Abstract
Coxsackievirus B3 (CVB3), a small single-stranded RNA virus, belongs to the Picornaviridae family. Its infection is the most common cause of myocarditis, with no vaccine available. Gastrointestinal mucosa is the major entry port for CVB3; therefore, the induction of local immunity in mucosal tissues may help control initial viral infections and alleviate subsequent myocardial injury. Here we evaluated the ability of high-mobility group box 1 (HMGB1) encapsulated in chitosan particles to enhance the mucosal immune responses induced by the CVB3-specific mucosal DNA vaccine chitosan-pVP1. Mice were intranasally coimmunized with 4 doses of chitosan-pHMGB1 and chitosan-pVP1 plasmids, at 2-week intervals, and were challenged with CVB3 4 weeks after the last immunization. Compared with chitosan-pVP1 immunization alone, coimmunization with chitosan-pHMGB1 significantly (P < 0.05) enhanced CVB3-specific fecal secretory IgA levels and promoted mucosal T cell immune responses. In accordance, reduced severity of myocarditis was observed in coimmunized mice, as evidenced by significantly (P < 0.05) reduced viral loads, decreased myocardial injury, and increased survival rates. Flow cytometric analysis indicated that HMGB1 enhanced dendritic cell (DC) recruitment to mesenteric lymph nodes and promoted DC maturation, which might partly account for its mucosal adjuvant effect. This strategy may represent a promising approach to candidate vaccines against CVB3-induced myocarditis.
INTRODUCTION
Coxsackieviruses belong to the Picornaviridae family with single-stranded RNA genomes and are implicated in various clinical manifestations, ranging from mild to severe life-threatening diseases (1–3). Among them, coxsackievirus B3 (CVB3) is considered to be the most common cause of viral myocarditis (4). Previous studies showed that about 5 to 50% of cases of myocarditis and its end stage of dilated cardiomyopathy, as well as congestive heart failure, are attributable to CVB3 infection (5–7). Although a few vaccine candidates have been reported to be effective in a murine CVB3-induced myocarditis model (8–10), no prophylactic or therapeutic reagent is available for the clinic to date. Thus, development of new efficient vaccines is needed urgently.
Various types of CVB-specific vaccines have been examined in animal models, including inactivated or attenuated virus vaccines (11, 12), recombinant protein vaccines (13), DNA vaccines (14), and virus-like particle vaccines (8). Considering that CVB3 mainly invades hosts through the gastrointestinal tract, it is critical to induce mucosal immune responses to limit virus infection in the initial stage and at the primary site.
Compared with other candidates, mucosal vaccines are more likely to induce immune tolerance rather than activation, due to their limited immunogenicity and the special mucosal environment. Therefore, discovery of safe effective mucosal adjuvants is critical to the success of mucosal vaccines. High-mobility group box 1 (HMGB1), also known as amphoterin, was originally identified as a highly conserved nonhistone DNA-binding factor expressed by all nucleated eukaryotic cells (15). In addition to functioning as a nuclear DNA chaperone and a cytosolic autophagy-promoting molecule, HMGB1 is considered a damage-associated molecular pattern (DAMP) molecule similar to interleukin-33 (IL-33) and possesses diverse roles in immunity (16). It has been reported that HMGB1 not only evokes innate immune responses through binding to Toll-like receptor 2/4 (TLR2/4) or the receptor for advanced glycation end products (RAGE) (17, 18) but also modulates adaptive immune responses by promoting T cell proliferation and activation (19, 20). These characteristics suggest that HMGB1 may be used as an adjuvant.
We previously reported that intranasal immunization with a chitosan-pVP1 DNA vaccine produced moderate levels of mucosal IgA as well as decent gamma interferon (IFN-γ)-positive T cell responses. In accordance, a significant (P < 0.05) reduction of viral load was observed and about 42% of immunized mice survived a lethal CVB3 challenge; in contrast, the survival rate of the control group (pVP1) was only 16.7% (10).
In this study, mice were coimmunized with chitosan-pHMGB1 to further increase the mucosal immune responses elicited by chitosan-pVP1 and to improve the protection against viral myocarditis. We found that chitosan-pHMGB1 coimmunization increased the level of CVB3-specific fecal secretory IgA (SIgA), promoted mucosal T cell proliferation and IFN-γ-secreting cell production, and protected 70% of mice against a lethal viral challenge, which was higher than 40% in the chitosan-pVP1 group. The immune enhancement of chitosan-pHMGB1 may be partly attributable to the ability of pHMGB1 to recruit and to promote the maturation of mucosal dendritic cells (DCs).
MATERIALS AND METHODS
Animals and virus.
Male, inbred, 6-week-old, BALB/c (H-2d) mice were obtained from the Experimental Animal Center of the Chinese Academy of Science (Shanghai, China). All animals were housed in pathogen-free mouse colonies, and all animal experiments were performed according to guidelines for the care and use of laboratory animals. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Medical Laboratory Animals (21). The protocol was approved by the Ethics Committee of Soochow University (permit number 2010036). CVB3 (Nancy strain) was a gift from Yingzhen Yang (Key Laboratory of Viral Heart Diseases, Zhongshan Hospital, Shanghai Medical College of Fudan University) and was maintained by passage through HeLa cells grown in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 0.2% penicillin-streptomycin.
Expression of VP1 and HMGB1 plasmids in 293T cells.
The murine HMGB1 expression plasmid pCAGGS-HMGB1-HA (pHMGB1) was kindly provided by Tadatsugu Taniguchi at the University of Tokyo, and pcDNA3.1-VP1 (pVP1) has been described previously (10). Plasmids were extracted from Escherichia coli (DH5a) grown overnight by using the Qiagen EndoFree Plasmid Mega kit. For cell transfection assays, plasmids were dissolved in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml. For chitosan-DNA vaccine preparation, plasmids were dissolved in 5 mM Na2SO4 at a concentration of 400 μg/ml. In transfection assays, 293T cells (5 × 105 cells) at about 60 to 70% confluence were transfected with 3 μg of pHMGB1 or pVP1 in 35-mm dishes by using Lipofectamine (Invitrogen), according to the manufacturer's protocol. The same amount of empty vector pCAGGS-HA (for pHMGB1 transfection) or pcDNA3.1 (for pVP1 transfection) was used for negative controls.
Forty-eight hours after transfection, cells were harvested and lysed in cold lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, and 0.5% NP-40. The cellular debris was depleted by centrifugation at 6,000 × g for 5 min. Equal amounts of protein for all samples were loaded onto 12% SDS-PAGE gels, separated by electrophoresis, and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were probed with anti-HMGB1 (N-terminal) antibody (1 mg/ml; dilution, 1:2,000; Sigma), mouse anti-enterovirus VP1 antibody (110 μg/ml; dilution, 1:500; Dako), or anti-mouse β-actin antibody (100 μg/ml; dilution, 1:1,000; Cell Signaling Technology), followed by goat anti-rabbit IgG-horseradish peroxidase (1 mg/ml; dilution, 1:5,000; Southern Biotech) or goat anti-mouse IgA antibody (heavy and light chain specific, 1 mg/ml; dilution, 1:5,000; Southern Biotech). The signals were developed using Thermo Scientific SuperSignal West Pico chemiluminescent substrate. β-Actin was used as the internal marker for loading control.
Chitosan-DNA preparation.
Chitosan (molecular weight of about 390,000) was obtained from Fluka BioChemika. The deacetylation degree of the chitosan was approximately 85%. DNA was purified from Escherichia coli (DH5a) by using the Plasmid Mega preparation kit (Qiagen). Chitosan-DNA nanoparticles were generated as described previously (10).
Immunization.
Groups of mice were mildly anesthetized with pentobarbital (40 mg/kg body weight, administered intraperitoneally) and immunized intranasally with chitosan-pVP1 plus chitosan-pHMGB1, chitosan-pVP1, chitosan-pHMGB1, or chitosan-pcDNA3.1 (mock immunization) 4 times, on a biweekly schedule, with a dose of 50 μg for each plasmid (Table 1). In groups immunized with pVP1 alone, pHMGB1 alone, or empty plasmid, the mice received an additional 50 μg pcDNA3.1 so that the total DNA amount was 100 μg. Serum and fecal pellets were collected 2 weeks following the final immunization, as described previously (10). Two weeks following the final immunization, the body temperatures of the mice were detected with an infrared camera (FLIR i5; FLIR Systems, Inc.) and the thicknesses of their foot pads were measured with a vernier caliper. Body weight and behavior were also monitored during the process of immunization.
Table 1.
Vaccine groupsa
| Group no. | Vaccine |
|---|---|
| 1 | Chitosan-pcDNA3.1 (mock) |
| 2 | Chitosan-pVP1 |
| 3 | Chitosan-pHMGB1 |
| 4 | Chitosan-pVP1 plus chitosan-pHMGB1 |
The mice in each group were immunized 4 times biweekly at a dose of 50 μg of each plasmid. The mice that received pVP1 alone, pHMGB1, or empty plasmid also received an additional 50 μg pcDNA3.1 so that the total DNA amount was 100 μg.
Viral infection and myocarditis histopathological analysis.
The myocarditis model in BALB/c mice in our study was established by intraperitoneal injection of 3 times the 50% lethal dose (LD50) of CVB3 (Nancy strain from Yingzhen Yang, as described above), as described previously (22). Seven days later, heart tissues were collected, sectioned, and stained with hematoxylin and eosin. The extent of myocardial lesions was quantified as described previously (22). To further evaluate the immune protection effect, mice were challenged with a lethal dose of CVB3 (5 times the LD50), and the survival rate was monitored for up to 28 days.
Evaluation of CVB3-specific antibody responses and detection of neutralizing antibody titers.
The titers and neutralizing titers of CVB3-specific serum IgG and fecal SIgA were detected by enzyme-linked immunosorbent assay (ELISA) and microneutralization assays, as described previously (22).
ELISPOT assays and T cell proliferation.
Two weeks after the final immunization, mice were euthanized via decapitation. Mesenteric lymph nodes (MLNs) were isolated immediately, and single-cell suspensions were prepared by gentle mechanical disaggregation through sterile 100-μm nylon cell strainers (BD Falcon). Cells were resuspended in RPMI 1640 medium containing 10% FBS and 50 U/ml IL-2 and were plated at 1 × 106 cells/well. IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assays were performed as described previously (22). Medium alone and 2 μg/ml concanavalin A (ConA) were incubated with cells as negative and positive controls, respectively. For ConA stimulation, 3 × 105 cells/well were added to the plate.
Cell proliferation was measured with a colorimetric immunoassay based on bromodeoxyuridine (BrdU) incorporation. A BrdU ELISA (Roche) was performed according to the manufacturer's instructions. Briefly, cells were seeded in 96-well plates (5 × 105 cells/well) with 10 μg/ml recombinant VP1 protein or 2 μg/ml ConA (as a positive control) and were cultured for 48 h. Then, 10 μl of the BrdU labeling solution in RPMI 1640 medium (dilution, 1:100) was added to each well. The plates were cultured at 37°C with 5% CO2 for another 24 h, to allow BrdU incorporation into the cells. Subsequently, the supernatant in each well was removed by pipetting. After being washed twice, the plates were incubated with FixDenat reagent (200 μl/well; Roche Applied Science) for 30 min at room temperature and then were washed 3 times. Anti-BrdU peroxidase (100 μl/well; dilution, 1:100) was added, and the cells were kept at room temperature for 60 min. After the removal of unbound antibody conjugates, 100 μl of substrate solution was added. The resulting mixture was allowed to stand for 20 min, and the reaction was completed by adding 2 N H2SO4 (25 μl/well). Plates were mixed on a shaker for 1 min. Absorbance at 370 nm was detected on a microplate reader (Bio-Tek).
Quantification of viral loads in heart tissues.
Seven days after the challenge with CVB3 at 3 times the LD50, mice were euthanized via decapitation. Heart tissues were immediately collected, weighed, homogenized with liquid nitrogen using a mortar and pestle, and then resuspended in TRIzol reagent (Invitrogen) for viral RNA detection or in 10% FBS-RPMI 1640 medium for PFU measurement. The viral loads in heart tissues were determined by quantitative real-time reverse transcription-PCR and PFU assays, as described previously (23, 24).
Flow cytometric analysis.
Single-cell suspensions of MLN cells were prepared and resuspended in PBS containing 1% bovine serum albumin (BSA), at a concentration of 1 × 106 cells/ml. Cells were incubated for 1 h at 4°C with anti-CD80 antibody labeled with phycoerythrin (0.2 mg/ml; dilution, 1:100; BD Pharmingen) and anti-mouse CD11c antibody labeled with PerCP-Cy5.5 (0.2 mg/ml; dilution, 1:200; Biolegend). After being washed twice with PBS containing 1% BSA, the stained cells were resuspended in PBS and analyzed with a FACSCanto II flow cytometer (Becton, Dickinson).
Statistical analysis.
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed with analysis of variance (ANOVA) followed by Tukey's post hoc test. Survival rates were analyzed with the Kaplan-Meier test, using Prism version 4.01 (GraphPad Software Inc.). Differences with P values of <0.05 were considered statistically significant.
RESULTS
Efficient expression of pHMGB1 and pVP1 in vitro.
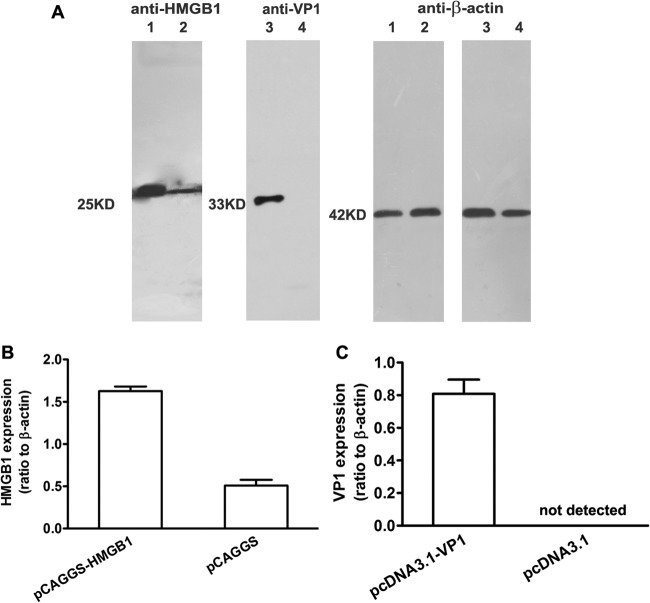
To verify the transcriptional efficiency of pHMGB1 and pVP1 in vitro, the plasmids were transfected into 293T cells using Lipofectamine. Western blot results showed increased expression of HMGB1 in pHMGB1-transfected cells, compared with their counterparts transfected with empty vector, at a predicted molecular weight of 25,000 (Fig. 1). In addition, robust expression of VP1 was detected in pVP1-transfected cells at a molecular weight of 33,000. These data demonstrated the high expression efficiencies of pHMGB1 and pVP1 plasmids. Next, these two plasmids were encapsulated with chitosan for intranasal administration.
Fig 1.
Expression of VP1 and HMGB1 plasmids in vitro. 293T cells were transfected with various plasmids using Lipofectamine for 48 h, and then cell lysates were subjected to Western blot analysis using anti-VP1, anti-HMGB1, or anti-β-actin antibody. (A) In vitro expression of pHMGB1 and pVP1 plasmids. Lane 1, 293T cells transfected with pCAGGS-HMGB1-HA; lane 2, 293T cells transfected with pCAGGS-HA; lane 3, 293T cells transfected with pcDNA3.1-VP1; lane 4, 293T cells transfected with pcDNA3.1. Molecular mass markers are also shown. (B and C) Quantification of pHMGB1 (B) and pVP1 (C) expression by densitometry. Experiments were repeated three times, with similar results. All of the experimental data were pooled and are presented together.
Enhancement of CVB3-specific mucosal immunity by coimmunization with chitosan-pHMGB1 and chitosan-pVP1.
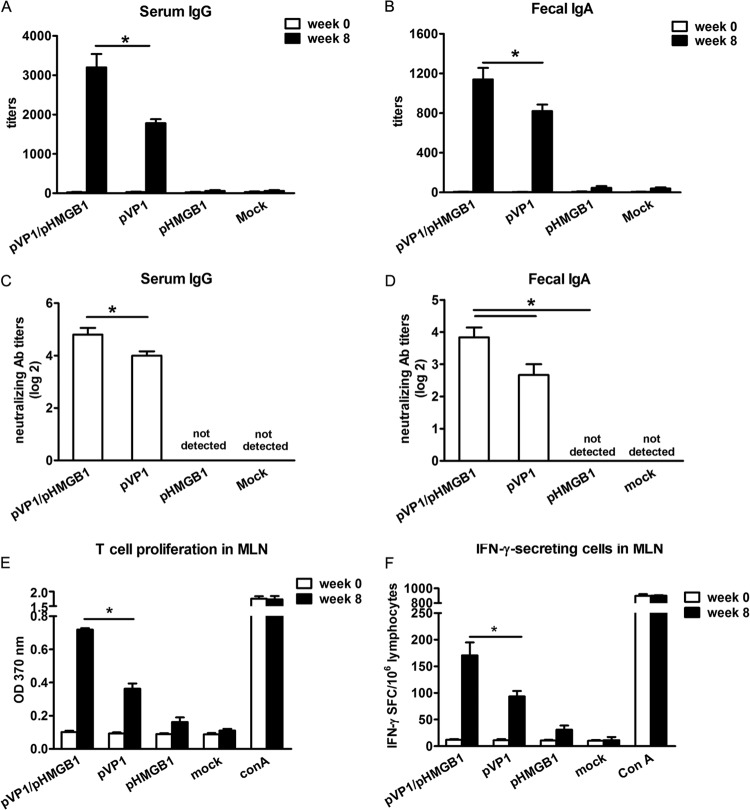
Groups of mice were intranasally immunized with chitosan-pVP1 plus chitosan-pHMGB1, chitosan-pVP1, chitosan-pHMGB1, or chitosan-pcDNA3.1 (mock immunization) 4 times, on a biweekly schedule, with a dose of 50 μg for each plasmid. Two weeks following the last immunization, serum IgG and fecal SIgA levels were measured by ELISA. As shown in Fig. 2A, pVP1 administration induced a high level of CVB3-specific serum IgG, with a titer of about 1,800; coimmunization with pHMGB1 further increased the serum IgG production, and the titer reached about 3,200 (P < 0.05). In addition, pHMGB1 coimmunization significantly (P < 0.05) enhanced the level of CVB3-specific fecal SIgA, compared with pVP1 (titers of 1,100 versus 800) (Fig. 2B). More interestingly, the virus-neutralizing abilities of both serum IgG and fecal SIgA were dramatically increased (Fig. 2C and D), indicating that pHMGB1 coimmunization robustly enhanced not only the levels but also the quality of systemic and mucosal antibody responses. No obvious differences in the levels and virus-neutralizing titers of serum IgG and fecal SIgA were observed for the pHMGB1- and mock-immunized groups. CVB3-specific mucosal T cell proliferation and IFN-γ-secreting cell frequency also increased in the pHMGB1 coimmunization group, about 1.8-fold and 1.6-fold, respectively, over those of the pVP1 group (P < 0.05) (Fig. 2E and 2F). These data indicated that HMGB1 coimmunization comprehensively augmented CVB3-specific immune responses at mucosal sites.
Fig 2.
Enhanced CVB3-specific mucosal immune responses with coimmunization with pHMGB1. (A) Serum IgG titers. (B) Fecal SIgA titers. (C) Serum IgG neutralizing antibody (Ab) titers. (D) Fecal SIgA neutralizing antibody titers. (E) T cell proliferation responses in MLNs. (F) Frequency of IFN-γ-secreting cells in MLNs. Each group contained 8 mice. Experiments were repeated three times, with similar results. All of the experimental data were pooled and are presented together. OD, optical density; SFC, spot-forming cells. ∗, P < 0.05.
Alleviation of CVB3-induced viral myocarditis with chitosan-pHMGB1 coimmunization.
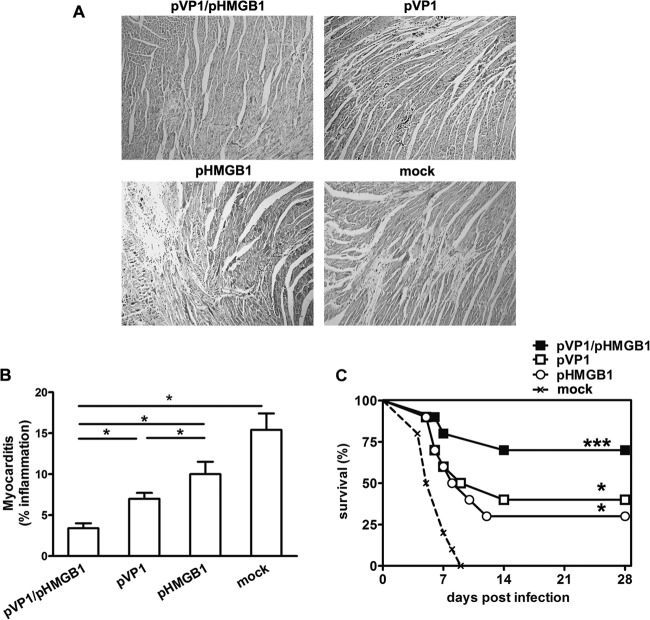
Seven days after CVB3 infection, myocardial injury was detected by histopathological observation. This showed that, compared with the other groups, HMGB1 greatly reduced the inflammatory infiltration in heart tissues. Only tiny areas of inflammation were observed in mice from the pVP1/pHMGB1 group (Fig. 3A), while areas of more-severe inflammation were seen in pVP1-, pHMGB1-, and mock-immunized mice. To further confirm the improved immunoprotection conferred by pHMGB1 coimmunization, mice were challenged with a lethal dose of CVB3 (5 times the LD50) and 28-day survival rates were observed. As shown in Fig. 3C, all of the mock-immunized mice died within 10 days after the viral challenge, while 40% of the mice in the pVP1 group survived the lethal challenge (P < 0.05). Interestingly, 30% of the mice in the pHMGB1 group also survived up to 28 days postinfection, although no specific immune responses were evidenced. A further enhanced survival rate (70%) was observed for the pHMGB1-coimmunized mice, although no statistically significant difference was observed in comparison with the pVP1 or pHMGB1 group. Survival rates of all treated groups were significantly (P < 0.05) higher than that of the mock-immunized group.
Fig 3.
Alleviation of CVB3-induced myocarditis by pHMGB1 coimmunization. (A) One representative heart section is shown for 5 mice per group (magnification, ×100). (B) Myocarditis was assessed as the percentage of the heart section with inflammation, compared with the overall size of the heart section, with the aid of a microscope eyepiece grid. Each group contained 5 mice. ∗, P < 0.05. (C) Survival rates of mice were observed until day 28 following challenge with a lethal dose of CVB3 (5 times the LD50). Each group contained 10 mice. Experiments were repeated three times with similar results. All of the experimental data were pooled and are presented together. ∗, P < 0.05; ∗∗∗, P < 0.001, versus the mock-immunized group.
Reduction of viral loads in heart tissues by coimmunization with chitosan-pHMGB1.
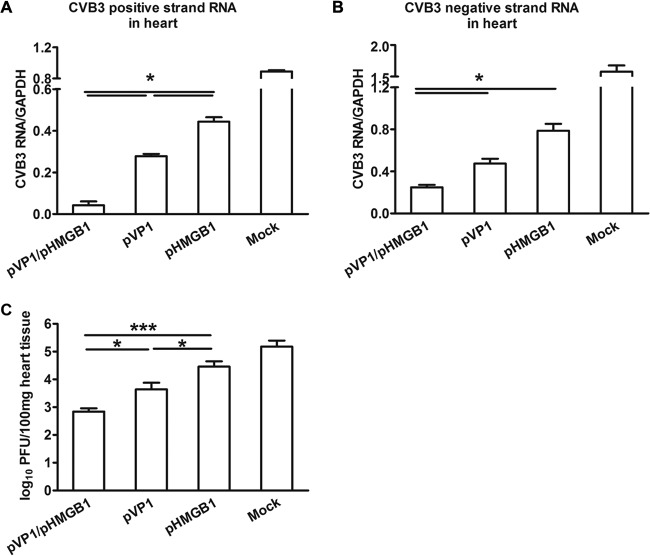
Seven days postinfection, the viral loads in heart tissues were measured by real-time PCR and PFU assays. The results showed that both the positive- and negative-strand RNAs of CVB3 were significantly (P < 0.05) reduced in the pVP1/pHMGB1 group, compared with the other groups (Fig. 4A and B). Consistently, the minimal myocardial viral load determined by standard PFU assays was shown in pVP1/pHMGB1-coimmunized mice (Fig. 4C), indicating better control of the virus.
Fig 4.
Reduced viral loads in heart with coimmunization with pHMGB1. Four weeks following the last immunization, mice were infected with 3 times the LD50 of CVB3; 7 days later, the viral loads in heart were detected by real-time PCR. (A) Levels of CVB3 positive-strand RNA. (B) Levels of CVB3 negative-strand RNA. (C) Myocardial viral loads. Each group contained 5 mice. Experiments were repeated three times, with similar results. All of the experimental data were pooled and are presented together. ∗, P < 0.05; ∗∗∗, P < 0.001.
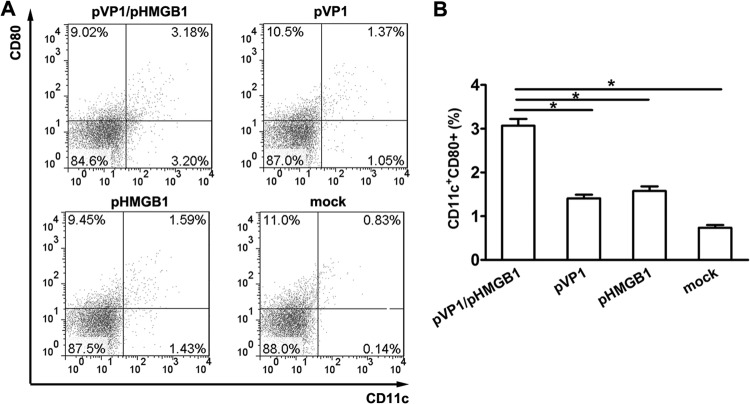
Promotion of recruitment and maturation of MLN DCs by chitosan-pHMGB1 coimmunization.
To further explore the immune-enhancing mechanism of HMGB1, the percentages and maturation states of MLN dendritic cells (DCs) were analyzed. As shown in Fig. 5A, the percentage of CD11c+ DCs in the mock-immunized group was only 0.94%, which increased to 2.42% in the pVP1 group and 3.02% in the pHMGB1 group. A further significant (P < 0.05) increase was achieved with pHMGB1 coimmunization; the percentage increased to 6.38%, about 2.1- to 6.8-fold greater than the values for the other groups, indicating the DC-recruiting ability of pHMGB1. In addition, pHMGB1 coimmunization promoted the maturation of mucosal DCs, as evidenced by a higher percentage of CD11c+ CD80+ cells in the pVP1/pHMGB1 group than in the pVP1 group (3.18% versus 1.37%) or the pHMGB1 group (3.18% versus 1.59%). No significant difference in the percentages of CD80+ MLN cells was observed among these groups.
Fig 5.
Increased DC recruitment and maturation in MLNs with coimmunization with pHMGB1. (A) Representative flow cytometric results for 5 mice per group. (B) Statistical analysis of the frequency of CD11c+ CD80+ cells in MLNs. Each group contained 8 mice. Experiments were repeated three times, with similar results. All of the experimental data were pooled and are presented together. ∗, P < 0.05.
DISCUSSION
An ideal vaccine against mucosa-invasive pathogens should simultaneously induce systemic immune responses, to clear pathogens in the blood, and mucosal immune responses, to block pathogen colonization, to eliminate infected cells, and to limit microbial spread. It has been well accepted that mucosal vaccines are most efficient in inducing mucosal immunity (25). Therefore, the development of novel mucosal vaccines attracts strong interest from immunologists and vaccine researchers.
Due to their unique ability to induce humoral and cellular immune responses, DNA vaccines have been considered a promising immunization approach. However, they are rarely used as mucosal vaccines due to many factors, such as mucociliary clearance, the presence of degradative enzymes, pH extremes (in the gastrointestinal tract), low permeability, and metabolic degradation. To overcome such obstacles, a series of mucosal delivery systems have been developed (26). In this study, we used chitosan to deliver a DNA vaccine mucosally. Chitosan is able to bind negatively charged cell surfaces and mucus, open tight junctions, and obtain paracellular transport across the epithelium (27). It can easily form nanoparticles with plasmid DNA (28) and protect the latter from nuclease degradation (29). In addition, chitosan was reported to be an immune stimulator, increasing the activation of DCs and T cells (30). It has also been shown to promote the secretion of chemokines and cytokines (31, 32). Thus, it could be an efficient mucosal delivery system for vaccines (33). Premaletha et al. (34) reported that oral delivery of hepatitis B virus surface antigen-loaded chitosan microspheres enhanced antigen stability. Liu et al. (35) showed that a tumor DNA vaccine encapsulated in alginic acid-coated chitosan and administered orally could efficiently reach Peyer's patches, induce robust active CD8+ T cells, and significantly inhibit tumor growth.
Another important approach to increase the potency of DNA vaccines is the use of adjuvant. HMGB1 has been recently identified as a damage-associated molecular pattern (DAMP) molecule and could interact with pattern recognition receptors such as RAGE and TLRs (36). It has been reported to be involved in host responses to infections, injuries, tumors, and inflammation by promoting cytokine production (37), recruiting immune cells (38), and modulating DC migration and maturation (39). These characteristics make HMGB1 a potential vaccine adjuvant.
In this study, we found that pHMGB1 coimmunization significantly (P < 0.05) enhanced CVB3-specific mucosal immune responses and conferred improved protection against viral myocarditis, compared with the pVP1 vaccine. This may be partly attributable to the DC-attracting and maturation-promoting abilities of HMGB1 at mucosal sites. Our data showed that coimmunization with HMGB1 recruited DCs with activated phenotypes to MLNs. We speculated that HMGB1 may attract mature DCs by interacting with RAGE, as reported previously (40).
Interestingly, we observed that a low level of protection was achieved in mice immunized with pHMGB1 alone, compared with mock-immunized mice. HMGB1 was reported to be an innate adjuvant that could activate antigen-presenting cells (APCs) in autoimmune diseases and graft rejection conditions (41). It was also shown that HMGB1-deficient mice developed greatly reduced levels of antigen-specific antibodies and T cell responses when immunized with antigens in the presence of lipopolysaccharide (LPS) (42). In agreement with earlier studies, our data indicated that immunization with pHMGB1 alone could result in the recruitment and maturation of mucosal DCs, which may help amplify host resistance to CVB3-induced myocarditis. However, the HMGB1-mediated protection seems to be dependent on preexposure, because mice that received HMGB1 after CVB3 infection developed more-severe viral myocarditis. The increased levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-6, and IL-12 were responsible for the aggravated disease progression (X. Zha, X. Li, Y. Yue, and S. Xiong, unpublished data).
Apart from the effects on DCs, HMGB1 may perform its adjuvant function in other ways, such as enhancing macrophage activation and proinflammatory cytokine secretion (43). Furthermore, it is thought that plasmid DNA containing unmethylated CpG dinucleotide can trigger strong innate immunity through DNA sensors such as TLR9 (44), and this process could be accelerated and augmented by HMGB1. These abilities to promote plasmid recognition by TLR9 and subsequently to provide an inflammatory environment also may facilitate the induction of mucosal immunity.
One concern in using HMGB1 as an adjuvant is its involvement in the pathogenesis of arthritis and sepsis (45, 46). However, this is largely dependent on the amount of HMGB1 used. Only sufficient amounts of HMGB1 could facilitate autoreactive T and B cell activation (47, 48) and accelerate autoimmune diseases. Furthermore, the delivery method also greatly influences the effects of HMGB1 on autoimmune disease initiation and development. Arthritis can be induced by intra-articular injection of recombinant HMGB1 protein in animal models (49), indicating that high local levels of HMGB1 in joint tissues are critical for arthritis induction.
In this study, an HMGB1-encoding eukaryotic expression plasmid was administered to mice by the intranasal route. Two weeks following the final immunization, the indices of body temperature, body weight, and foot pad thickness were measured for pHMGB1-coimmunized mice, to evaluate the potential side effects of pHMGB1. We found that the coimmunized mice had body temperatures (36.89 ± 0.6°C versus 36.6 ± 0.6°C), body weights (29.7 ± 1.0 g versus 30.1 ± 0.6 g), and foot pad thicknesses (2.40 ± 0.05 mm versus 2.36 ± 0.03 mm) comparable to those of pVP1-immunized mice. In addition, no shivering or lameness was observed, indicating that no obvious symptoms of arthritis or inflammation were induced by pHMGB1 administration. We speculated that, with the intranasal immunization approach, most chitosan-DNA particles were taken up and expressed by mucosal cells. Only very limited amounts of pHMGB1 arrived and were expressed in local joints or reached the circulation, amounts that were not sufficient to induce arthritis, systemic inflammation, or sepsis. Our results are in agreement with those of previous reports using an HMGB1 plasmid as a DNA vaccine adjuvant. Muthumani et al. (50) showed that coimmunization of mice with 3 doses of 25 μg pHMGB1 by intramuscular electroporation substantially increased antigen-specific IFN-γ+ CD8+ T cells without side effects such as the induction of arthritis, systemic inflammation, or sepsis, further indicating the safety of HMGB1.
CD8+ T cells have been proposed to play complex roles in CVB3-induced myocarditis. Some studies showed that these cells may not facilitate viral clearance, even exacerbating heart injury (51). On the other hand, Opavsky et al. (52) reported that CVB3-infected CD8−/− mice exhibited lower survival rates than their immunocompetent counterparts, which is in contrast to the notion of detrimental CD8+ T cells in viral myocarditis. In addition, in vivo depletion of CD8+ T lymphocytes from CD4 knockout mice alleviated myocarditis with an increase in myocardial virus titers (53), suggesting the important role of CD8+ T cells in virus clearance. The discordance between these studies may partly be ascribed to the heterogeneity of CD8+ T cells. Two distinct cytotoxic T lymphocyte (CTL) populations, i.e., autoimmune CTLs and virus-specific CTLs, would be induced in CVB3-infected mice. The former destroys uninfected myocardiocytes by recognizing cardiac myosin and aggravates myocarditis (54), while the latter clears infected myocardiocytes and controls virus replication. Although both populations participate in pathogenesis, the lesions caused by autoimmune CTLs were more extensive and necrotizing than those of virus-specific CTLs, indicating that the former are the primary pathogenic effector cells in CVB3-induced myocarditis. The virus-specific CD8+ T cells function as a double-edged sword for viral myocarditis. On one hand, they limit virus propagation; on the other hand, they cause damage to infected myocardiocytes. Their impact on myocarditis depends mainly on their activation time course and the numbers of infected myocardiocytes. The existence of virus-specific CD8+ T cells at the beginning of infection could efficiently control virus propagation and prevent heavy myocardial damage. Therefore, induction of potent CTLs becomes important in the development of novel preventive vaccines against viral myocarditis (55). In our study, coimmunization of chitosan-pHMGB1 with chitosan-pVP1 significantly increased virus-specific CTL responses at the mucosal site and efficiently prevented CVB3-induced myocarditis.
Fusion of HMGB1 with the antigen VP1 offers an alternative strategy to enhance the immunogenicity of DNA vaccines, as they might recruit and target antigens to APCs more effectively (56). Interestingly, the immunological response induced by coimmunization with genes encoding antigen and adjuvant depends on how the two genes are linked. In a previous study, our group compared the mucosal immune responses induced by chitosan-VP1 DNA vaccine in different combinations with the adjuvant ltn gene. The two genes were either cloned in separate vectors or coexpressed as a fusion or bicistronic protein in the same vector before encapsulation in chitosan nanoparticles. It was found that coimmunization with VP1 and ltn genes in separate plasmids (1:1 ratio) was the best way to induce mucosal immunity (22). Hence, we coimmunized mice with two separate plasmids encoding adjuvant HMGB1 and antigen VP1.
In summary, we reported here that intranasal coimmunization of chitosan-pHMGB1 with chitosan-pVP1 could significantly (P < 0.05) recruit and promote maturation of mucosal DCs, lead to enhanced mucosal immune responses, and alleviate viral myocarditis. This study may provide a useful strategy to develop a novel mucosal vaccine against viral myocarditis as well as other mucosa-infectious pathogens.
ACKNOWLEDGMENTS
We thank Tadatsugu Taniguchi at the University of Tokyo for providing pCAGGS-HMGB1-HA.
This work was supported by grants from the National Natural Science Foundation of China (grants 81072413, 31170878, 31370894, 31270973, and 31270977), the Major State Basic Research Development Program of China (grant 2013CB530501), the Jiangsu Pan-Deng Project (grant BK2010004), the Natural Science Foundation of the Jiangsu higher education institutions (grant 12KJB310015), the Ph.D. Programs Foundation of the Ministry of Education of China (grant 20113201120011), the Jiangsu Provincial Innovative Research Team, the Jiangsu 333 Project for cultivation of high-level talents, the Qing Lan Project of the Jiangsu higher education institutions, Priority Academic Program Development of the Jiangsu higher education institutions, and the Program for Changjiang Scholars and Innovative Research Team in University (grant PCSIRT-IRT1075).
The authors have no conflicting financial interests.
Footnotes
Published ahead of print 11 September 2013
REFERENCES
- 1.Calabrese F, Rigo E, Milanesi O, Boffa GM, Angelini A, Valente M, Thiene G. 2002. Molecular diagnosis of myocarditis and dilated cardiomyopathy in children: clinicopathologic features and prognostic implications. Diagn. Mol. Pathol. 11:212–221 [DOI] [PubMed] [Google Scholar]
- 2.Godeny EK, Gauntt CJ. 1986. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J. Immunol. 137:1695–1702 [PubMed] [Google Scholar]
- 3.Leonard EG. 2004. Viral myocarditis. Pediatr. Infect. Dis. J. 23:665–666 [DOI] [PubMed] [Google Scholar]
- 4.Martino TA, Liu P, Sole MJ. 1994. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ. Res. 74:182–188 [DOI] [PubMed] [Google Scholar]
- 5.Kandolf R, Klingel K, Zell R, Selinka HC, Raab U, Schneider-Brachert W, Bultmann B. 1993. Molecular pathogenesis of enterovirus-induced myocarditis: virus persistence and chronic inflammation. Intervirology 35:140–151 [DOI] [PubMed] [Google Scholar]
- 6.Gangaplara A, Massilamany C, Brown DM, Delhon G, Pattnaik AK, Chapman N, Rose N, Steffen D, Reddy J. 2012. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-α-reactive CD4 T cells in A/J mice. Clin. Immunol. 144:237–249 [DOI] [PubMed] [Google Scholar]
- 7.Fairweather D, Stafford KA, Sung YK. 2012. Update on coxsackievirus B3 myocarditis. Curr. Opin. Rheumatol. 24:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Parham NJ, Zhang F, Aasa-Chapman M, Gould EA, Zhang H. 2012. Vaccination with coxsackievirus B3 virus-like particles elicits humoral immune response and protects mice against myocarditis. Vaccine 30:2301–2308 [DOI] [PubMed] [Google Scholar]
- 9.Henke A, Jarasch N, Martin U, Wegert J, Wildner A, Zell R, Wutzler P. 2008. Recombinant coxsackievirus vectors for prevention and therapy of virus-induced heart disease. Int. J. Med. Microbiol. 298:127–134 [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Shen Y, Jiang Z, Wang Y, Chu Y, Xiong S. 2004. Intranasal delivery of chitosan-DNA vaccine generates mucosal SIgA and anti-CVB3 protection. Vaccine 22:3603–3612 [DOI] [PubMed] [Google Scholar]
- 11.See DM, Tilles JG. 1994. Efficacy of a polyvalent inactivated-virus vaccine in protecting mice from infection with clinical strains of group B coxsackieviruses. Scand. J. Infect. Dis. 26:739–747 [DOI] [PubMed] [Google Scholar]
- 12.Chapman NM, Ragland A, Leser JS, Hofling K, Willian S, Semler BL, Tracy S. 2000. A group B coxsackievirus/poliovirus 5′ nontranslated region chimera can act as an attenuated vaccine strain in mice. J. Virol. 74:4047–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fohlman J, Ilback NG, Friman G, Morein B. 1990. Vaccination of Balb/c mice against enteroviral mediated myocarditis. Vaccine 8:381–384 [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Jeon ES, Lim BK, Kim SM, Chung SK, Kim JM, Park SI, Jo I, Nam JH. 2005. Immunogenicity of a DNA vaccine for coxsackievirus B3 in mice: protective effects of capsid proteins against viral challenge. Vaccine 23:1672–1679 [DOI] [PubMed] [Google Scholar]
- 15.Goodwin GH, Sanders C, Johns EW. 1973. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 38:14–19 [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251 [DOI] [PubMed] [Google Scholar]
- 17.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. 2006. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26:174–179 [DOI] [PubMed] [Google Scholar]
- 18.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. 2002. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 62:4805–4811 [PubMed] [Google Scholar]
- 19.Sundberg E, Fasth AE, Palmblad K, Harris HE, Andersson U. 2009. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology 214:303–309 [DOI] [PubMed] [Google Scholar]
- 20.Bianchi ME, Manfredi AA. 2007. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 220:35–46 [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health 1998. Guide for the care and use of medical laboratory animals. Ministry of Health, Beijing, People's Republic of China [Google Scholar]
- 22.Yue Y, Xu W, Xiong S. 2012. Modulation of immunogenicity and immunoprotection of mucosal vaccine against coxsackievirus B3 by optimizing the coadministration mode of lymphotactin adjuvant. DNA Cell Biol. 31:479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue Y, Xu W, Hu L, Jiang Z, Xiong S. 2009. Enhanced resistance to coxsackievirus B3-induced myocarditis by intranasal co-immunization of lymphotactin gene encapsulated in chitosan particle. Virology 386:438–447 [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z, Xu W, Li K, Yue Y, Xu L, Ye F, Xiong S. 2008. Remission of CVB3-induced viral myocarditis by in vivo Th2 polarization via hydrodynamics-based interleukin-4 gene transfer. J. Gene Med. 10:918–929 [DOI] [PubMed] [Google Scholar]
- 25.Dlugonska H, Grzybowski M. 2012. Mucosal vaccination: an old but still vital strategy. Ann. Parasitol. 58:1–8 [PubMed] [Google Scholar]
- 26.Sabirov A, Metzger DW. 2008. Mouse models for the study of mucosal vaccination against otitis media. Vaccine 26:1501–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. 2001. Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 52:139–144 [DOI] [PubMed] [Google Scholar]
- 28.Goldmann K, Ensminger SM, Spriewald BM. 2012. Oral gene application using chitosan-DNA nanoparticles induces transferable tolerance. Clin. Vaccine Immunol. 19:1758–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Qian F, He X, Wang F, Ren D, He Y, Li K, Sun S, Yin C. 2007. Novel chitosan derivative nanoparticles enhance the immunogenicity of a DNA vaccine encoding hepatitis B virus core antigen in mice. J. Gene Med. 9:253–264 [DOI] [PubMed] [Google Scholar]
- 30.Porporatto C, Bianco ID, Correa SG. 2005. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J. Leukoc. Biol. 78:62–69 [DOI] [PubMed] [Google Scholar]
- 31.Guzman-Morales J, Ariganello MB, Hammami I, Thibault M, Jolicoeur M, Hoemann CD. 2011. Biodegradable chitosan particles induce chemokine release and negligible arginase-1 activity compared to IL-4 in murine bone marrow-derived macrophages. Biochem. Biophys. Res. Commun. 405:538–544 [DOI] [PubMed] [Google Scholar]
- 32.Baek KS, Won EK, Choung SY. 2007. Effects of chitosan on serum cytokine levels in elderly subjects. Arch. Pharm. Res. 30:1550–1557 [DOI] [PubMed] [Google Scholar]
- 33.Slutter B, Bal SM, Que I, Kaijzel E, Lowik C, Bouwstra J, Jiskoot W. 2010. Antigen-adjuvant nanoconjugates for nasal vaccination: an improvement over the use of nanoparticles? Mol. Pharm. 7:2207–2215 [DOI] [PubMed] [Google Scholar]
- 34.Premaletha K, Licy CD, Jose S, Saraladevi A, Shirwaikar A, Shirwaikar A. 2012. Formulation, characterization and optimization of hepatitis B surface antigen (HBsAg)-loaded chitosan microspheres for oral delivery. Pharm. Dev. Technol. 17:251–258 [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Lv D, Liu S, Gong J, Wang D, Xiong M, Chen X, Xiang R, Tan X. 2013. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: effect against breast cancer in mice. PLoS One 8:e60190. 10.1371/journal.pone.0060190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira-Machado JA, Volpe CM, Veloso CA, Chaves MM. 2011. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opin. Ther. Targets 15:1023–1035 [DOI] [PubMed] [Google Scholar]
- 37.Pittet JF, Koh H, Fang X, Iles K, Christiaans S, Anjun N, Wagener BM, Park DW, Zmijewski JW, Matthay MA, Roux J. 2013. HMGB1 accelerates alveolar epithelial repair via an IL-1β- and αvβ6 integrin-dependent activation of TGF-β1. PLoS One 8:e63907. 10.1371/journal.pone.0063907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu H, Li J, Wang S, Liu K, Wang L, Huang L. 2013. Hmgb1-TLR4-IL-23-IL-17A axis promote ischemia-reperfusion injury in a cardiac transplantation model. Transplantation 95:1448–1454 [DOI] [PubMed] [Google Scholar]
- 39.Gougeon ML, Melki MT, Saidi H. 2012. HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells. Cell Death Differ. 19:96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manfredi AA, Capobianco A, Esposito A, De Cobelli F, Canu T, Monno A, Raucci A, Sanvito F, Doglioni C, Nawroth PP, Bierhaus A, Bianchi ME, Rovere-Querini P, Del Maschio A. 2008. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J. Immunol. 180:2270–2275 [DOI] [PubMed] [Google Scholar]
- 41.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA. 2004. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 5:825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F, Klinman D, Gioannini T, Weiss JP, Furusawa T, Bustin M, Oppenheim JJ. 2012. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J. Exp. Med. 209:157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Gazzar M. 2007. HMGB1 modulates inflammatory responses in LPS-activated macrophages. Inflamm. Res. 56:162–167 [DOI] [PubMed] [Google Scholar]
- 44.Rottembourg D, Filippi CM, Bresson D, Ehrhardt K, Estes EA, Oldham JE, von Herrath MG. 2010. Essential role for TLR9 in prime but not prime-boost plasmid DNA vaccination to activate dendritic cells and protect from lethal viral infection. J. Immunol. 184:7100–7107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I. 2003. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 48:971–981 [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Zhu S, Zhou R, Li W, Sama AE. 2008. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev. Mol. Med. 10:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cully M. 2013. Connective tissue diseases: HMGB1 helps elicit anti-dsDNA antibody production in SLE. Nat. Rev. Rheumatol. 9:321. [DOI] [PubMed] [Google Scholar]
- 48.Robinson AP, Caldis MW, Harp CT, Goings GE, Miller SD. 2013. High-mobility group box 1 protein (HMGB1) neutralization ameliorates experimental autoimmune encephalomyelitis. J. Autoimmun. 43:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, Erlandsson-Harris H, Tarkowski A. 2003. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 48:1693–1700 [DOI] [PubMed] [Google Scholar]
- 50.Muthumani G, Laddy DJ, Sundaram SG, Fagone P, Shedlock DJ, Kannan S, Wu L, Chung CW, Lankaraman KM, Burns J, Muthumani K, Weiner DB. 2009. Co-immunization with an optimized plasmid-encoded immune stimulatory interleukin, high-mobility group box 1 protein, results in enhanced interferon-gamma secretion by antigen-specific CD8 T cells. Immunology 128:e612–e620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gebhard JR, Perry CM, Harkins S, Lane T, Mena I, Asensio VC, Campbell IL, Whitton JL. 1998. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am. J. Pathol. 153:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opavsky MA, Penninger J, Aitken K, Wen WH, Dawood F, Mak T, Liu P. 1999. Susceptibility to myocarditis is dependent on the response of αβ T lymphocytes to coxsackieviral infection. Circ. Res. 85:551–558 [DOI] [PubMed] [Google Scholar]
- 53.Henke A, Huber S, Stelzner A, Whitton JL. 1995. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 69:6720–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huber SA, Lodge PA. 1984. Coxsackievirus B-3 myocarditis in Balb/c mice: evidence for autoimmunity to myocyte antigens. Am. J. Pathol. 116:21–29 [PMC free article] [PubMed] [Google Scholar]
- 55.Park JH, Kim DS, Cho YJ, Kim YJ, Jeong SY, Lee SM, Cho SJ, Yun CW, Jo I, Nam JH. 2009. Attenuation of coxsackievirus B3 by VP2 mutation and its application as a vaccine against virus-induced myocarditis and pancreatitis. Vaccine 27:1974–1983 [DOI] [PubMed] [Google Scholar]
- 56.Williman J, Young S, Buchan G, Slobbe L, Wilson M, Pang P, Austyn J, Preston S, Baird M. 2008. DNA fusion vaccines incorporating IL-23 or RANTES for use in immunization against influenza. Vaccine 26:5153–5158 [DOI] [PubMed] [Google Scholar]