol. Lett. 45:107–108 [DOI] [PubMed] [Google Scholar]
- 19.Curry PS, Elkin BT, Campbell M, Nielsen K, Hutchins W, Ribble C, Kutz SJ. 2011. Filter-paper blood samples for ELISA detection of Brucella antibodies in caribou. J. Wildl. Dis. 47:12–20 [DOI] [PubMed] [Google Scholar]
- 20.Dubay SA, Rosenstock SS, Stallknecht DE, deVos JC., Jr 2006. Determining prevalence of bluetongue and epizootic hemorrhagic disease viruses in mule deer in Arizona (USA) using whole blood dried on paper strips compared to serum analyses. J. Wildl. Dis. 42:159–163 [DOI] [PubMed] [Google Scholar]
- 21.Yu C, Zimmerman C, Stone R, Engle RE, Elkins W, Nardone GA, Emerson SU, Purcell RH. 2007. Using improved technology for filter paper-based blood collection to survey wild Sika deer for antibodies to hepatitis E virus. J. Virol. Methods 142:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 43:83–91 [Google Scholar]
- 23.Nielsen K, Ewalt DR. 2010. Bovine brucellosis. Manual of diagnostic tests and vaccines for terrestrial animals 2010. Office International Des Epizooties, Paris, France [Google Scholar]
- 24.Thompson I, McGiven J, Sawyer J, Thirlwall R, Commander N, Stack J. 2009. Competitive electrochemiluminescence wash and no-wash immunoassays for detection of serum antibodies to smooth Brucella strains. Clin. Vaccine Immunol. 16:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood PR, Rothel JS. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125–135 [DOI] [PubMed] [Google Scholar]
- 27.Grunow R, Miethe P, Conlan W, Finke EJ, Friedewald S, Porsch-Özcurumez M. 2008. Rapid detection of Francisella tularensis by the immunoaffinity assay Abicap in environmental and human samples. J. Rapid Methods Autom. Microbiol. 16:30–54 [Google Scholar]
- 28.Fan L, Lee JA. 2012. Managing the effect of hematocrit on DBS analysis in a regulated environment. Bioanalysis 4:345–347 [DOI] [PubMed] [Google Scholar]
- 29.Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, Cox J, Abeku T, Bousema T, Ghani AC, Drakeley C, Riley E. 2008. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malaria J. 7:195. 10.1186/1475-2875-7-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mei JV, Alexander JR, Adam BW, Hannon WH. 2001. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 131:1631S–1636S [DOI] [PubMed] [Google Scholar]
- 31.Skogstrand K, Thysen AH, Jørgensen CS, Rasmussen EM, Andersen Å, Lillebaek BT, Hougaard DM, Houen G. 2012. Antigen-induced cytokine and chemokine release test for tuberculosis infection using adsorption of stimulated whole blood on filter paper and multiplex analysis. Scand. J. Clin. Lab. Invest. 72:204–211 [DOI] [PubMed] [Google Scholar]
- 32.Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. 2000. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine 12:1712–1716 [DOI] [PubMed] [Google Scholar]
- 33.Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Nørgaard-Pedersen B, Hougaard DM. 2008. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J. Immunol. Methods 336:78–84 [DOI] [PubMed] [Google Scholar]
- 34.Skogstrand K, Thorsen P, Nørgaard-Pedersen B, Schendel DE, Sørensen LC, Hougaard DM. 2005. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 51:1854–1866 [DOI] [PubMed] [Google Scholar]
- 35.Behets F, Kashamuka M, Pappaioanou M, Green TA, Ryder RW, Batter V, George JR, Hannon WH, Quinn TC. 1992. Stability of human immunodeficiency virus type 1 antibodies in whole blood dried on filter paper and stored under various tropical conditions in Kinshasa, Zaire. J. Clin. Microbiol. 30:1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Akker R, Kooy H, van der Meyden HP, Lumey BH. 1990. Recovery of HIV antibodies in eluates from plasma and erythrocytes dried on filter paper and stored under various conditions. AIDS 4:90–91 [PubMed] [Google Scholar]
- 37.Ades AE, Parker S, Berry T, Holland FJ, Davison CF, Cubitt D, Hjelm M, Peckham CS, Wilson AH, Hudson CN, Briggs M, Tedder RS. 1991. Prevalence of maternal HIV-1 infection in Thames regions: results from anonymous unlinked neonatal testing. Lancet 337:1562–1565 [DOI] [PubMed] [Google Scholar]
- 38.Peckham CS, Ades AE, O'Connor C, Tedder RS, Briggs M, Parra-Mejia N, Hjelm M, Wilcox AH. 1990. Prevalence of maternal HIV infection based on unlinked anonymous testing of newborn babies. Lancet 335:516–519 [DOI] [PubMed] [Google Scholar]


