Abstract
Enterotoxigenic Escherichia coli (ETEC) is a primary cause of traveler's diarrhea for which there is no licensed vaccine. This phase 1 trial determined the safety and immunogenicity of a recombinantly produced double mutant heat-labile enterotoxin (dmLT) of ETEC. It was administered as a single oral dose of dmLT in escalating doses of 5 μg, 25 μg, 50 μg, and 100 μg, followed by a 72-h inpatient observation, outpatient visits at 8, 14, and 28 days, and telephone calls at 2 and 6 months postvaccination. Safety was assessed by frequency of adverse events, and immune responses determined after immunization included dmLT-specific serum IgA and IgG, fecal IgA, antibody-secreting cells (ASC), and antibodies in lymphocyte supernatant (ALS) responses. All doses were well tolerated by the 36 healthy adults enrolled. Immune responses were limited in the 5- and 25-μg dose recipients. The 50-μg dose recipients trended toward stronger responses than the 100-μg dose recipients by serum IgA (67% versus 33%, P = 0.22), serum IgG (58% versus 33%, P = 0.41), and fecal IgA (58% versus 33%, P = 0.41). By day 14 postvaccination, there were significantly more positive responders (≥4-fold increase from baseline) among the 50- versus 100-μg dose recipients for serum IgA (P = 0.036) but not serum IgG (P = 0.21). In conclusion, a single oral dose of dmLT was well tolerated and immunogenic, with immune responses plateauing at the 50-μg dose. (This clinical trial is registered at www.clinicaltrials.gov, registration number NCT01147445.)
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is one of six recognized diarrheagenic E. coli strains and causes approximately 200 million diarrheal episodes and 380,000 deaths annually worldwide (1–3). Although it is the commonest cause of traveler's diarrhea and a leading cause of diarrhea among children in areas where ETEC is endemic, there is no licensed vaccine to prevent enteric illness caused by these organisms (4–6). Pathogenic ETEC strains produce either a small, poorly immunogenic, peptide heat-stable toxin (ST) and/or the highly immunogenic heat-labile toxin (LT), which has been studied as a potential vaccine antigen (7).
LT is an oligomeric protein which is structurally, functionally, and antigenically similar to the cholera toxin (CT) of Vibrio cholerae and consists of a single enzymatically active subunit (A subunit) and five identical receptor binding subunits (B subunits). The B subunits bind GM1 and other galactose-containing cell surface components of the intestinal epithelial cell and facilitate the cell membrane penetration of the A subunit, which further dissociates into two smaller peptides through proteolysis and disulfide reduction. The A1 subunit represents the active moiety responsible for catalyzing ADP ribosylation of GTP-binding cell proteins, with upregulation of intracellular cyclic AMP (cAMP) leading to hypersecretion of water and electrolytes into the lumen of the small intestine, causing a voluminous secretory diarrhea and potentially severe dehydration. LT-expressing ETEC strains have been shown to be better colonizers and more virulent in animal models (8–10). The LT protein is a potent immunogen that has been shown to be a protective antigen in animal models (11) and in limited human field trials (12, 13) and has been extensively studied as a robust immunostimulating adjuvant (14, 15). All these studies indicate its potential as both an important ETEC antigen and a mucosal adjuvant for coadministered antigens.
To address safety concerns of an LT protein-based vaccine, research has focused on site-directed mutagenesis to eliminate the ADP-ribosylating enzymatic activity of the A subunit (16, 17). A single mutant LT (mLT) containing a glycine substitution at position 192 by arginine (R192G) was created to disrupt the enzymatic and toxic activity of LT. In preclinical studies, the LTR192G protein demonstrated reduced toxicity while retaining adjuvant properties (18). In a phase 1 trial, doses of 50 μg or less of LTR192G were found to be safe, but the 100-μg dose resulted in diarrhea (>1 liter within 24 h) among 2 of 12 recipients (19). In additional trials, 25 μg of LTR192G was associated with cases of mild, self-limited diarrhea when coadministered with other antigens (20, 21). For comparison, only 5 μg of native LT is necessary to induce diarrhea in humans (22).
Therefore, a second-generation derivative, double mutant LT (dmLT), was created through the additional substitution of alanine for leucine at amino acid position 211 (L211A) at a putative pepsin-sensitive proteolytic cleavage site (23). In preclinical testing, LTR192G/L211A (or dmLT) demonstrated no enterotoxicity (in the patent mouse assay) and retained adjuvanticity. We hypothesized that dmLT would demonstrate a further reduction in toxicity (diarrhea) without reducing immunogenicity in humans. The purpose of this study was to determine the safety and immunogenicity of orally administered dmLT, an important ETEC antigen, in humans for the first time.
MATERIALS AND METHODS
Vaccine.
LTR192G/L211A, or dmLT, is a genetically modified derivative of wild-type ETEC heat-labile enterotoxin created by replacing the arginine at amino acid position 192 with glycine and the leucine at amino acid position 211 with alanine (23). The dmLT protein toxoid was produced according to cyclic GMP (cGMP) specifications at the Pilot BioProduction Facility, Walter Reed Army Institute of Research (WRAIR; Silver Spring, MD) (24). The final vaccine was formulated as a lyophilized product, containing 700 μg of vaccine protein in a 3-ml, sterile, multidose, Wheaton serum vial and was stored at −20°C after production. The study product also contained sodium phosphate buffer supplemented with 5% lactose to stabilize the product during the freezing and drying processes. Stability testing on dmLT (lot 1575, manufactured July 2009) was performed to ensure structural integrity using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with and without trypsin treatment every 3 months for 1 year and then every 6 months for up to 42 months after it was manufactured. This testing demonstrated that dmLT remained structurally unchanged and retained its activity as an immunogen and adjuvant for a coadministered antigen (tetanus toxoid) in mice.
Study design.
Healthy adults 18 to 45 years of age from Baltimore and Cincinnati were recruited to participate in this first-in-human phase 1 trial of single oral vaccination with dmLT. In an open-label, dose-ascending design, 6 subjects each were enrolled into cohorts 1 to 4 and administered dmLT at 5 μg, 25 μg, 50 μg, and 100 μg, respectively. In a fifth cohort, 12 subjects were enrolled and randomized 1:1 for double-blinded administration of 50- or 100-μg doses of dmLT. Eligible subjects were nonpregnant, healthy volunteers that provided informed consent and satisfied the protocol-defined inclusion and exclusion criteria (registered at http://clinicaltrials.gov/show/NCT01147445). They were admitted to the inpatient unit 1 day prior to vaccination for acclimatization. The next day, subjects fasted for 90 min after a light breakfast and then ingested 120 ml of bicarbonate solution (2 g NaHCO3). Within 5 min, subjects drank 30 ml of bicarbonate buffer solution containing the specified dose of dmLT. Following ingestion, subjects fasted for another 90 min. Vaccinated subjects remained in the inpatient unit another 72 h (through day 3) for the observation of any reactogenicity and the grading of all stools. Discharge from the inpatient unit was contingent upon a subject remaining healthy and meeting all discharge criteria (i.e., normal stool, vital signs, and laboratory results). Outpatient clinic follow-up visits were scheduled on days 8, 14, and 28, and telephone interviews were conducted at 2 and 6 months after vaccination. The study was reviewed and approved by the local Institutional Review Boards of University of Maryland, Baltimore, and Cincinnati Children's Hospital Medical Center and by Western Institutional Review Board (Olympia, WA).
Safety evaluation.
During the inpatient stay, all stools were assessed (time of onset, number, volume, and grading of consistency) for the occurrence of diarrhea. Additional reactogenicity that was recorded for the primary outcome of safety included nausea, vomiting, abdominal pain, and anorexia. Clinical safety laboratories were evaluated on days 3 and 28 and included hematology (white blood cells [WBC], absolute neutrophil count [ANC], Hgb, and platelets), coagulation (prothrombin time [PT], partial thromboplastin time [PTT], international normalized ratio [INR], C-reactive protein [CRP], and fibrinogen), and chemistry parameters (sodium, potassium, chloride, bicarbonate, blood urea nitrogen [BUN], creatinine, aspartate transaminase [AST], and alanine aminotransferase [ALT]). Adverse events (AEs), including serious adverse events (SAEs), were graded according to standardized criteria starting after vaccination, through the inpatient stay, at each follow-up clinic visit (days 8, 14, and 28), and at months 2 and 6, by telephone. A Division of Microbiology and Infectious Diseases, National Institutes of Health-appointed Safety Monitoring Committee reviewed safety data through day 28 prior to recommending the initiation of each subsequent dose cohort.
Immune response assays. (i) Serum antibody ELISA.
Serum IgA and IgG antibodies specific for dmLT (1 μg/ml, cGMP lot 1575; WRAIR) were tested by enzyme-linked immunosorbent assay (ELISA) as previously described (25). The endpoint titers (EU/ml) were calculated through linear regression curves as the inverse of the serum dilution that produces an optical density at 450 nm (OD450) of 0.2 above the mean of the blanks. All samples were tested in duplicate and with a positive calibrated control. A positive response was defined as an increase of antibody titer of ≥4-fold over baseline.
(ii) Fecal total and dmLT-specific IgA.
Stool supernatants were tested for total IgA and dmLT-specific IgA by ELISA as previously described (25). Total and dmLT-specific IgA concentrations were calculated by interpolation of the regression-corrected absorbance values produced by serially diluted stool samples and extrapolation from a standard curve of known concentrations of human IgA (Calbiochem). In order to adjust for individual variations in the IgA content of stools, the ratio of specific IgA to total IgA antibody was calculated, and a positive response was defined as a ≥4-fold increase of the ratio over the baseline ratio. The threshold of detection for the assay was 3 ng/ml; therefore, any prevaccination values below this level of detection were designated the value of 3 ng/ml for the calculation of fold increases postvaccination.
(iii) ASC assays.
Circulating IgG and IgA dmLT-specific antibody-secreting cells (ASC) were measured by enzyme-linked immunosorbent spot assay (ELISpot) using freshly isolated peripheral blood mononuclear cells (PBMC), as previously published (25). The frequency of ASC was expressed as the number of IgA or IgG spot-forming cells (SFC) per 106 PBMC. A positive ASC response was defined as ≥8 SFC per 106 cells.
(iv) ALS.
Purified PBMC were resuspended in complete RPMI and incubated in 24-well plates (1 × 107 cells/ml, 1 ml/well) at 37°C and 5% CO2 for 72 h. Following incubation, culture supernatants were collected and stored at −20°C until tested by ELISA for the presence of dmLT-specific antibodies as described above. An increase of antibodies in lymphocyte supernatant (ALS) titers of ≥2-fold over baseline was considered a positive response.
Statistical analysis and sample size.
Although no formal sample size calculation was performed, the number of subjects was selected to be appropriate for a first-in-human study. AEs and reactogenicity are summarized using the number and percentage of subjects who experienced each event overall. A Fisher exact test was performed to test the comparability of the treatment groups. Geometric mean antibody titers and rates of seroconversions were compared between two groups by Wilcoxon rank-sum test. All reported P values are two-sided using the 0.05 level of significance. All data analyses and statistical computations were conducted with SAS, version 9.2.
RESULTS
Thirty-six healthy adults were enrolled with a mean age of 31.4 years (range, 19 to 44), of whom 19 (52.8%) were males, 35 (97.2%) reported being non-Hispanic, 22 (61.1%) were black, and 12 (33.3%) were white (Table 1). All 36 subjects (100%) completed the inpatient phase of the study, with 35 subjects (97.2%) completing outpatient visits through day 28, and 33 (91.7%) completed the month 6 telephone call. One subject did not submit the day 14 and day 28 serum and stool samples.
Table 1.
Demographic and baseline characteristics, by dose
| Demographic | Valuec |
||||
|---|---|---|---|---|---|
| 5 μg (n = 6) | 25 μg (n = 6) | 50 μga (n = 12) | 100 μgb (n = 12) | Total (n = 36) | |
| Gender, no. (%) | |||||
| Male | 3 (50) | 1 (16.7) | 7 (58.3) | 8 (66.7) | 19 (52.8) |
| Female | 3 (50) | 5 (83.3) | 5 (41.7) | 4 (33.3) | 17 (47.2) |
| Ethnicity, no. (%) | |||||
| Non-Hispanic or Non-Latino | 6 (100) | 6 (100) | 11 (91.7) | 12 (100) | 35 (97.2) |
| Hispanic or Latino | 0 | 0 | 1 (8.3) | 0 | 1 (2.8) |
| Race, no. (%) | |||||
| Black/African American | 2 (33.3) | 5 (83.3) | 8 (66.7) | 7 (58.3) | 22 (61.1) |
| White | 4 (66.7) | 1 (16.7) | 3 (25) | 4 (33.3) | 12 (33.3) |
| Multiracial | 0 | 0 | 1 (8.3) | 0 | 1 (2.8) |
| Other/unknown | 0 | 0 | 0 | 1 (8.3) | 1 (2.8) |
| Age (yrs) | |||||
| Mean (SD) | 29.0 (8.7) | 28.8 (8.8) | 33.1 (7.3) | 32.3 (7.8) | 31.4 (7.8) |
| Median | 29.0 | 27.5 | 35.0 | 30.5 | 30.5 |
| Min, max | 19, 43 | 20, 41 | 19, 43 | 21, 44 | 19, 44 |
| Age group, no. (%) | |||||
| 18–30 yrs | 4 (66.7) | 4 (66.7) | 4 (33.3) | 6 (50) | 18 (50) |
| >30 yrs | 2 (33.3) | 2 (33.3) | 8 (66.7) | 6 (50) | 18 (50) |
Six subjects were dosed with 50 μg in cohort 3 and another six were dosed in cohort 5.
Six subjects were dosed with 100 μg in cohort 4 and another six were dosed in cohort 5.
The denominator for percentages is the no. of subjects in the population for each dose.
Safety.
Thirty-three (91.7%) subjects experienced at least one AE. Of the total 103 AEs reported, 98 were mild, 3 were moderate, and 2 were severe and serious but not associated with the vaccine (a hospitalization for a trauma-induced fracture and a death from a heroin overdose). A single subject experienced two moderate-grade AEs, a torn left ankle ligament and back strain; the other moderate AE was a neck strain. Of the 98 mild-grade AEs, 39 were possibly associated, of which all but one were transient laboratory abnormalities. There was no dose-limiting toxicity by any measure of safety.
There were no diarrheal episodes in any dosage group during the 8 days postvaccination (Table 2). One subject (50 μg) experienced mild abdominal pain on day 1, and another subject (100 μg) had mild abdominal pain with mild vomiting (no nausea) on day 2; these AEs were only for a single day. In total, 5 subjects experienced 6 mild-grade reactogenicity events, 3 of which occurred between days 4 to 8 postvaccination (after the inpatient period). Among the clinical laboratory testing, there were no instances of greater than mild (grade 1) abnormalities which were deemed associated with vaccination. The laboratory abnormalities were transient and self-corrected.
Table 2.
Maximum reactogenicity during the 8 days postvaccination
| Symptom | No. of subjects with symptom/total no. of subjectsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Days 0–3, inpatient |
Days 4–8, outpatient |
|||||||
| 5 μg | 25 μg | 50 μg | 100 μg | 5 μg | 25 μg | 50 μg | 100 μg | |
| Diarrheab | 0/6 | 0/6 | 0/12 | 0/12 | 0/5 | 0/6 | 0/12 | 0/12 |
| Abdominal pain | 0/6 | 0/6 | 1/12 | 1/12 | 1/5 | 0/6 | 0/12 | 0/12 |
| Anorexia | 0/6 | 0/6 | 0/12 | 0/12 | 0/5 | 0/6 | 0/12 | 0/12 |
| Nausea | 0/6 | 0/6 | 0/12 | 0/12 | 0/5 | 2/6 | 0/12 | 0/12 |
| Vomiting | 0/6 | 0/6 | 0/12 | 1/12 | 0/5 | 0/6 | 0/12 | 0/12 |
| Fever | 0/6 | 0/6 | 0/12 | 0/12 | 0/5 | 0/6 | 0/12 | 0/12 |
In total, 5 subjects experienced 6 mild-grade reactogenicity events.
Defined as ≥2 loose stools over 24 h.
Immunogenicity.
The immune response was evaluated by dmLT-specific IgA and IgG antibody in serum, feces (IgA only), and lymphocyte supernatants, as well as dmLT-specific ASC.
The anti-dmLT serum IgA and IgG geometric mean titer (GMT) and 95% confidence intervals were calculated for each dose group (Table 3). The immune response did not follow a linear dose-response curve. Whereas the 5-μg and 25-μg doses were generally poorly immunogenic, the 50-μg and 100-μg doses resulted in robust serum immune responses. The highest postvaccination GMT was found in the 50-μg dose group at all measured time points (day 8, 14, and 28) for both serum IgA and IgG (Table 3). However, due to small sample sizes, the differences between the 50-μg and 100-μg doses were not statistically significant (using a two-sided Mann-Whitney U-test), in either the serum IgA (day 8, P = 0.29; day 14, P = 0.14; day 28, P = 0.25) or IgG (day 8, P = 0.34; day 14, P = 0.25; day 28, P = 0.44) levels.
Table 3.
Summary of serum IgA and IgG responses, by dose
| Response | GMT, EU/ml (95% CI)a |
|||
|---|---|---|---|---|
| 5 μg (n = 5 subjects)b | 25 μg (n = 6 subjects) | 50 μg (n = 12 subjects) | 100 μg (n = 12 subjects) | |
| Anti-dmLT serum IgA | ||||
| Baseline | 124 (25, 620) | 130 (77, 219) | 211 (119, 373) | 311 (185, 525) |
| Day 8 | 167 (28, 994) | 264 (46, 1,527) | 822 (358, 1,885) | 591 (233, 1,501) |
| Day 14 | 177 (32, 977) | 263 (51, 1,350) | 1,490 (497, 4,464) | 675 (280, 1,631) |
| Day 28 | 164 (33, 827) | 328 (109, 990) | 1,030 (368, 2,882) | 800 (412, 1,553) |
| Total no. of responders (%)c | 0/5 (20) | 1/6 (16.7) | 8/12 (66.7) | 4/12 (33.3) |
| Anti-dmLT serum IgG | ||||
| Baseline | 369 (158, 864) | 424 (107, 1,675) | 573 (250, 1,315) | 514 (286, 924) |
| Day 8 | 487 (136, 1,744) | 627 (89, 4,414) | 1,872 (526, 6,655) | 1,063 (359, 3,148) |
| Day 14 | 615 (111, 3,404) | 756 (93, 6,138) | 3,494 (776, 15,723) | 1,215 (352, 4,190) |
| Day 28 | 583 (117, 2,913) | 853 (111, 6,536) | 3,120 (697, 13,977) | 1,739 (574, 5,272) |
| Total no. of responders (%)c | 1/5 (20) | 1/6 (16.7) | 7/12 (58.3) | 4/12 (33.3) |
Values are GMT (95% CI) unless otherwise stated.
A nonassociated death occurred prior to the collection of the day 8 specimen.
A 4-fold increase in antibody over baseline, at any time postvaccination.
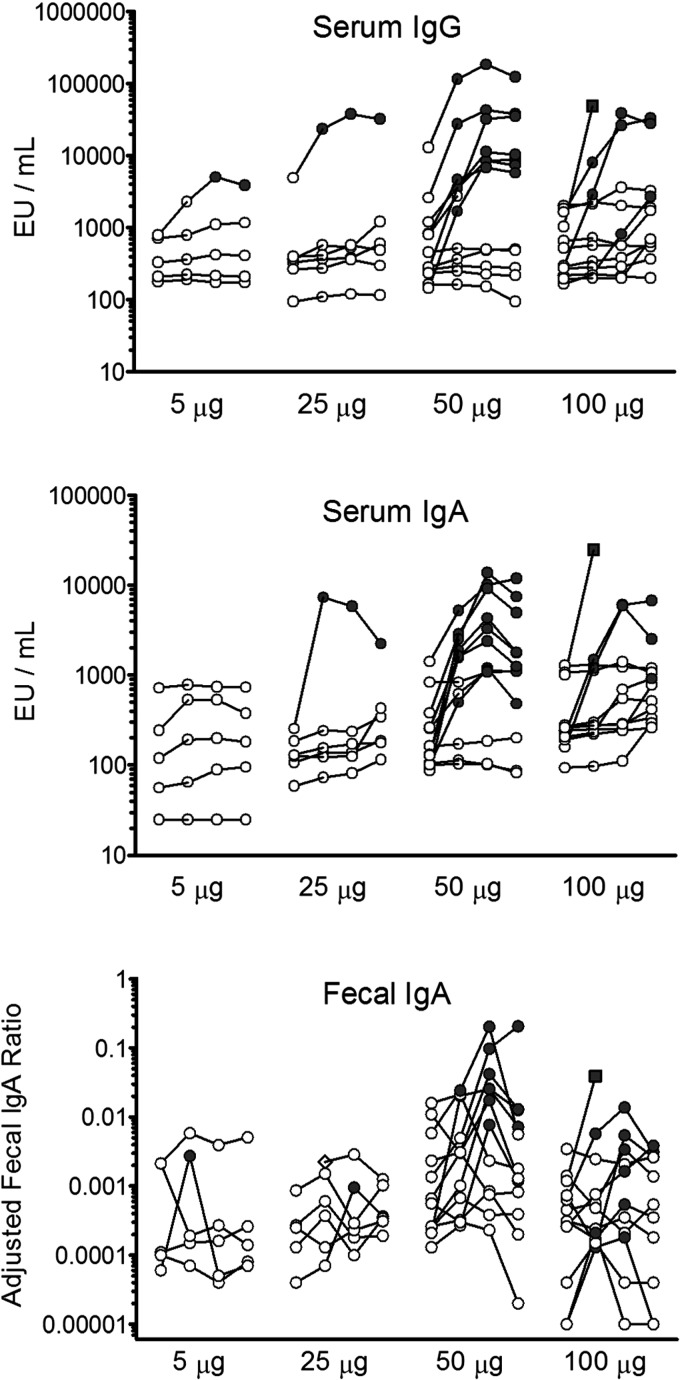
The peak serum antibody response occurred between 14 and 28 days postvaccination (Fig. 1, top two panels). When comparing positive responses among subjects receiving the 50-μg and 100-μg doses, defined as a ≥4-fold increase compared to the baseline, there was a significantly higher response rate (using a two-sided Fisher exact test) for serum IgA (day 8, P = 0.40; day 14, P = 0.036; day 28, P = 0.10) than for serum IgG (day 8, P = 0.67; day 14, P = 0.21; day 28, P = 0.21). The adjusted dmLT-specific fecal IgA responses generally followed the serum immune response pattern, with the most frequent responses seen in the 50-μg and 100-μg dosing groups (Fig. 1, bottom).
Fig 1.
Individual serum anti-dmLT IgG (top) and IgA (middle) and adjusted fecal IgA (bottom) responses for each of the four doses administered (5 μg, 25 μg, 50 μg, and 100 μg). Within each dose group, the four circles denote (from left to right) the following four time points: baseline and 8, 14, and 28 days postvaccination. A positive response was defined by ≥4-fold increases compared to the baseline (indicated by the filled symbols). The diamond indicates a subject that did not submit a baseline sample (bottom; fecal IgA in the 25-μg dose group), and the square indicates a subject that submitted only the baseline and day 8 samples (100-μg dose group in all 3 panels).
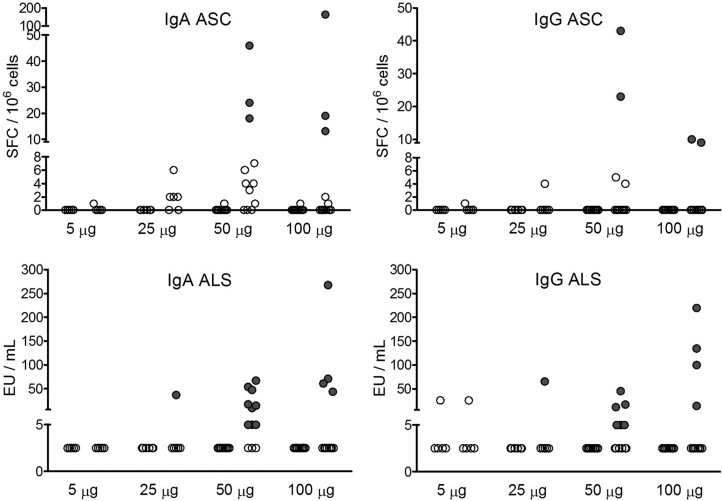
The presence of circulating dmLT-specific IgA and IgG ASC provides additional information on the mucosal immune response to vaccination. Using our definition of ASC response (≥8 SFC/106 cells), there were no ASC responses with the 5-μg and 25-μg doses. However, the groups that received 50-μg and 100-μg doses exhibited positive IgA and IgG ASC responses (Fig. 2, top). The dmLT-specific IgA and IgG ALS responses were also observed among subjects who demonstrated either a serum or ASC response (Fig. 2, bottom). When exploring for an association between mucosal and systemic (serum) immune responses, a strong correlation was found between the peak dmLT-specific IgA titers in serum and the IgA ASC response (Spearman's rank correlation coefficient r = 0.73, P < 0.0001) (data not shown). The frequency and magnitude of individual immune responses, according to vaccine dosage levels, were tabulated for those subjects that demonstrated a positive response in at least one of the study assays (Table 4). Overall, compared to 100 μg, more 50-μg dose recipients seroconverted for both systemic and mucosal responses (Table 4). Among the systemic responses, 8 (67%) versus 4 (33%) subjects seroconverted for serum IgA (P = 0.22), and 7 (58%) versus 4 (33%) subjects seroconverted for serum IgG (P = 0.41). A similar pattern was observed in the mucosal responses, with 9 (75%) versus 4 (33%) subjects seroconverting for IgA ALS (P = 0.10), 6 (50%) versus 4 (33%) seroconverting for IgG ALS (P = 0.68), and 7 (58%) versus 4 (33%) subjects seroconverting for fecal IgA (P = 0.41). There was no difference between the two groups for IgA and IgG ASC, with 3 (25%) and 2 subjects (17%) seroconverting in both groups, respectively (both P = 1.0).
Fig 2.
dmLT-specific IgA (top left) and IgG (top right) ASC and IgA (bottom left) and IgG (bottom right) ALS responses at baseline (prevaccination) and 8 days (postvaccination) for each of the four doses administered (5 μg, 25 μg, 50 μg, and 100 μg). A positive ASC response is defined as ≥8 spot-forming cells (SFC)/106 cells, and a positive ALS response is defined as a ≥2-fold increase compared to the baseline (indicated by filled circles).
Table 4.
Summary of systemic and mucosal immune responsesc
| Dosage cohort | Systemic responses in seruma |
Mucosal responses |
|||||
|---|---|---|---|---|---|---|---|
| ASCb |
ALSa |
Fecala | |||||
| IgA | IgG | IgA | IgG | IgA | IgG | IgA | |
| 5 μgd | – | 6.4 | – | – | – | – | 42 |
| 25 μge | – | – | – | – | – | – | 22.1 |
| 28.8 | 7.7 | – | – | 14.8 | 26.2 | ND | |
| 50 μgf | 48.8 | 60.6 | 46 | – | 26.8 | 2 | – |
| 11.8 | 32.2 | – | – | 3.8 | 2 | 58.5 | |
| 25.1 | 16.5 | – | 23 | 2 | 6.9 | 99 | |
| 8.3 | 14.3 | 18 | 43 | 21.6 | 18.2 | 123.1 | |
| 4.4 | – | – | – | 6.9 | – | 63.3 | |
| – | – | – | – | 2 | – | – | |
| 24.2 | 9.6 | 24 | – | 2 | 4.8 | – | |
| 9.1 | 8.3 | – | – | 19 | – | 34.6 | |
| – | – | – | – | – | – | 7.5 | |
| 106.7 | 43.8 | – | – | 2 | 2 | 152.4 | |
| 100 μgg | – | – | – | – | 28.4 | 40.1 | 5.7 |
| 30.5 | 20.2 | 13 | 9 | 24.4 | 53.9 | 51.2 | |
| 37.5 | 130.9 | 19 | – | 17.4 | 5.8 | 4.9 | |
| 4.4 | 16.0 | – | – | – | – | – | |
| 24.4 | 47.3 | 163 | 10 | 107 | 87.8 | 33 | |
Peak fold increase above baseline.
SFC/106 cells.
–, a negative response for that assay; ND, not done (the baseline was not available for calculating the fold increase).
Four of 5 (80%) were complete nonresponders.
Four of 6 (67%) were complete nonresponders.
Two of 12 (17%) were complete nonresponders.
Seven of 12 (58%) were complete nonresponders.
DISCUSSION
Given the importance of LT as a candidate ETEC vaccine antigen and its documented adjuvant properties for coadministered antigens, research over the last 20 years has been devoted to the development of detoxified or attenuated forms of the protein that retain their antigenicity and adjuvant properties but lack significant local or systemic toxicity. Active and cleavage site mutants like LTK63 and LT192G have been the most extensively studied (15, 18, 26). Although both are good immunogens, LT192G appears to have more robust adjuvant potential since it also enhances mucosal and systemic responses to orally administered vaccine antigens. Unfortunately, these vaccine-adjuvant combinations showed unacceptable levels of local reactogenicity (20, 21). Ideally, an attenuated form of LT is needed that will have little to no enterotoxicity on its own, thus allowing it to be evaluated as an antigen and adjuvant by both the traditional oral route and more novel routes of vaccine delivery (e.g., intramuscular, intradermal, sublingual, subcutaneous). Furthermore, it should not interact with coadministered vaccine antigens to yield significantly more reactogenic formulations. Successful attenuation of LT could help facilitate and accelerate the movement of dmLT from a promising candidate vaccine antigen and adjuvant into more active clinical evaluation.
This phase 1 trial demonstrates that a single oral dose of dmLT is safe and minimally reactogenic at doses up to 100 μg. A predecessor single mutant LT (LTR192G) vaccine elicited severe diarrhea (>1 liter of stool over 24 h) at the 100-μg dose (19), whereas the native LT protein can elicit diarrhea at the 5-μg dose. Therefore, this study appears to confirm that a second mutation (L211A) results in a further attenuation of the diarrheagenic nature of the enterotoxin. Because there was no dose-limiting toxicity or diarrhea induced at doses up to 100 μg, it is important to carefully consider the immunogenicity data.
Our immunogenicity results show that the greatest proportion of systemic and mucosal immune responses occurred with the 50-μg dmLT dose, while the 100-μg dose group did not demonstrate significant further increases. Although the current data appear to demonstrate a trend for lower responses beyond the 50-μg dose, this conclusion is limited by the small sample sizes. Nonetheless, 8 of 12 of the 50-μg dose recipients demonstrated simultaneous serum and mucosal immune responses (Table 3), supporting our view that these were true vaccine-induced responses.
The fecal IgA results may have been limited by the lower limit of detection (LLD) of our assay. If we had used half the LLD (1.5 ng/ml) for these low calculated levels, then we may have been able to declare 2 additional responders in the 100-μg group, but these 2 subjects had no other response by the other assays. We report the results according to the LLD, as per our original analysis plan, to avoid the potential for false conversions. For future studies, we will be further optimizing the assay to allow a lower detection limit.
On the whole, the data suggest that the 50-μg dose may be the most optimal, when used as a solitary oral immunogen. It is possible that additional sequential doses may elicit a higher frequency of responses to the lower dosage levels. In a prior single- and ascending-dose phase 1 trial evaluating the safety and immunogenicity of native LT and the single mutant, LTR192G, both serum and mucosal immune responses tended to plateau at the 25-μg dose rather than at the 50-μg dose and were not improved by higher (50 and 100 μg) oral doses (21, 27). Consequently, these earlier results serve as further evidence that the dose response observed with dmLT in this study is an accurate reflection of the immunogenicity of this protein.
Pragmatically, the dmLT protein is being developed for use as an adjuvant or in combination with other ETEC antigens, a property not evaluated in this study. Results from preclinical studies have been encouraging (28). Candidate ETEC vaccines inducing both anti-LT and anticolonization factor responses have been more protective than vaccines inducing anti-LT responses alone in both animals and human field trials (12). Therefore, to fully protect from ETEC-associated diarrhea, an effective vaccine may need to include LT and up to seven fimbrial colonization factors. Several animal and human studies have also evaluated LT as a potential mucosal adjuvant for coadministration with candidate vaccine antigens against a wide range of bacterial, fungal, and viral pathogens (15). When oral doses of 5 and 10 μg of native LT were administered with a recombinant Helicobacter pylori urease protein to human subjects, the combination adjuvanted the anti-urease response; however, 16 of 24 subjects (67%) developed diarrhea (29). In a subsequent study, lower doses of LT were evaluated (0.1 μg, 0.5 μg, and 2.5 μg) with the prototype urease vaccine, and mild diarrhea (1 to 4 loose stools) occurred after the first oral immunization in 50% (6 of 12) of the volunteers exposed to the 2.5-μg doses (30).
A well-tolerated mucosal adjuvant in combination with other oral vaccines should impart several advantages, including ease of administration, elicitation of mucosal immunity, and higher acceptance by children and their families. Furthermore, there is evidence that dmLT can elicit Th17-type T cell responses, providing further justification for continued product development (31). One potential disadvantage of oral administration of the current dmLT vaccine is the need to buffer gastric acidity by preadministering sodium bicarbonate, which may be unpalatable to some recipients. Recent animal studies that suggest that dmLT may be given by the sublingual route without a buffer need to be confirmed in a phase 1 trial (32). In our study, none of the 36 subjects expressed discomfort, nausea, or vomiting after the administration of the buffer or vaccine.
In conclusion, this study demonstrated that a single oral dose of 100 μg of the genetically attenuated ETEC dmLT is safe, well tolerated, and reasonably immunogenic. A dose of 50 μg induced mucosal and systemic dmLT-specific immune responses in most immunized individuals that appeared to plateau (or decline somewhat) at the highest (100-μg) dose tested. It is not known how effective the 50-μg dose may be, when given alone or in combination with other ETEC antigens, in preventing wild-type ETEC-induced diarrhea. However, these encouraging results warrant further investigation of dmLT in future phase 1 and 2 trials of new ETEC vaccines or with other enteric vaccine candidates where preclinical studies indicate that the inclusion of dmLT improves immunogenicity and protective efficacy.
ACKNOWLEDGMENTS
This work was supported by the Clinical Research Unit (CRU; NO1-AI-40014, WHC) of the National Institute of Allergy and Infectious Diseases (NIAID) Food & Waterborne Diseases Integrated Research Network (FWD IRN) contract.
None of the authors have a commercial or other association that might pose a conflict of interest.
We are grateful for the contributions of Carol Tacket, University of Maryland School of Medicine, toward the planning and preparation for this study and for the recruitment and care of subjects provided by Jane Cowan, Melissa Billington, Sandra Getlein, and Lisa Chrisley at the University of Maryland and Amy Hoeper, Michelle Dickey, Colleen Fitzpatrick, and Tara Foltz at the Cincinnati Children's Hospital Medical Center. We also thank personnel from the Applied Immunology Laboratory at the Center for Vaccine Development, University of Maryland, for performing serological measurements and from Advanced Biosciences Laboratories in Rockville, MD (ABL), for performing dmLT dose verification assays. We also thank NIH/NIAID colleagues Stephanie Zafonte and Mirjana Nesin for their support on the project, and we acknowledge Shahida Baqar for her continued support for the study and reviewing the manuscript. We also extend a special thanks to the EMMES Corporation, Rockville, MD, for data management and data analysis.
Footnotes
Published ahead of print 18 September 2013
REFERENCES
- 1.Steffen R, Castelli F, Dieter Nothdurft H, Rombo L, Jane Zuckerman N. 2005. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. J. Travel Med. 12:102–107 [DOI] [PubMed] [Google Scholar]
- 2.Wenneras C, Erling V. 2004. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J. Health Popul. Nutr. 22:370–382 [PubMed] [Google Scholar]
- 3.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178 [DOI] [PubMed] [Google Scholar]
- 4.Black RE. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl 1):S73–S79 [DOI] [PubMed] [Google Scholar]
- 5.Black RE. 1993. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine 11:100–106 [DOI] [PubMed] [Google Scholar]
- 6.Steffen R, Tornieporth N, Clemens SA, Chatterjee S, Cavalcanti AM, Collard F, De Clercq N, DuPont HL, von Sonnenburg F. 2004. Epidemiology of travelers' diarrhea: details of a global survey. J. Travel Med. 11:231–237 [DOI] [PubMed] [Google Scholar]
- 7.Walker RI, Steele D, Aguado T. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545–2566 [DOI] [PubMed] [Google Scholar]
- 8.Allen KP, Randolph MM, Fleckenstein JM. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect. Immun. 74:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berberov EM, Zhou Y, Francis DH, Scott MA, Kachman SD, Moxley RA. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 72:3914–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenn GM, Francis DH, Danielsen EM. 2009. Toxin-mediated effects on the innate mucosal defenses: implications for enteric vaccines. Infect. Immun. 77:5206–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahren CM, Svennerholm AM. 1982. Synergistic protective effect of antibodies against Escherichia coli enterotoxin and colonization factor antigens. Infect. Immun. 38:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vaccines 11:677–694 [DOI] [PubMed] [Google Scholar]
- 13.Ellingsworth L. 2011. Transcutaneous immunization and the travelers' diarrhea vaccine system: a phase III pivotal efficacy study. Abstr. 6th Int. Conf. Vaccines Enteric Dis. 2011, Cannes, France, 14 to 16 September 2011 [Google Scholar]
- 14.da Hora VP, Conceicao FR, Dellagostin OA, Doolan DL. 2011. Non-toxic derivatives of LT as potent adjuvants. Vaccine 29:1538–1544 [DOI] [PubMed] [Google Scholar]
- 15.Freytag LC, Clements JD. 2005. Mucosal adjuvants. Vaccine 23:1804–1813 [DOI] [PubMed] [Google Scholar]
- 16.Dickinson BL, Clements JD. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lycke N, Tsuji T, Holmgren J. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277–2281 [DOI] [PubMed] [Google Scholar]
- 18.Cheng E, Cardenas-Freytag L, Clements JD. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38–49 [DOI] [PubMed] [Google Scholar]
- 19.Oplinger M, Baqar S, Trofa A, Clements J, Gibbs P, Pazzaglia G, Bourgeois A, Scott DA. 1997. Safety and immunogenicity in volunteers of a new candidate oral mucosal adjuvant, LT(R192G), abstr G-10, p 193 Prog. Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 20.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tribble D, Baqar S, Thompson S. 2008. Development of a human vaccine, p 429–444 In Nachamkin ISCM, Blaser MJ. (ed), Campylobacter, 3rd ed. ASM Press, Washington, DC [Google Scholar]
- 22.Levine MM, Kaper JB, Black RE, Clements ML. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47:510–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 18:546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, Clements JD, Nabors GS. 2010. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole-cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine 28:1404–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S. 2004. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine 22:4385–4389 [DOI] [PubMed] [Google Scholar]
- 26.Baudner BC, Giudice GD. 2010. Determining the activity of mucosal adjuvants. Methods Mol. Biol. 626:261–285 [DOI] [PubMed] [Google Scholar]
- 27.Baqar S, Nour El Din AA, Scott DA, Bourgeois AL, Mourad AS, Kleinosky MT, Oplinger MJ, Murphy JR. 1997. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin. Diagn. Lab. Immunol. 4:375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464 [DOI] [PubMed] [Google Scholar]
- 29.Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthesy-Theulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath TP, Blum AL. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804–812 [DOI] [PubMed] [Google Scholar]
- 30.Banerjee S, Medina-Fatimi A, Nichols R, Tendler D, Michetti M, Simon J, Kelly CP, Monath TP, Michetti P. 2002. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut 51:634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leach S, Clements JD, Kaim J, Lundgren A. 2012. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One 7:e51718. 10.1371/journal.pone.0051718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjokvist Ottsjo L, Flach CF, Clements J, Holmgren J, Raghavan S. 2013. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect. Immun. 81:1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]