Abstract
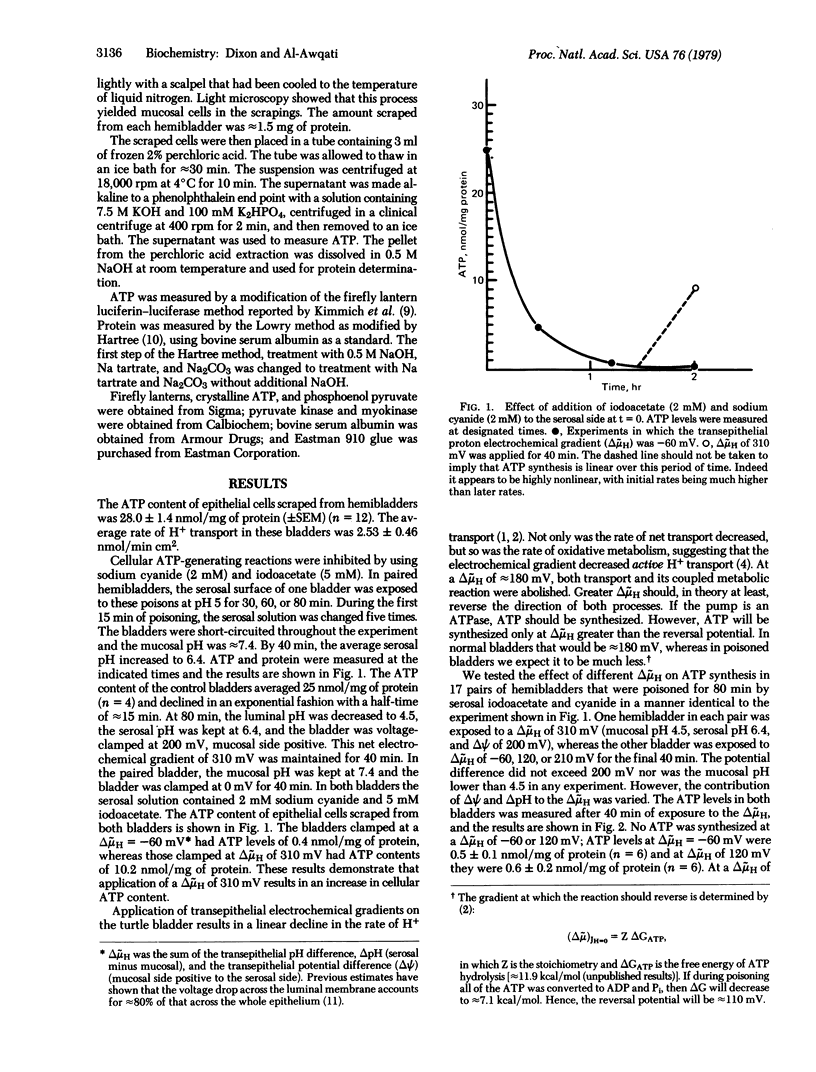
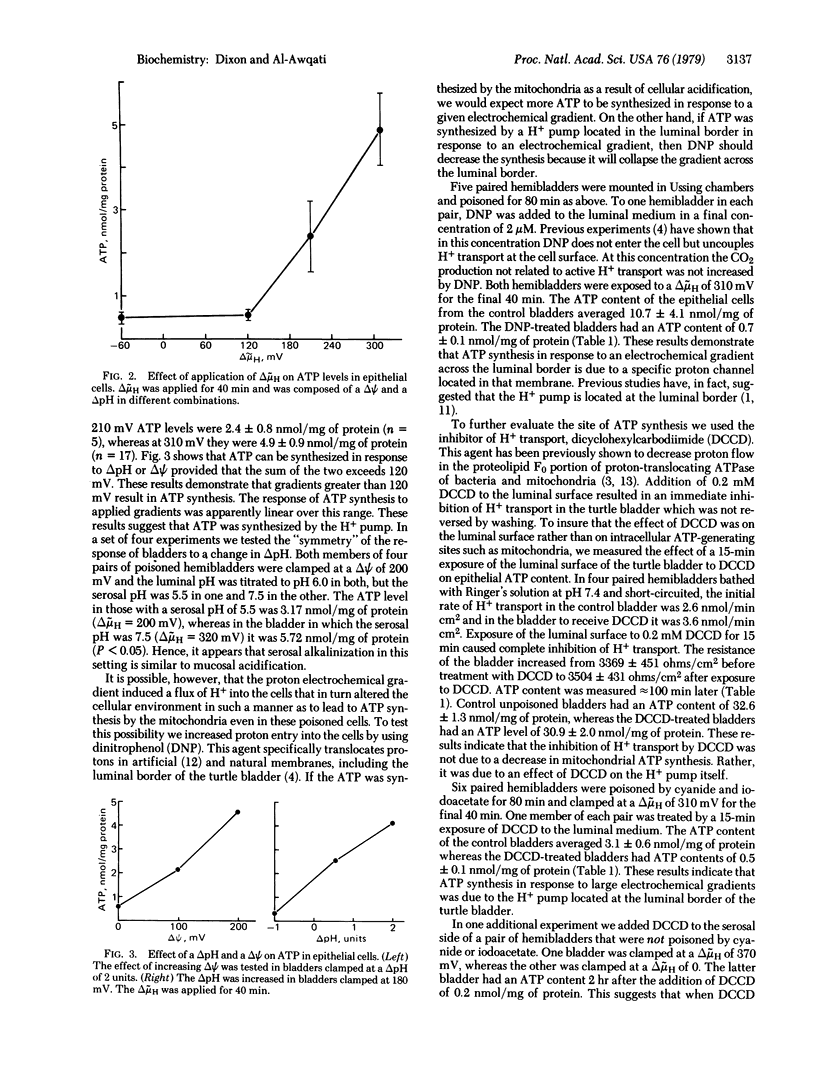
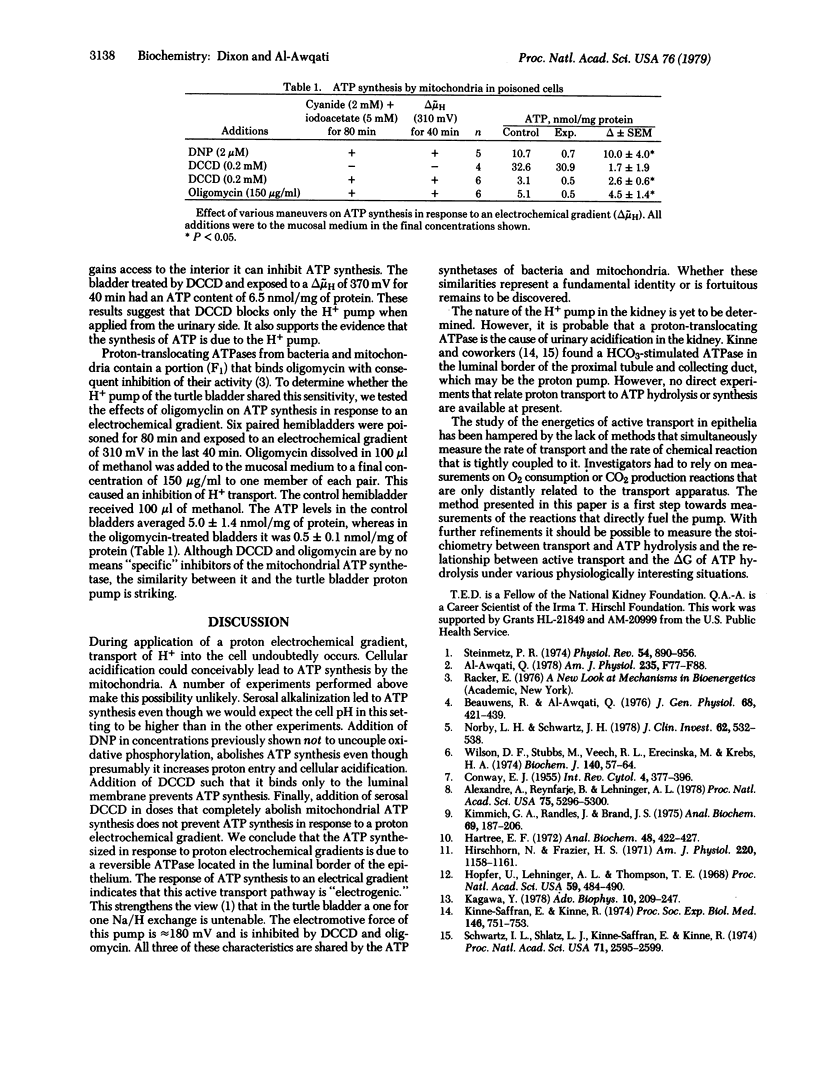
Adverse proton electrochemical gradients (delta muH) applied across the turtle urinary bladder decrease active H+ transport in this epithelium. A delta muH of 180 mV abolishes both transport and its tightly coupled metabolic reaction. Larger gradients should, in theory, reverse the direction of H+ transport and the metabolic reaction leading to synthesis of ATP if the pump is an ATPase, or cause an increase in the oxidized state of a redox pair if it is a redox pump. To distinguish between these two possibilities, we measured ATP levels in epithelial cells that were poisoned to inhibit cellular mechanisms of ATP synthesis. At delta muH of 120 mV or less no ATP synthesis was found. At delta muH of greater than 120 mV there was a linear increase in ATP synthesis. Dinitrophenol, a H+ carrier, prevented synthesis at delta muH of 310 mV. Dicyclohexylcarbodiimide, an inhibitor of H+ transport that works at the cell surface, prevented ATP synthesis at delta muH of 310 mV. These results demonstrate that a reversible proton-translocating ATPase in the mucosal border of the bladder is the H+ pump responsible for urinary acidification.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. H + transport in urinary epithelia. Am J Physiol. 1978 Aug;235(2):F77–F88. doi: 10.1152/ajprenal.1978.235.2.F77. [DOI] [PubMed] [Google Scholar]
- Alexandre A., Reynafarje B., Lehninger A. L. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5296–5300. doi: 10.1073/pnas.75.11.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauwens R., Al-Awqati Q. Active H+ transport in the turtle urinary bladder. Coupling of transport to glucose oxidation. J Gen Physiol. 1976 Oct;68(4):421–439. doi: 10.1085/jgp.68.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hirschhorn N., Frazier H. S. Intracellular electrical potential of the epithelium of turtle bladder. Am J Physiol. 1971 May;220(5):1158–1161. doi: 10.1152/ajplegacy.1971.220.5.1158. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y. Proton translocating ATPase: its pump, gate, and channel. Adv Biophys. 1978;10:209–247. [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kinne-Saffran E., Kinne R. Presence of bicarbonate stimulated ATPase in the brush border microvillus membranes of the proximal tubule. Proc Soc Exp Biol Med. 1974 Jul;146(3):751–753. doi: 10.3181/00379727-146-38186. [DOI] [PubMed] [Google Scholar]
- Norby L. H., Schwartz J. H. Relationship between the rate of H+ transport and pathways of glucose metabolism by turtle urinary bladder. J Clin Invest. 1978 Sep;62(3):532–538. doi: 10.1172/JCI109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I. L., Shlatz L. J., Kinne-Saffran E., Kinne R. Target cell polarity and membrane phosphorylation in relation to the mechanism of action of antidiuretic hormone. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2595–2599. doi: 10.1073/pnas.71.7.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular mechanisms of urinary acidification. Physiol Rev. 1974 Oct;54(4):890–956. doi: 10.1152/physrev.1974.54.4.890. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]


