Abstract
The genomic DNA of ixodid ticks from western Canada was tested by PCR for the presence of Rickettsia. No rickettsiae were detected in Ixodes sculptus, whereas 18% of the I. angustus and 42% of the Dermacentor andersoni organisms examined were PCR positive for Rickettsia. The rickettsiae from each tick species were characterized genetically using multiple genes. Rickettsiae within the D. andersoni organisms had sequences at four genes that matched those of R. peacockii. In contrast, the Rickettsia present within the larvae, nymphs, and adults of I. angustus had novel DNA sequences at four of the genes characterized compared to the sequences available from GenBank for all recognized species of Rickettsia and all other putative species within the genus. Phylogenetic analyses of the sequence data revealed that the rickettsiae in I. angustus do not belong to the spotted fever, transitional, or typhus groups of rickettsiae but are most closely related to “Candidatus Rickettsia kingi” and belong to a clade that also includes R. canadensis, “Candidatus Rickettsia tarasevichiae,” and “Candidatus Rickettsia monteiroi.”
INTRODUCTION
The Rickettsia are obligate Gram-negative intracellular bacteria of arthropods (e.g., ticks, mites, fleas, and lice), many species of which are the causative agents of human disease, such as spotted fever and typhus (1–3). There are at least 30 recognized species within the genus (2); however, a number of other putative species have also been recently proposed based on molecular characterization of rickettsiae at three or four genes (4–7). The genus Rickettsia has been divided into two main groups, the spotted fever group (SFG) and the typhus group (TG), based on clinical (e.g., pathogenic effects), ecological (e.g., vector species and geographical distribution), and phenotypic characteristics (e.g., optimal growth temperatures, DNA G+C content, and relative cross-reactivity with the somatic antigens of different strains of Proteus) (1). This separation of Rickettsia into SFG and TG has been confirmed by phylogenetic analyses of sequence data of several rickettsial genes (1, 8, 9) and the complete genomes of nine species (10). The SFG has been further divided into four species groups: the R. akari, R. helvetica, R. massiliae, and R. rickettsii groups (1, 2). The R. akari species group, comprising R. akari, R. felis, and R. australis (1, 2), has been referred to as a transitional group (TRG) between the SFG and TG rickettsiae (10, 11); however, this classification was considered controversial (2). The results of a recent molecular study (11), based on the phylogenetic analysis of 113 core proteins from 22 taxa within the genus Rickettsia, revealed that R. helvetica does not belong to the SFG and may represent a sister taxon to the TRG rickettsiae. There are also at least two species of Rickettsia that do not belong to the SFG, TRG, or TG: R. canadensis and R. bellii (1, 2). R. canadensis, which has morphological similarities to both the SFG, TRG, and TG rickettsiae (3), represents a sister taxon to these three groups of rickettsiae, while R. bellii is a sister taxon to all rickettsiae that use parasitic arthropods as vectors (1).
Ticks are the most important vectors of SFG rickettsiae (1–3). In North America, there are 34 species of Ixodes (12), at least six of which, I. scapularis, I. pacificus, I. cookei, I. dentatus, I. brunneus, and I. texanus, have been shown to contain SFG rickettsiae (6, 12). Other ticks in North America, such as Dermacentor andersoni and D. variabilis, are also vectors and reservoirs of SFG rickettsiae (i.e., R. peacockii and R. montanensis, respectively) (13–15). Both D. andersoni and D. variabilis are also known vectors of R. rickettsii, the causative agent of Rocky Mountain spotted fever (16). All of these species of Ixodes and Dermacentor use rodents and/or insectivores as hosts for some part of their life cycle (12, 17).
Recently, a novel Rickettsia organism was detected in rotund ticks, Ixodes kingi, feeding on northern pocket gophers (Thomomys talpoides) in Saskatchewan, Canada (7). Phylogenetic analyses of sequence data for four genes revealed that this putative new species, provisionally named “Candidatus Rickettsia kingi,” did not belong to the SFG, TRG, or TG of rickettsiae but represented a sister taxon to R. canadensis and “Candidatus Rickettsia tarasevichiae” (7), the latter of which occurs in I. persulcatus from Russia and Japan (3). R. canadensis has been reported from several tick species (i.e., Haemaphysalis leporispalustris, D. andersoni, D. variabilis, and Ambylomma americanum) in Canada and the United States (3). The distributional range of I. kingi in western Canada overlaps that of D. andersoni and D. variabilis and several species of Ixodes, including the sculptured tick, I. sculptus, and the vole tick, I. angustus (17–20). Although I. angustus has been implicated as a vector of pathogenic bacteria, such as Borrelia burgdorferi (21), there are no reports of the presence of Rickettsia in this tick species or in I. sculptus. Therefore, the aim of the present study was to determine if I. angustus and/or I. sculptus in western Canada contain rickettsiae, and if so, whether the bacteria belong to the SFG, TRG, TG, or the clade containing R. canadensis, “Candidatus Rickettsia tarasevichiae,” and “Candidatus Rickettsia kingi.”
MATERIALS AND METHODS
DNA extraction, PCR, and single-strand conformation polymorphism (SSCP).
Total genomic DNA (gDNA) was extracted and purified from the complete bodies of 378 individual ticks using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany) and the modifications described previously (7, 22). All ticks were identified to the species level by morphological examination and genetic characterization (22, 23), the latter of which served as a control for the quality of the gDNA extracted from each tick. Tick specimens included I. angustus and D. andersoni collected in July of 2005, 2006, and 2007 from four species of rodents (Myodes gapperi, Microtus longicaudus, Phenacomys intermedius, and Callospermophilus lateralis) and one species of insectivore (Sorex cinereus) within the Kootenay National Park (NP), British Columbia (BC), Canada; I. sculptus, I. kingi, and D. andersoni collected in June and July of 2009 and 2010 from Richardson's ground squirrels (Spermophilus richardsonii) near Beechy, Saskatchewan (SK), Canada; and I. sculptus collected in July 2007 from a 13-lined ground squirrel (Ictidomys tridecemlineatus) near Clavet in SK (Table 1).
Table 1.
Numbers of Ixodes angustus, I. sculptus, I. kingi, and Dermacentor andersoni organisms positive for infection with Rickettsiaa
| Locality (coordinates) and tick species | Life cycle stage | No. of organisms tested | No. (%) PCR positive |
|---|---|---|---|
| Kootenay NP, BC (49°44′N, 112°50′W) | |||
| I. angustus | Larva | 176 | 45 (26) |
| Nymph | 68 | 3 (4) | |
| Adult | 24 | 1 (4) | |
| D. andersoni | Adult | 2 | 1 (50) |
| Beechy, SK (50°53′N, 107°23′W) | |||
| I. sculptus | Larva | 34 | 0 |
| Nymph | 21 | 0 | |
| Adult | 3 | 0 | |
| I. kingi | Larva | 1 | 0 |
| Nymph | 4 | 0 | |
| Adult | 1 | 0 | |
| D. andersoni | Nymph | 20 | 17 (85) |
| Adult | 20 | 17 (85) | |
| Clavet, SK (51°95′N, 106°45′W) | |||
| I. sculptus | Nymph | 4 | 0 |
Results were determined by using PCR analyses of the rickettsial 17-kDa antigen gene.
The presence/absence of Rickettsia DNA in each tick was tested by nested PCR (n-PCR) targeting part (434 bp) of the rickettsia-specific 17-kDa antigen gene using primers 17K-5 (5′-GCTTTACAAAATTCTAAAAACCATATA-3′) and 17K-3 (5′-TGTCTATCAATTCACAACTTGCC-3′) for the first phase and primers 17kD1 (5′-GCTCTTGCAACTTCTATGTT-3′) and 17kD2 (5′-CATTGTTCGTCAGGTTGGCG-3′) for the second phase (24), as well as the protocols and cycling conditions described previously (7). All PCR-positive samples were then subjected to SSCP analyses (25) to prescreen for genetic variation. This mutation-scanning technique can be used to differentially display genetic variation between DNA sequences that are 150 to 450 bp in size and that differ by one or more nucleotides (25). Representative amplicons (n = 5) of each different SSCP profile type were purified (26) prior to DNA sequencing using primers 17kD1 and 17kD2 in separate reactions. Amplicons from phase one of the n-PCR of three rickettsia-infected I. angustus ticks were also purified and subjected to automated DNA sequencing using primers 17K-5 and 17K-3.
To confirm the presence of rickettsial DNA in I. angustus, a second PCR assay, targeting 491 bp of the outer membrane protein A gene (ompA), was conducted on the gDNA samples that were PCR positive for the 17-kDa antigen gene. PCRs were carried out using primers Rr190.70p (5′-ATGGCGAATATTTCTCCAAAA-3′) and Rr190.602n (5′-AGTGCAGCATTCGCTCCCCCT-3′) (27) under the following conditions: 95°C for 5 min; 30 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 30 s; and a final cycle of 72°C for 8 min. SSCP was used to compare the rickettsial ompA amplicons derived from all PCR-positive I. angustus, seven “Candidatus Rickettsia kingi,” and two rickettsial-infected D. andersoni individuals. The ompA amplicons from two I. angustus individuals were then purified and subjected to DNA sequencing using primers Rr190.70p and Rr190.602n.
Four additional genetic markers were used to characterize the rickettsiae in I. angustus and D. andersoni. First, a 1,060-bp fragment of the citrate synthase gene (gltA) was amplified from the gDNA of two rickettsia-infected I. angustus larvae and one rickettsia-infected D. andersoni nymph using the primers CS2dF (5′-ATGACCAATGAAAATAATAAT-3′) and RpCS.1258n (5′-ATTGCAAAAAGTACAGTGAACA-3′) (8, 27) and the following conditions: 95°C for 5 min; 30 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min; and a final cycle of 72°C for 8 min. The amplicons were purified and sequenced using primers CS2dF and RpCS.1258n. Part (1,332 bp) of the prokaryotic 16S rRNA gene of six rickettsia-infected ticks (i.e., four I. angustus and two D. andersoni ticks) was then amplified using primers Rick-16S-F3 (5′-ATCAGTACGGAATAACTTTTA-3′) and Rick-16S-R4 (5′-TGCCTCTTGCGTTAGCTCAC-3′) under the following conditions: 95°C for 5 min; 30 cycles of 95°C for 45 s, 58°C for 45 s, and 72°C for 45 s; and then 72°C for 5 min. Primers Rick-16S-F3 and Rick-16S-R4 were designed specifically to amplify the 16S rRNA gene of Rickettsia, because the primers most often used for this purpose in other studies (i.e., primers fd1 and rp2 [28]) also coamplified the 16S rRNA gene of other bacteria present within the ticks. The purified 16S rRNA gene amplicons were subjected to DNA sequencing using primers Rick-16-F3 and Rick-16-R4. In addition, part (488 bp) of the surface cell antigen 1 (sca1) gene of three rickettsia-infected ticks was amplified using primers SCA1-F2 (5′-GGTGATGAAGAAGAGTCTC-3′) and SCA1-R2 (5′-CTCTTTAAAATTATGTTCTAC-3′) under the following conditions: 95°C for 5 min; 35 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 30 s; and then 72°C for 5 min. Purified amplicons from three I. angustus larvae were subjected to DNA sequencing using primers SCA1-F2 and SCA1-R2. Amplification of sca1 was not achieved for any gDNA samples from D. andersoni (n = 5). Amplification of 812 bp of the outer membrane protein B gene (ompB) using the primers 120.3599 (5′-TACTTCCGGTTACAGCAAAGT-3′) and 120.2788 (5′-AAACAATAATCAAGGTACTGT-3′) was also attempted. PCR conditions of Roux and Raoult (29) were used, except that the number of cycles was increased to 35 and the annealing temperature was raised from 50 to 52°C.
Negative controls (i.e., no gDNA) were included in each PCR assay conducted. In addition, the gDNA of “Candidatus Rickettsia kingi” from I. kingi (7), R. peacockii from D. andersoni, and R. montanensis from D. variabilis (15) were included in each PCR assay and SSCP analysis as positive controls. The amplicons of these positive controls were also sequenced for each gene region to confirm that the correct target genes had been successfully amplified.
Sequence analyses.
BLAST searches (GenBank) were performed on the DNA sequences of each gene to determine the genetic similarity of the rickettsiae in I. angustus and D. andersoni to the different taxa within the genus Rickettsia. For each gene region, DNA sequences were aligned manually with those of Rickettsia species available in GenBank (see Table S1 in the supplemental material). Phylogenetic analyses were performed separately on the sequence data of each gene, and on the concatenated sequence data of all five genes, using the neighbor joining (NJ), maximum likelihood (ML), and maximum parsimony (MP) methods in PAUP (30). Given that there are no ompA sequences for the TG rickettsiae (9), alignment gaps were used in the concatenated data set to represent the lack of ompA sequences in R. typhi and R. prowazekii. For the MP analyses, characters were treated as unordered and were equally weighted, and alignment gaps were treated as missing characters. Heuristic searches with TBR branch swapping were used to infer the shortest trees. The lengths, consistency indices (CI) excluding uninformative characters, and retention indices (RI) of the most parsimonious trees were recorded. The sequences of Orientia tsutsugamushi and Midichloria mitochondrii were used as outgroups in the MP analyses of the 16S rRNA gene and gltA (respectively), while the sequences of R. bellii were used as the outgroup in the MP analyses of the 17-kDa gene, sca1, and the concatenated sequence data. Midpoint rooting was used in the MP analysis of the ompA sequence data. Bootstrap analyses (1,000 replicates for the NJ analyses and 100 replicates for MP analyses) were conducted to determine the relative support for clades in the consensus trees.
Ethics statement.
This work was approved by the University of Saskatchewan's Animal Research Ethics Board and adhered to the Canadian Council on Animal Care guidelines for humane use.
Nucleotide sequence accession numbers.
The nucleotide sequences of the different genes for representative samples of the rickettsiae obtained in the present study have been deposited in GenBank under accession numbers HF935068 to HF935081.
RESULTS
Three hundred seventy-eight ticks were each tested for the presence of Rickettsia DNA by n-PCR of the 17-kDa antigen gene, of which 84 were positive (Table 1). Each of these amplicons had a single band of the expected size (∼450 bp) on 1.5% agarose–Tris-borate-EDTA gels. No bands were detected on agarose gels for the negative-control samples. Rickettsia DNA was not detected in any of the I. kingi ticks from Beechy (SK) or the I. sculptus ticks from Beechy and Clavet (SK). In contrast, 49 (18%) of the 268 I. angustus individuals from Kootenay NP (BC) and 35 (83%) of the 42 D. andersoni organisms collected from two localities (Kootenay NP and Beechy) were PCR positive for Rickettsia DNA (Table 1). A significantly (χ22 = 18.0; P < 0.001) greater proportion of I. angustus larvae were PCR positive for Rickettsia DNA than I. angustus nymphs or adults. Significantly more D. andersoni individuals were PCR positive for Rickettsia DNA than I. angustus individuals (χ21 = 77.8; P < 0.001), but there was no significant difference (χ21 = 0.8; P > 0.05) in the proportion of D. andersoni nymphs and adults containing rickettsiae (Table 1).
The SSCP banding patterns (i.e., profiles) of the rickettsial 17-kDa gene amplicons derived from D. andersoni individuals collected from Kootenay NP and Beechy were identical to one another and to that of the R. peacockii control samples (data not shown). A BLAST search of the 17-kDa gene sequences of the Rickettsia in D. andersoni revealed that they were genetically identical to the sequence of R. peacockii (GenBank accession no. CP001227). The DNA sequences of the rickettsiae detected in D. andersoni from the two locations were also identical in sequence to those of R. peacockii for gltA (accession no. DQ100162) and ompB (accession no. CP001227), and they were 99.9% similar (i.e., at 1,214 of 1,215 bp) to the 16S rRNA gene sequence of R. peacockii (accession no. DQ062433). However, no sca1 amplicons were obtained for the rickettsiae in D. andersoni or for the R. peacockii controls.
The SSCP banding patterns of the rickettsial 17-kDa gene amplicons from 49 I. angustus individuals were identical to one another but differed from those of the positive-control samples “Candidatus Rickettsia kingi,” R. peacockii, and R. montanensis (Fig. 1). The DNA sequences (394 bp) of three representative I. angustus amplicons revealed that they differed at one nucleotide position from the 17-kDa gene sequence of “Candidatus Rickettsia kingi” (accession no. HE647694). When sequences of a slightly larger fragment (497 bp) of the 17-kDa gene were obtained (i.e., using 17K5/17K3 amplicons), the rickettsiae in I. angustus all had identical sequences but differed from that of “Candidatus Rickettsia kingi” at 3 (0.6%) nucleotide positions (Table 2). A BLAST search revealed that the 17-kDa gene sequence of the rickettsiae in I. angustus differed at 9 to 60 (0.7 to 12.2%) nucleotide positions compared to the 17-kDa sequences of other taxa within the genus Rickettsia (Table 2).
Fig 1.
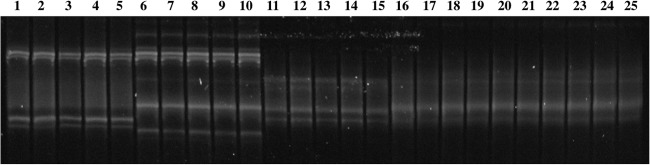
SSCP profiles of amplicons of the rickettsial 17-kDa gene for representative control samples (R. peacockii, lanes 1 to 5; R. montanensis, lanes 6 to 10; “Candidatus Rickettsia kingi,” lanes 11 to 15) and the rickettsiae in Ixodes angustus (lanes 16 to 25).
Table 2.
Sequence similarity of the partial 16S rRNA gene, 17-kDa gene, gltA, ompA, and sca1 of Rickettsia detected in I. angustus to those of other rickettsiae
| Gene and Rickettsia organism(s)a | % sequence similarity (bp) |
|---|---|
| 17-kDa gene | |
| “Candidatus Rickettsia kingi” | 99.4 (494 of 497) |
| R. canadensis | 94.0 (471 of 501) |
| “Candidatus Rickettsia monteiroi”b | 92.4 (404 of 437) |
| R. bellii | 81.6 (407 of 499) |
| TG rickettsiae | 81.9–83.3 (408–415 of 498) |
| TRG rickettsiae | 88.3–88.7 (439–441 of 498) |
| SFG rickettsiae | 87.8–89.0 (437–443 of 498) |
| ompA | |
| “Candidatus Rickettsia kingi” | 99.4 (488 of 491) |
| R. canadensis | 86.7 (430 of 495) |
| TRG rickettsiae | 81.1–83.6 (401–441 of 493) |
| SFG rickettsiae | 81.0–86.3 (402–428 of 496) |
| gltA | |
| “Candidatus Rickettsia kingi” | 99.9 (1,059 of 1,060) |
| “Candidatus Rickettsia tarasevichiae” | 98.7 (1,046 of 1,060) |
| R. canadensis | 97.5 (1,034 of 1,060) |
| “Candidatus Rickettsia monteiroi”b | 96.5 (1,008 of 1,045) |
| R. bellii | 85.4 (908 of 1,063) |
| TG rickettsiae | 88.8–89.3 (941–947 of 1,060) |
| TRG rickettsiae | 89.9–91.5 (952–970 of 1,060) |
| SFG rickettsiae | 89.3–92.7 (947–983 of 1,060) |
| 16S rRNA gene | |
| “Candidatus Rickettsia kingi” | 99.7 (1,328 of 1,332) |
| R. canadensis | 98.6 (1,313 of 1,332) |
| “Candidatus Rickettsia tarasevichiae”b | 99.3 (1,281 of 1,290) |
| “Candidatus Rickettsia monteiroi”b | 97.9 (1,304 of 1,317) |
| R. bellii | 99.3 (1,323 of 1,332) |
| TG rickettsiae | 97.9–98.0 (1,304–1,306 of 1,332) |
| TRG rickettsiae | 98.1–99.2 (1,307–1,321 of 1,332) |
| SFG rickettsiae | 98.6–99.1 (1,313–1,320 of 1,332) |
| sca1 | |
| “Candidatus Rickettsia kingi” | 100 (488 of 488) |
| R. canadensis | 96.3 (470 of 488) |
| “Candidatus Rickettsia monteiroi”b | 95.5 (426 of 446) |
| R. bellii | 82.6 (403 of 488) |
| TG rickettsiae | 87.9–88.1 (429–430 of 488) |
| TRG rickettsiae | 92.0–94.3 (449–460 of 488) |
| SFG rickettsiae | 92.4–94.7 (451–462 of 488) |
Accession numbers of taxa are available in Table S1 in the supplemental material.
Only a partial sequence is available for this taxon.
The results of a second PCR assay, targeting ompA, confirmed the presence of rickettsial DNA in the 49 I. angustus individuals that were PCR positive for the 17-kDa antigen gene. Each ompA amplicon consisted of a single band of the expected size (∼533 bp) on 1.5% agarose-TBE gels, while no bands were detected for the negative-control samples. The SSCP profiles of all ompA amplicons from I. angustus were identical to one another but differed from those of seven “Candidatus Rickettsia kingi” amplicons and two R. peacockii amplicons. The rickettsiae in I. angustus had a unique sequence for ompA (491-bp) compared to the sequences of “Candidatus Rickettsia kingi” and other taxa within the genus Rickettsia (Table 2).
Amplicons were obtained for the rickettsiae detected in I. angustus for three of the four additional target regions: gltA, 16S rRNA gene, and sca1. No amplicons were obtained for ompB. The gltA sequences (1,060 bp) of two representative samples of the rickettsiae from I. angustus were identical to one another but differed in sequence from the gltA sequences of species within the genus Rickettsia (Table 2). The closest match in sequence was to the gltA sequence of “Candidatus Rickettsia kingi.” The rickettsiae in I. angustus also had a unique sequence for the 16S rRNA gene (1,332 bp) compared to the sequences of this gene for other species within the genus Rickettsia (Table 2). The DNA sequences of sca1 amplicons derived from the gDNA of three rickettsia-infected I. angustus strains were identical to those of “Candidatus Rickettsia kingi” but differed in sequence from those of other taxa in the genus Rickettsia (Table 2).
The phylogenetic trees produced from the NJ analyses of the sequence data for each of the four genes (i.e., 17-kDa gene, gltA, ompA, and sca1) revealed strong to total statistical support (bootstrap values of 98 to 100%) for the inclusion of the Rickettsia in I. angustus within a clade that contained “Candidatus Rickettsia kingi,” “Candidatus Rickettsia monteiroi,” “Candidatus Rickettsia tarasevichiae,” and R. canadensis, to the exclusion of members of the SFG and TG rickettsiae (see Fig. S1 to S4 in the supplemental material). There was also support (i.e., bootstrap values of 78 to 100%) for the placement of the rickettsiae in I. angustus within the R. canadensis clade in the MP analyses of the sequence data for gltA, ompA, and the 17-kDa gene. However, there was limited resolution of the relationships of taxa in the NJ and MP analyses of the 16S rRNA gene sequence data, except that the rickettsiae in I. angustus represented the sister taxon to “Candidatus Rickettsia kingi” (see Fig. S5 in the supplemental material). The NJ analysis of the concatenated sequence data of the five genes produced a tree with the Rickettsia in I. angustus representing the sister taxon to “Candidatus Rickettsia kingi” (Fig. 2). There was also total statistical support (100% bootstrap value) for the placement of these two taxa in a clade that contained R. canadensis, “Candidatus Rickettsia monteiroi,” and “Candidatus Rickettsia tarasevichiae.” The MP analysis of the same data set (i.e., 538 cladistically informative characters) produced two equally most parsimonious trees (the strict consensus tree is not shown), with a length of 1,602, a CI of 0.61, and an RI of 0.75. As with the NJ tree, there was 100% bootstrap support for the inclusion of the rickettsiae in I. angustus within a clade that included R. canadensis, “Candidatus Rickettsia kingi,” “Candidatus Rickettsia monteiroi,” and “Candidatus Rickettsia tarasevichiae” (Fig. 2). In both the NJ and MP analyses, there was strong to total support (bootstrap values of 99 to 100%) for the SFG and TG rickettsiae each representing a monophyletic clade. The topology of the phylogenetic tree produced from the ML analyses of the concatenated sequence data (Fig. 3) was very similar to that of the NJ tree (Fig. 2), with total (100%) bootstrap support for the inclusion of the rickettsiae in I. angustus within a clade that included R. canadensis, “Candidatus Rickettsia kingi,” “Candidatus Rickettsia monteiroi,” and “Candidatus Rickettsia tarasevichiae.” One significant difference in the topology of the ML and NJ trees was the placement of R. helvetica. In the NJ tree (Fig. 2), R. helvetica represented a sister taxon to all members of the SFG, whereas in the ML tree (Fig. 3), there was some statistical support (bootstrap value of 83%) for this species representing a sister taxon to both the SFG and the TRG rickettsiae.
Fig 2.
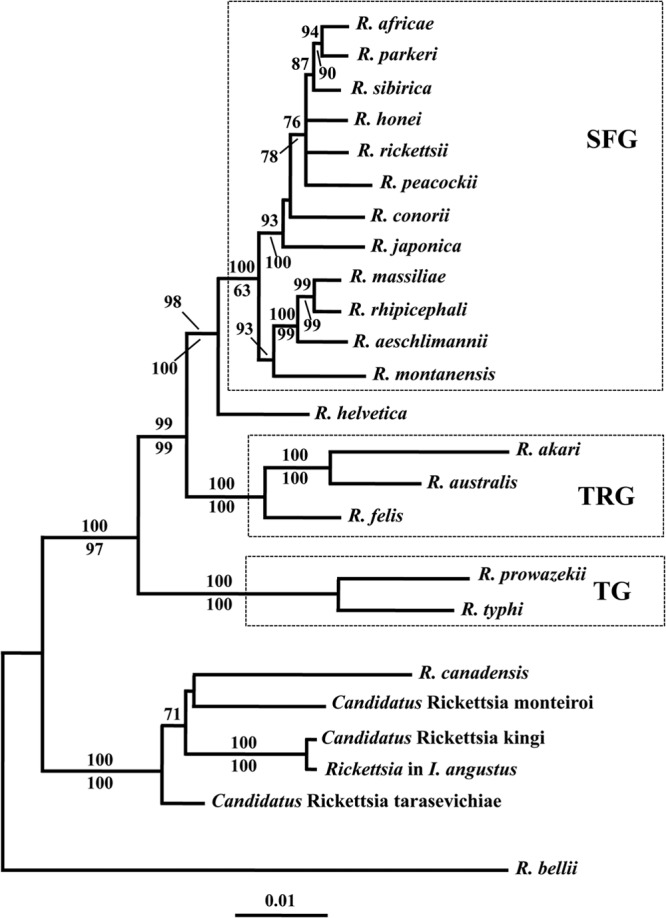
Phylogenetic tree depicting the relationships of the rickettsiae in Ixodes angustus with other species of Rickettsia based on neighbor-joining analyses of concatenated sequence data of five genes (17-kDa gene, ompA, gltA, 16S rRNA gene, and sca1). SFG, TG, and TRG refer to the spotted fever group, typhus group, and transitional group of Rickettsia, respectively. We have not included R. helvetica within the SFG based on the findings of a recent study (11) that considered the position of this species within the genus Rickettsia as incertae sedis. The scale bar represents the inferred substitutions per nucleotide site. The relative support for clades in the tree produced from the NJ and MP analyses are indicated above and below branches, respectively.
Fig 3.
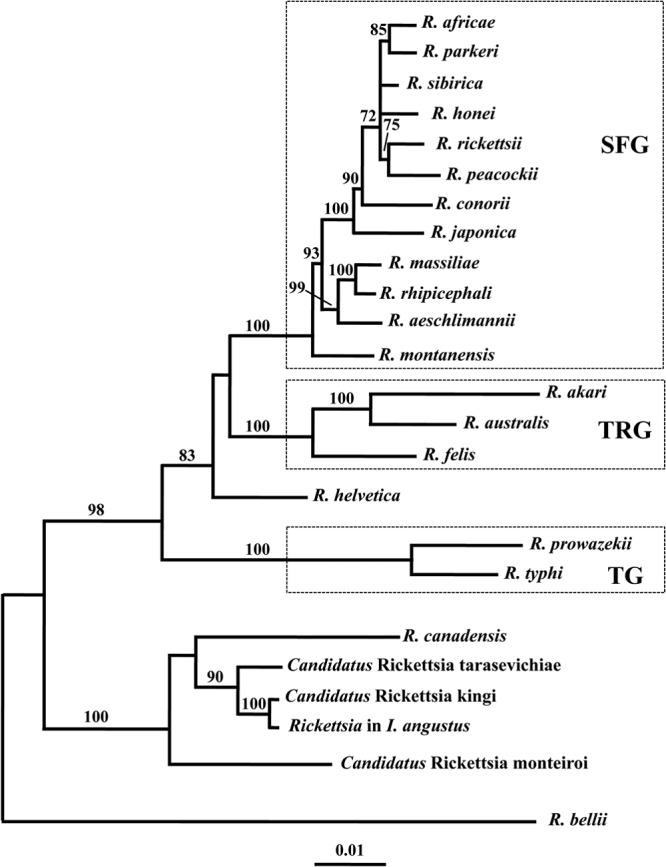
Phylogenetic tree depicting the relationships of the rickettsiae in Ixodes angustus with other species of Rickettsia based on maximum likelihood analyses of concatenated sequence data. SFG, TG, and TRG refer to the spotted fever group, typhus group, and transitional group of Rickettsia, respectively. The scale bar represents the inferred substitutions per nucleotide site. The relative support for clades in the tree are indicated above branches.
DISCUSSION
Many tick species are vectors of Rickettsia (1–3); however, in the present study, rickettsial DNA was not detected in any of the 62 I. sculptus individuals collected from 17 Richardson's ground squirrels (Spermophilus richardsonii) at Beechy (SK) and a 13-lined ground squirrel (Ictidomys tridecemlineatus) at Clavet (SK). This was markedly different from the presence of rickettsiae in 85% of the D. andersoni individuals feeding on two Richardson's ground squirrels at Beechy, both of which were also parasitized by I. sculptus and I. kingi. It is possible that I. sculptus does not represent a suitable host for Rickettsia; however, individuals from different localities throughout the large geographical range of this tick species in North America (19, 31, 32) need to be tested, because the prevalence of rickettsiae can vary significantly among tick populations. For example, the prevalence of R. montanensis in D. variabilis adults from different populations in Saskatchewan ranges from 0 to 33% (15). Geographical heterogeneity in rickettsial prevalence among tick populations also may explain the absence of rickettsiae from the I. kingi organisms feeding on Richardson's ground squirrels at Beechy compared to a 69% prevalence of “Candidatus Rickettsia kingi” in I. kingi (n = 87) individuals feeding on northern pocket gophers (Thomomys talpoides) at Clavet, which is situated 135 km northeast of Beechy (7). However, the sample size of I. kingi individuals at Beechy was very small, consisting of six individuals, each of which was feeding on a different host individual.
The rickettsiae detected in the D. andersoni individuals parasitizing Richardson's ground squirrels from Beechy and in the one D. andersoni adult on a golden-mantled ground squirrel (Callospermophilus lateralis) in the Kootenay NP (BC) were identified as R. peacockii based on genetic characterization at four loci: the 17-kDa gene, gltA, ompB, and the 16S rRNA gene. The SSCP banding patterns of all 35 R. peacockii amplicons of the 17-kDa gene were identical to one another. There were no sequence differences among amplicons of R. peacockii for the 17-kDa gene. PCR-SSCP has been shown previously to be a powerful and effective method to prescreen for genetic variation among rickettsial DNAs derived from the total gDNA of individual ticks, particularly when combined with DNA sequencing of multiple samples of each SSCP profile type (7, 15). For example, a previous study (15) demonstrated a lack of variation in the SSCP profiles of gltA amplicons derived from 386 R. peacockii-infected D. andersoni adults collected from localities up to 450 km apart (i.e., from Lethbridge, Alberta, Canada, to Outlook, SK, Canada) and among gltA amplicons derived from 66 R. montanensis-infected D. variabilis adults collected from localities up to 780 km apart (i.e., from Buffalo Pound Provincial Park, SK, Canada, to Kenora, Ontario, Canada) (15). In contrast, the gltA amplicons for R. peacockii had an SSCP profile different from that of R. montanensis that corresponded to three nucleotide differences in their gltA sequences (15). The detection of R. peacockii in D. andersoni from Beechy and Kootenay NP (i.e., localities ∼425 km apart) in the present study is consistent with previous findings of this species of Rickettsia in D. andersoni from other localities in western North America (13, 15). The 85% prevalence of R. peacockii in D. andersoni nymphs and adults feeding on Richardson's ground squirrels at Beechy was also consistent with the high prevalence (i.e., 96%) of this rickettsial species in questing D. andersoni adults at Saskatchewan Landing Provincial Park (SK) (15), situated ∼50 km to the southwest. Attempts were made to characterize the R. peacockii in D. andersoni and the R. peacockii control samples at the sca1 locus, because this gene has been recommended as a target for species delineation and inferring phylogenetic relationships of taxa within the genus Rickettsia (9). Sequences of the variable region (488 bp) of sca1 have been determined for all recognized members of the TG, TRG, and SFG rickettsiae (except for R. peacockii), R. canadensis, and R. bellii (9). In the present study, no sca1 amplicons were obtained for R. peacockii, whereas amplicons (488 bp) were obtained and sequenced for several representatives of “Candidatus Rickettsia kingi” and the rickettsiae within I. angustus. This suggests that sca1 is absent from R. peacockii; therefore, it may reduce the effectiveness of this gene for species delineation for all taxa within the genus Rickettsia. BLAST searches comparing the sequences for conserved regions of the sca1 gene in several species of Rickettsia to the sequence of the complete genome for R. peacockii available from GenBank (accession no. CP001227) also indicate that sca1 is absent from R. peacockii.
Although no rickettsiae were detected in I. sculptus from Saskatchewan, 18% of the 268 I. angustus ticks feeding on red-backed voles and on a small number of shrews, golden-mantled ground squirrels, and deer mice within the Kootenay NP (BC) were found to be PCR positive for Rickettsia. All life cycle stages of I. angustus were found to contain the rickettsiae; however, a significantly greater proportion of larvae were PCR positive for Rickettsia than nymphs or adult ticks. The results of the SSCP analyses of the amplicons of the 17-kDa gene of the rickettsiae present within all 49 PCR-positive I. angustus samples revealed that they had identical banding patterns (i.e., profiles) but differed markedly from the SSCP profiles of the control samples: R. peacockii, R. montanensis, and “Candidatus Rickettsia kingi” (i.e., rickettsiae present in the total gDNA of D. andersoni, D. variabilis, and I. kingi, respectively). Subsequent DNA sequencing of the 17-kDa gene amplicons (394-bp) for representative samples of the rickettsiae from I. angustus revealed that they had identical sequences but differed in sequence from “Candidatus Rickettsia kingi” at one nucleotide position and from R. peacockii and R. montanensis by 51 and 52 nucleotide positions, respectively. Two additional nucleotide differences were detected between the rickettsiae from I. angustus and “Candidatus Rickettsia kingi” within a larger fragment (497 bp) of the 17-kDa gene. Furthermore, the ompA amplicons of all 49 PCR-positive I. angustus individuals had identical SSCP profiles but differed from the SSCP profiles of ompA for all control samples, including the amplicons of seven “Candidatus Rickettsia kingi” individuals. The ompA sequences of the rickettsiae from I. angustus (491 bp) were found to be different from the ompA sequences of “Candidatus Rickettsia kingi” at three nucleotide positions. A BLAST search of the sequence data further revealed that the rickettsiae in I. angustus had novel sequences for ompA and the 17-kDa gene compared to the sequences of these genes for all recognized and putative species of Rickettsia. Given this, the rickettsiae in I. angustus were genetically characterized at three additional gene loci (i.e., gltA, sca1, and the 16S rRNA gene). These rickettsiae had sca1 sequences identical to that of “Candidatus Rickettsia kingi” but differed in sequence from all other taxa within the genus Rickettsia. Attempts were also made to characterize the rickettsiae in I. angustus for an ∼800-bp fragment of ompB, a gene present in SFG, TRG, and TG rickettsiae (1, 29). However, no amplicons could be obtained for the rickettsiae in I. angustus, whereas we were able to amplify this gene for R. peacockii present within the total gDNA of D. andersoni. The inability to amplify ompB for the rickettsiae in I. angustus may be associated with the specific PCR assay used (e.g., the primers, annealing temperature, etc.); however, there is no ompB gene in R. canadensis (1, 29), and it could not be amplified for “Candidatus Rickettsia kingi” (7).
Phylogenetic analyses conducted on the concatenated sequence data of five genes (i.e., the 17-kDa gene, gltA, ompA, 16S rRNA gene, and sca1) revealed that the species of Rickettsia detected in I. angustus was not a member of the SFG, TRG, or TG rickettsiae but was most closely related to “Candidatus Rickettsia kingi.” It belonged to a clade that contained R. canadensis and three other putative species of Rickettsia: “Candidatus Rickettsia kingi,” “Candidatus Rickettsia tarasevichiae,” and “Candidatus Rickettsia monteiroi.” Although the rickettsiae in I. angustus represent a sister taxon of R. canadensis, a potential human pathogen (2, 3), and occurs in a tick species (i.e., I. angustus) that has been reported to occasionally bite humans (21, 31, 33), it remains to be determined if this bacterium is of any significance with respect to human health.
The magnitude of the sequence differences between the rickettsiae in I. angustus and “Candidatus Rickettsia kingi” for the 17-kDa gene (0.6%), ompA (1.6%), gltA (0.1%), and the 16S rRNA gene (0.3%) were similar to the minimum levels of sequence differences (i.e., 0.7, 1.2, 0.1, and 0.2%, respectively) that distinguish closely related species of Rickettsia within the SFG (1). In addition, no sequence variation was detected in the 17-kDa gene among “Candidatus Rickettsia kingi” amplicons derived from 60 I. kingi individuals (7) or among the rickettsial amplicons derived from 49 I. angustus individuals, despite the three nucleotide differences between the sequences of the two taxa. Given this and that the rickettsiae in I. angustus had novel sequences at four genes compared to those of all recognized and putative species of Rickettsia (including “Candidatus Rickettsia kingi”), this taxon is provisionally named “Candidatus Rickettsia angustus” in accordance with the recommended nomenclature for new rickettsiae that have not been established in pure culture (1). Although “Candidatus Rickettsia angustus” and “Candidatus Rickettsia kingi” have been detected in different tick species with broad overlapping geographical ranges in western Canada (18–20), I. kingi is primarily a tick of the prairies, whereas I. angustus has been recorded on hosts in more forested habitats (19). Thus, there may be limited opportunities for cross-transmission of “Candidatus Rickettsia angustus” and “Candidatus Rickettsia kingi” to I. kingi and I. angustus, respectively. Furthermore, both tick species also parasitize different species of mammalian hosts in western Canada; I. kingi feeds primarily on ground squirrels, pocket gophers, prairie dogs, kangaroo mice, weasels, badgers, and dogs (7, 18, 19), whereas I. angustus is common on voles, shrews, mice, red squirrels, pikas, moles, wood rats, and rabbits (19, 20, 34, 35). Therefore, further work is needed to determine if “Candidatus Rickettsia angustus” represents a species different from “Candidatus Rickettsia kingi.”
Supplementary Material
ACKNOWLEDGMENTS
We thank Yeen Ten Hwang (Saskatchewan Ministry of Environment) for providing the ticks from Kootenay National Park, British Columbia, Canada.
Financial support for this work was provided (to N.B.C.) from the Natural Sciences and Engineering Research Council of Canada and the Canadian Foundation for Innovation. A Margaret McKay scholarship and a University of Saskatchewan Graduate Scholarship provided financial support to C.A.A.
This work was performed in the Faculty of Biology of the University of Saskatchewan, Saskatchewan, Canada.
Footnotes
Published ahead of print 27 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02286-13.
REFERENCES
- 1.Fournier P-E, Raoult D. 2009. Current knowledge on phylogeny and taxonomy of Rickettsia spp. Ann. N. Y. Acad. Sci. 1166:1–11 [DOI] [PubMed] [Google Scholar]
- 2.Merhej V, Raoult D. 2011. Rickettsial evolution in the light of comparative genomics. Biol. Rev. Camb. Philos. Soc. 86:379–405 [DOI] [PubMed] [Google Scholar]
- 3.Mediannikov O, Paddock CD, Parola P. 2007. Chapter 12. Other rickettsiae of possible or undetermined pathogenicity, p 163–178 In Raoult D, Parola P. (ed), Rickettsial diseases, 1st ed. Informa Healthcare, London, United Kingdom [Google Scholar]
- 4.Paddock CD, Fournier P-E, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Loftis AD, Varela-Stokes A. 2010. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 76:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacheco RC, Moraes-Filho J, Marcili A, Richtzenhain LJ, Szabó MPJ, Catroxo MHB, Bouyer DH, Labruna MB. 2011. Rickettsia monteiroi sp. nov., infecting the tick Amblyomma incisum in Brazil. Appl. Environ. Microbiol. 77:5207–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan JN, Lu CR, Bender WG, Smoak RM, III, Zhong J. 2011. Molecular detection and identification of Rickettsia species in Ixodes pacificus in California. Vector Borne Zoonotic Dis. 11:957–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstead CA, Chilton NB. 2013. Detection of a novel Rickettsia (Alphaproteobacteria: Rickettsiales) in rotund ticks (Ixodes kingi) from Saskatchewan, Canada. Ticks Tick-Borne Dis. 4:202–206 [DOI] [PubMed] [Google Scholar]
- 8.Roux V, Rydkina E, Eremeeva M, Raoult D. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 47:252–261 [DOI] [PubMed] [Google Scholar]
- 9.Ngwamidiba M, Blanc G, Raoult D, Fournier P-E. 2006. Sca1, a previously undescribed paralog from autotransporter protein-encoding genes in Rickettsia species. BMC Microbiol. 6:12. 10.1186/1471-2180-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillespie JJ, Beler MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, Sobral BS, Azad AF. 2007. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS One 3:E266. 10.1371/journal.pone.0000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driscoll T, Gillespie JJ, Nordberg EK, Azad AF, Sobral BW. 2013. Bacterial DNA sifted from the Trichoplax adhaerens (Animalia: Placozoa) genome project reveals a putative rickettsial endosymbiont. Genome Biol. Evol. 5:621–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan SA. 2001. Ticks (class Arachnida: order Acarina), p 72–106 In Samuel WM, Pybus MJ, Kocan AA. (ed), Parasitic diseases of wild mammals, 2nd ed. Iowa State University Press, Ames, IA [Google Scholar]
- 13.Niebylski ML, Schrumpf ME, Burgdorfer W, Fischer ER, Gage KL, Schwan TG. 1997. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int. J. Syst. Bacteriol. 47:446–452 [DOI] [PubMed] [Google Scholar]
- 14.Ammerman NC, Swanson KI, Anderson JM, Schwartz TR, Seaberg EC, Glass GE, Norris DE. 2004. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg. Infect. Dis. 10:1478–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dergousoff SJ, Gajadhar AJA, Chilton NB. 2009. Prevalence of Rickettsia species in Canadian populations of Dermacentor andersoni and D. variabilis. Appl. Environ. Microbiol. 75:1786–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgdorfer W. 1975. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol. 12:269–278 [DOI] [PubMed] [Google Scholar]
- 17.Dergousoff SJ, Galloway TD, Lindsay LR, Curry PS, Chilton NB. 2013. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 50:510–520 [DOI] [PubMed] [Google Scholar]
- 18.Gregson JD. 1971. Studies on two populations of Ixodes kingi Bishopp (Ixodidae). Can. J. Zool. 49:591–597 [DOI] [PubMed] [Google Scholar]
- 19.Gregson JD. 1956. The Ixodoidea of Canada. Science Service, Entomology Division, Canada Department of Agriculture, Ottawa, Canada [Google Scholar]
- 20.Robbins RG, Keirans JE. 1992. Systematics and ecology of the subgenus Ixodiopsis (Acari: Ixodidae: Ixodes). Thomas Say Foundation monographs, vol 14, p 14–26 Entomological Society of America, Lanham, MD [Google Scholar]
- 21.Damrow T, Freedman H, Lane RS, Preston KL. 1989. Is Ixodes (Ixodiopsis) angustus a vector of Lyme disease in Washington State? West. J. Med. 150:580–582 [PMC free article] [PubMed] [Google Scholar]
- 22.Dergousoff SJ, Chilton NB. 2007. Differentiation of three species of ixodid tick, Dermacentor andersoni, D. variabilis and D. albipictus, by PCR-based approaches using markers in ribosomal DNA. Mol. Cell. Probes 21:343–348 [DOI] [PubMed] [Google Scholar]
- 23.Anstead CA, Chilton NB. 2011. Ticks feeding on northern pocket gophers (Thomomys talpoides) in central Saskatchewan and the unexpected detection of Ixodes scapularis larvae. J. Vector Ecol. 36:355–360 [DOI] [PubMed] [Google Scholar]
- 24.Heise SR, Elshahed MS, Little SE. 2010. Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. J. Med. Entomol. 47:258–268 [DOI] [PubMed] [Google Scholar]
- 25.Gasser RB, Hu M, Chilton NB, Campbell BE, Jex AJ, Otranto D, Cafarchia C, Beveridge I, Zhu X. 2006. Single-strand conformation polymorphism (SSCP) for the analysis of genetic variation. Nat. Protoc. 1:3121–3128 [DOI] [PubMed] [Google Scholar]
- 26.Dergousoff SJ, Chilton NB. 2012. Association of different genetic types of Francisella-like organisms with the Rocky Mountain wood tick (Dermacentor andersoni) and the American dog tick (Dermacentor variabilis) in localities near their northern distributional limits. Appl. Environ. Microbiol. 78:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regnery RL, Spruill CL, Plikaytis BD. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux V, Raoult D. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 50:1449–1455 [DOI] [PubMed] [Google Scholar]
- 30.Swofford DL. 2003. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4 Sinauer Associated, Sunderland MA [Google Scholar]
- 31.Durden LA, Keirans JE. 1996. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification key, distribution, hosts, and medical/veterinary importance. Thomas Say Foundation. Ent. Soc. Am [Google Scholar]
- 32.Salkeld DJ, Eisen RJ, Antolin MF, Stapp P, Eisen L. 2006. Host usage and seasonal activity patterns of Ixodes kingi and I. sculptus (Acari: Ixodidae) nymphs in a Colorado prairie landscape, with a summary of published North American host records for all life stages. J. Vector Ecol. 31:168–180 [DOI] [PubMed] [Google Scholar]
- 33.Estrada-Peña A, Jongejan F. 1999. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 23:685–715 [DOI] [PubMed] [Google Scholar]
- 34.Sorensen TC, Moses RA. 1998. Host preferences and temporal trends of the tick Ixodes angustus in north-central Alberta. J. Parasitol. 84:902–906 [PubMed] [Google Scholar]
- 35.Anstead CA, Hwang YT, Chilton NB. Ticks (Acari: Ixodidae) on small mammals in Kootenay National Park, British Columbia, Canada. J. Med. Entomol., in press [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


