Abstract
The bacterium Pseudonocardia dioxanivorans CB1190 grows on the cyclic ethers 1,4-dioxane (dioxane) and tetrahydrofuran (THF) as sole carbon and energy sources. Prior transcriptional studies indicated that an annotated THF monooxygenase (THF MO) gene cluster, thmADBC, located on a plasmid in CB1190 is upregulated during growth on dioxane. In this work, transcriptional analysis demonstrates that upregulation of thmADBC occurs during growth on the dioxane metabolite β-hydroxyethoxyacetic acid (HEAA) and on THF. Comparison of the transcriptomes of CB1190 grown on THF and succinate (an intermediate of THF degradation) permitted the identification of other genes involved in THF metabolism. Dioxane and THF oxidation activity of the THF MO was verified in Rhodococcus jostii RHA1 cells heterologously expressing the CB1190 thmADBC gene cluster. Interestingly, these thmADBC expression clones accumulated HEAA as a dead-end product of dioxane transformation, indicating that despite its genes being transcriptionally upregulated during growth on HEAA, the THF MO enzyme is not responsible for degradation of HEAA in CB1190. Similar activities were also observed in RHA1 cells heterologously expressing the thmADBC gene cluster from Pseudonocardia tetrahydrofuranoxydans K1.
INTRODUCTION
Increasing evidence and awareness of the prevalence of 1,4-dioxane (dioxane) and tetrahydrofuran (THF) contamination in groundwater, due mainly to their use as chlorinated solvent stabilizers, has prompted research into the development of remediation strategies to remove them from the environment. One strategy being investigated for treatment of these four-carbon cyclic ethers is biological degradation.
A number of pure and mixed cultures of bacteria and fungi have been reported to degrade dioxane aerobically (1–12), while only one study has reported anaerobic degradation (13). To date, nine microorganisms have been reported to be capable of growth on dioxane as a sole carbon and energy source (i.e., metabolism of dioxane), including Rhodococcus ruber 219 (1), Mycobacterium sp. strain PH-06 (12), Pseudonocardia dioxanivorans CB1190 (3, 14), Pseudonocardia benzenivorans B5 (11), the fungus Cordyceps sinensis (9), and four recently isolated strains identified as two Mycobacterium spp., a Pseudonocardia sp., and a Gram-negative Afipia sp. (15).
Proposed dioxane biodegradation pathways have largely been based on detected intermediates and products. The production of CO2 from the mineralization of dioxane was initially reported for CB1190 (3), and the intermediates ethylene glycol, glycolic acid, and oxalic acid were subsequently detected for the fungal isolate Cordyceps sinensis (9). Accumulation of β-hydroxyethoxyacetic acid (HEAA) was observed during cometabolic degradation of dioxane by Pseudonocardia sp. strain ENV478 initially grown on THF (10). HEAA and 12 additional intermediates of dioxane degradation were subsequently detected for strain CB1190, including 2-hydroxy-1,4-dioxane, 2-hydroxyethoxyacetaldehyde, 1,4-dioxane-2-one, 1,3-dihydroxyethoxyacetic acid, 2-hydroxyethoxy-2-hydroxyacetic acid, glycoaldehyde, glyoxal, and formic acid (16). Even though a dioxane degradation pathway was proposed based on these detected intermediates (16), genes likely associated with several enzymatic steps in the pathway were only recently identified (17). Transcriptional analyses showed that the putative THF monooxygenase (MO) gene cluster thmADBC in strain CB1190 was the only MO out of eight previously identified by genome sequencing (18) that was upregulated during growth on dioxane versus glycolate. However, unclear from this analysis was whether thmADBC is involved only in the oxidation of dioxane or if it is also responsible for the oxidation of HEAA, as proposed by Mahendra et al. (16).
Metabolism of THF has also been reported in nine bacterial strains, including Rhodococcus ruber strains 219 (1), M2 (19), and ENV425 (10), a variety of Pseudonocardia strains, including CB1190 (3), B5 (11), and ENV478 (10), Pseudonocardia sulfidoxydans (20), and Pseudonocardia tetrahydrofuranoxydans K1 (21, 22). In addition, three fungal isolates are capable of growth on THF, including Cordyceps sinensis (9), Aureobasidium pullulans (5), and Graphium sp. strain ATCC 58400 (7). Biochemical evidence from a number of studies indicates that the initial step in the aerobic biodegradation of THF, as with dioxane, is catalyzed by an MO reaction. Common among proposed metabolic pathways for THF degradation for R. ruber 219 (1), P. tetrahydrofuranoxydans K1 (22), and Graphium sp. (7) is the initial oxidation of THF to 2-hydroxytetrahydrofuran and the formation of succinate as a downstream intermediate. The four-subunit THF MO gene cluster (thmADBC) from strain K1 was cloned and sequenced and transcriptionally implicated in the oxidation of THF to 2-hydroxytetrahydrofuran (22). In addition to CB1190 and K1, homologs of the thm cluster have been found in Pseudonocardia strain ENV478 (10, 23) and Rhodococcus sp. strain YYL (24). In the fungus Graphium sp. ATCC 58400, a cytochrome P450 MO enzyme was suggested to catalyze the initial oxidation of THF (7).
In this study, the function of the THF MO in the degradation of dioxane and THF was examined. Transcriptomic microarray data from CB1190 grown on dioxane and THF and their respective intermediates were analyzed to identify differentially expressed genes involved in the metabolism of these cyclic ethers. Additionally, functionally active heterologous clones expressing the genes thmADBC from strains CB1190 and K1 were constructed and used to demonstrate the functional role of THF MO in the oxidation of dioxane and THF. The identification and functional characterization of THF MO will enable more effective detection and quantification of this important enzyme in the environment, leading to improved understanding of the fate and potential for bioremediation of cyclic ethers in the field.
MATERIALS AND METHODS
Laboratory strains and culture conditions.
The bacterial strains used in this study were P. dioxanivorans CB1190 (3, 14), P. tetrahydrofuranoxydans K1 (20), Rhodococcus jostii RHA1 (25), and electrocompetent Escherichia coli DH5α (New England BioLabs, Ipswich, MA). CB1190 cells were grown in ammonium mineral salts (AMS) liquid medium (3). The growth substrates dioxane, THF, succinate, glycolate, isopropanol, pyruvate, and glucose were added to the culture medium to achieve a final concentration of 5 mM, while HEAA was added to cultures at a final concentration of 1.5 mM. The potassium salt of HEAA was prepared by CanSyn Chemical Corporation (Toronto, ON, Canada). Cultures were incubated aerobically, with shaking at 150 rpm at 30°C.
Cell harvesting and RNA extraction for transcriptional studies.
CB1190 cells were harvested for transcriptomic microarray analysis, reverse transcriptase PCR (RT-PCR), and RT-quantitative PCR (RT-qPCR) using the methods described in Grostern et al. (17). Briefly, cells from replicate bottles were collected by filtration onto 0.22-μm-pore-size polyvinylidene difluoride (PVDF) Durapore membrane filters (Millipore, Billerica, MA). The cells from each filter were scraped with a sterile scalpel and transferred to 2-ml screw-top microcentrifuge tubes containing 1 g of 100-μm-diameter zirconia-silica beads (Biospec Products, Bartlesville, OK). Harvested cells were stored at −80°C until total nucleic acid extraction.
Nucleic acids were extracted using a modified version of the phenol method described previously (26). Briefly, each 2-ml microcentrifuge tube containing cells and zirconia-silica beads was filled with 250 μl of lysis buffer (50 mM sodium acetate, 10 mM EDTA [pH 5.1]), 100 μl of 10% sodium dodecyl sulfate, and 1.0 ml phenol, pH 8 (Sigma-Aldrich, St. Louis, MO). Cells were lysed by heating samples to 65°C for 2 min, bead beating with a Mini Bead Beater (Biospec Products) for 2 min, incubation at 65°C for 8 min, and bead beating for an additional 2 min. Cellular debris was collected by centrifugation (5 min at 14,000 × g), and the aqueous lysate was transferred to new microcentrifuge tubes. The lysate was extracted twice with 1 volume of phenol-chloroform-isoamyl alcohol (pH 8) (24:24:1, vol/vol/vol) and once with 1 volume of chloroform-isoamyl alcohol (24:1, vol/vol). Nucleic acids were precipitated with 0.1 volume of 3 M sodium acetate and 1 volume of ice-cold isopropanol, followed by incubation at −20°C overnight. The precipitate was collected by centrifugation (30 min at 21,000 × g at 4°C), washed once with 70% ethanol, and resuspended in 100 μl of nuclease-free water.
To obtain RNA, resuspended nucleic acids were initially separated with an AllPrep kit (Qiagen, Valencia, CA), and then the RNA was purified using an RNeasy kit (Qiagen). Following elution with 100 μl of RNase-free water, RNA was subjected to DNase I treatments using a DNA-free kit (Ambion, Carlsbad, CA), according to the manufacturer's instructions, until all of the contaminating DNA was removed (i.e., when qPCR analysis showed a threshold cycle [CT] value of >35). DNase-treated RNA was subjected to a final cleanup using an RNeasy kit before gene expression analyses were performed.
Analytical techniques.
Dioxane and THF concentrations were monitored by gas chromatography with a Varian 3400 gas chromatograph (GC) equipped with a flame ionization detector (FID), as described previously (11). The production and degradation of HEAA were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) using a hydrophilic interaction chromatography (HILIC) column. Culture samples were filtered before analysis, using 0.2-μm-pore-size syringe filters to remove cells. Standards and samples were measured on an Agilent Technologies 1200 Series LC system equipped with an Agilent Technologies Zorbax HILIC Plus column (4.6 mm by 100 mm; 3.5-μm particle size) coupled to an Agilent Technologies 6410 tandem triple quadrupole (QQQ) mass spectrometer (Santa Clara, CA). The LC solvents used were solvent A consisting of aqueous buffer and solvent B consisting of acetonitrile. The aqueous buffer was composed of 10 mM ammonium formate in water with formic acid to adjust acidity (pH 5) and filtered through a 0.45-μm-pore-size filter. The flow rate was 0.5 ml/min. The gradient was as follows: at time t = 0 min, 95% solvent B; at t = 10 min, 40% solvent B; at t = 13 min, 95% solvent B; at t = 15 min, 95% solvent B. The injection volume for samples and standards was 10 μl. Column effluent was introduced into the electrospray chamber, where the electrospray ionization (ESI) was set to 3,000 V in negative mode (ESI−). Nitrogen was used as the nebulizing gas at 30 lb/in2 and 325°C and a gas flow of 11 liters/min. Multiple reaction monitoring (MRM) was used to monitor the transitions from the parent ion of HEAA, m/z 119 [C4H7O4]− to the major product ions of m/z 119 [C4H7O4]−, m/z 101 [C4H5O2]−, m/z 75 [C2H3O3]−, and m/z 31 [CH3O]−. The collision gas used for MRM was argon, with a collision energy of 6 V and a fragmentor voltage of 70 V for all MRM transitions. The summation of the peak areas for all MRM transitions was used for quantification of standards and samples.
Microarray studies.
Transcriptomic microarray hybridization and imaging were performed on custom Affymetrix GeneChips (Santa Clara, CA) as previously described (17). All microarray data analyses were performed in the R statistical programming environment (www.r-project.org) using packages available from Bioconductor, version 2.9 (www.bioconductor.org) (27). Hybridization signal intensities for probe sets were calculated using the robust multiarray average (rma) function from the affy package (28). Identification of differentially expressed genes was accomplished using the limma package (29). Comparisons were made between groups of arrays in a contrast matrix, including all treatments (dioxane, glycolate, THF, and succinate) compared to the control (pyruvate), or direct comparisons were made between treatments (e.g., dioxane to glycolate or THF to succinate). Each biological triplicate for all growth conditions (THF and succinate in this study; dioxane, glycolate, and pyruvate in Grostern et al. [17]) was analyzed on a single microarray. Contrast matrices were applied to the linear fitted microarray data using the function “contrast.fit.” Estimated log fold changes (log2 FC) and differential expression statistics between contrasts were determined using an empirical Bayes method, and P values from linear modeling were adjusted to correct for multiple hypothesis testing using the Benjamini and Hochberg procedure (30) with a false discovery rate (FDR) of 1% and a log2 FC of ≥|1|.
RT-PCR and RT-qPCR analyses.
For RT-qPCR transcriptional analyses, CB1190 was initially grown on 5 mM glucose in AMS medium and harvested by filtration. The harvested cells were washed and resuspended in AMS medium to remove glucose. Each exposure treatment (dioxane, THF, and propane) and control (glucose) condition was tested in triplicate. Total RNA was isolated from cells after 8 h of exposure to either 5 mM dioxane, 5 mM THF, 20% (vol/vol of total headspace) propane, or 5 mM glucose (control). The isolated RNA was used to synthesize cDNA using a TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA). TaqMan chemistry was used for qPCRs targeting thmA (Psed_6976), prmA (Psed_0639), tpi (Psed_3417), thiC (Psed_6168), and rpoD (Psed_0376). The genes tpi, thiC, and rpoD were determined by the geNorm method (31) to be stably transcribed across all treatments and were thus used as internal references. qPCRs were performed in triplicate for each biological replicate on an Applied Biosystems StepOne Plus real-time PCR system and consisted of 1× Fast Universal Mix (Applied Biosystems), 2 μl of cDNA, each primer at 0.5 mM, and the probe at 145 nM. The cycling conditions were 95°C for 20 s and then 40 cycles of 95°C for 1 s, followed by annealing for 20 s at 60°C. The efficiency of each qPCR assay was determined with a serial dilution of cDNA derived from a dioxane treatment replicate. Gene expression was normalized using the method of Vandesompele et al. (31).
Cells for RT-PCR analysis of the thmADBC genes were grown on dioxane, isopropanol, or THF and harvested by centrifugation. RNA was isolated using an RNeasy kit. RT-PCR was performed using a Qiagen One Step RT-PCR kit. The primers used for amplification of each fragment can be found in Table S1 in the supplemental material.
Cloning and heterologous expression of THF MO genes in RHA1.
A 4.3-kb fragment corresponding to CB1190 genes Psed_6976 to Psed_6979 was inserted into plasmid pK18 (32) and then subcloned into plasmid pTip-QC2 (33), which was named pTip-CB110-thfmo. A detailed description of cloning procedures and the growth of RHA1 expressing Pseudonocardia THF MO can be found in Materials and Methods in the supplemental material. Cloning of the thmADBC gene cluster from P. tetrahydrofuranoxydans K1 was done as described above, except that the gene cluster was amplified directly from K1 gDNA and digested, along with vector pTip-QC1, using EcoRI and NcoI prior to ligation.
To perform oxidation assays, frozen aliquots of RHA1(pTip-CB1190-thfmo), RHA1(pTip-K1-thfmo), and RHA1(pTip-QC2) were thawed and resuspended in 1 ml of cold phosphate buffer. The transformation of dioxane and THF and the production of HEAA were tested in triplicate in 2-ml microcentrifuge tubes containing 900 μl of buffer and a 100-μl cell suspension. Each triplicate tube was amended with dioxane (2.5 mM) or THF (4.0 mM) from aqueous stocks. Buffer controls (no cells) were prepared in parallel. Tubes were incubated at 30°C with horizontal shaking at 150 rpm. At several time points, 200-μl samples were removed and analyzed for the disappearance of the amended compound, using GC-FID (for dioxane and THF) and LC-MS/MS (for HEAA).
MO acetylene inhibition assays.
CB1190 was grown on dioxane (10 mM) in AMS medium, cells were harvested by filtration with 0.22-μm-pore-size filters, and filters were washed with 20 mM phosphate buffer (pH 7) to remove residual dioxane. Five milligrams (wet weight) of cells was aliquoted into eight serum vials (10 ml; Bellco, Vineland NJ), the cells were resuspended in 2 ml of phosphate buffer (20 mM), and the vials were sealed with black butyl rubber stoppers. Acetylene (25%, vol/vol) was added to the headspace of half of the vials, and all vials were incubated for 45 min at 30°C with shaking at 150 rpm. Acetylene was then removed by bubbling all vials with N2 for 5 min. Dioxane (0.57 mM) or HEAA (0.57 mM) was added to two sets (acetylene-treated and non-acetylene-treated sets) of duplicate vials, and sodium formate (20 mM) was added to all vials as an external energy source. Duplicate no-cell controls were prepared in parallel. The vials were incubated at 30°C with horizontal shaking at 150 rpm. The concentrations of dioxane and HEAA in acetylene-exposed and non-acetylene-exposed cells and in abiotic controls were monitored over time.
Microarray data accession numbers.
Details of the experimental design and data for the transcriptomic analysis of THF- and succinate-grown strain CB1190 have been deposited in the NCBI Gene Expression Omnibus (GEO [http://www.ncbi.nlm.nih.gov/geo/]) and are accessible through GEO series accession number GSE48814. The CEL files containing the raw transcriptomic data for dioxane-, glycolate-, and pyruvate-grown CB1190 were submitted for a previous study (17) under the GEO series accession number GSE33197.
RESULTS AND DISCUSSION
Differential gene expression during growth on THF, succinate, and pyruvate.
A proposed reaction pathway for aerobic THF metabolism in CB1190 (Fig. 1), based on differential gene expression analysis of CB1190 grown on THF, succinate, and pyruvate, has been adapted from the pathways proposed by Bernhardt and Diekmann (1), Skinner et al. (7), and Thiemer et al. (22). Succinate was chosen as a growth substrate for differential gene expression analysis because it was previously identified as an intermediate of THF biodegradation in the bacterium P. tetrahydrofuranoxydans K1 (22), as well as in the fungus Graphium sp. ATCC 58400 (7). Transcriptomic microarray results showed that a total of 796 genes were differentially expressed when CB1190 was grown on THF versus succinate, with 210 upregulated and 586 downregulated (see Table S3 in the supplemental material). Relative to previously collected transcriptomics data of pyruvate-grown CB1190 (17), 669 and 553 genes were differentially expressed on THF and succinate, respectively (see Tables S4 and S5), with 220 upregulated on THF and 366 upregulated on succinate.
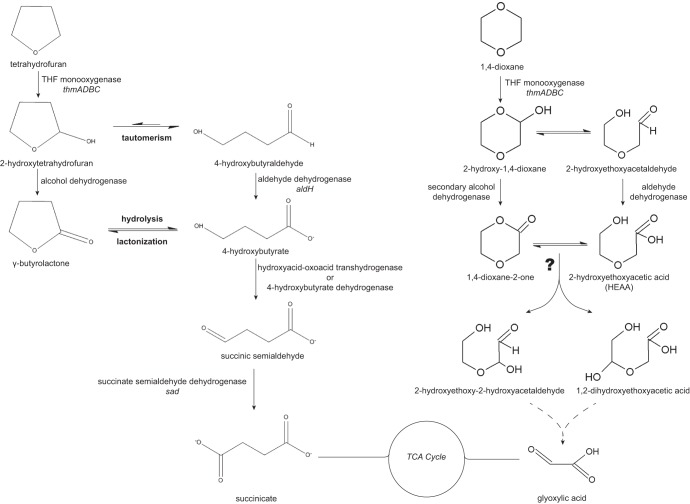
Fig 1.
THF and 1,4-dioxane degradation pathways. Proposed reaction pathways for THF and dioxane metabolism in CB1190, annotated with enzymes based on transcriptomic results in this study. The THF pathway is adapted from the pathways proposed by Bernhardt and Diekmann (1), Skinner et al. (7), and Thiemer et al. (22). The 1,4-dioxane pathway is adapted from Grostern et al. (17). The gene locus tags for the enzymes in the THF pathway are Psed_6976 to Psed_6979 for the THF monooxygenase thmADBC, Psed_0131 for alcohol dehydrogenase, Psed_6971 for hydroxyacid-oxoacid transhydrogenase, Psed_6970 for 4-hydroxybutyrate dehydrogenase, Psed_6975 for succinate semialdehyde dehydrogenase (sad), and Psed_6981 for aldehyde dehydrogenase (aldH). The locus tags are the same for the dioxane pathway, except for those for the secondary alcohol dehydrogenase (Psed_0131, Psed_2070, Psed_4156, or Psed_6971) and the aldehyde dehydrogenase (Psed_6971, Psed_6975, or Psed_6981). TCA, tricarboxylic acid cycle.
Among the genes upregulated on THF but not succinate, relative to pyruvate (Table 1; see also Table S2), is the annotated THF MO gene cluster thmDBC, Psed_6977-Psed_6979, located on plasmid pPSED02. In strain K1, thmADBC is thought to be involved in the hydroxylation of THF to 2-hydroxytetrahydrofuran since Northern blot analysis showed the thm cluster being transcribed during THF utilization (22). Although the CB1190 microarray used in this current study lacks a probe set for the gene encoding the α-subunit (thmA), RT-qPCR demonstrated that short-term (8-h) exposure of CB1190 cells to THF leads to upregulation of thmA transcription by 10-fold compared to transcription in unexposed cells. Short-term exposure to dioxane or propane was also shown to induce thmA expression by 24-fold or 6-fold, respectively. The gene expression of the α-subunit (thmA) was chosen for qPCR analysis because the α-subunit of the propane monooxygenase encoding the large hydroxylase component in R. jostii RHA1 was previously found to be the most highly expressed gene in its prmABCD cluster (34). Furthermore, RT-PCR of the putative THF MO genes thmADBC indicated that all of the genes in this cluster were cotranscribed during growth on THF (see Fig. S1 in the supplemental material). In addition to thmADBC, genes encoding an alcohol dehydrogenase GroES domain protein (Psed_0131), an aldehyde dehydrogenase (aldH, Psed_6981), and a Mn2+/Fe2+ transporter (Psed_6982) were also upregulated in the presence of THF relative to both succinate and pyruvate (Table 1). While the conversion of 2-hydroxytetrahydrofuran to γ-butyrolactone had been proposed to be catalyzed by an alcohol dehydrogenase (7), our results provide transcriptional evidence for the involvement of a specific alcohol dehydrogenase in THF degradation.
Table 1.
Transcription of CB1190 genes proposed to be involved in THF metabolism
| Gene locus tag | Gene name | Protein | Expression on THF vs pyruvate |
Expression on succinate vs pyruvate |
||
|---|---|---|---|---|---|---|
| log2 FC | Adjusted P value | log2 FC | Adjusted P value | |||
| Psed_0131 | Alcohol dehydrogenase GroES domain protein | 1.92 | 2.23E−05 | 0.53 | 9.87E−02 | |
| Psed_6970 | d-Lactate dehydrogenase (cytochrome) | 2.75 | 5.49E−05 | −0.18 | 7.86E−01 | |
| Psed_6971 | Hydroxyacid-oxoacid transhydrogenase | 2.17 | 7.06E−05 | 0.47 | 2.65E−01 | |
| Psed_6972 | GntR domain protein | 1.65 | 1.26E−03 | 0.26 | 6.35E−01 | |
| Psed_6974 | Ethyl tert-butyl ether degradation, EthD | 1.78 | 8.85E−07 | 0.94 | 2.42E−04 | |
| Psed_6975 | sad | Betaine-aldehyde dehydrogenase | 1.65 | 2.73E−07 | 0.73 | 4.01E−04 |
| Psed_6977 | thmD | Ferredoxin-NAD+ reductase | 2.47 | 2.42E−07 | 0.68 | 1.00E−02 |
| Psed_6978 | thmB | Methane/phenol/toluene hydroxylase | 1.58 | 3.75E−07 | 0.51 | 5.01E−03 |
| Psed_6979 | thmC | Monooxygenase component MmoB/DmpM | 2.21 | 6.61E−07 | 0.69 | 9.03E−03 |
| Psed_6981 | aldH | Aldehyde dehydrogenase | 3.38 | 1.67E−07 | 0.96 | 6.65E−03 |
| Psed_6982 | Mn2+/Fe2+ transporter, NRAMP family | 3.45 | 4.75E−06 | 0.21 | 7.34E−01 | |
Following abiotic hydrolysis of γ-butyrolactone to 4-hydroxybutyrate, the next enzymatic reaction in the THF degradation pathway is the transformation of 4-hydroxybutyrate to succinic semialdehyde, potentially catalyzed by a hydroxyacid-oxoacid transhydrogenase (35) or a 4-hydroxybutyrate dehydrogenase (36). According to the transcriptomic results, two genes (Psed_6970 and Psed_6971) located just upstream of the thm cluster on plasmid pPSED02 were upregulated in the presence of THF but not with succinate, relative to pyruvate (Table 1). Psed_6970 encodes a lactate dehydrogenase, a homolog of 4-hydroxybutyrate dehydrogenase, which has been reported to convert 4-hydroxybutyrate to succinic semialdehyde using NAD (37), while Psed_6971 encodes a hydroxyacid-oxoacid transhydrogenase. Also upregulated in the thm gene cluster on plasmid pPSED02 was the succinate semialdehyde dehydrogenase-encoding gene sad (Psed_6975) (Table 1), whose product is predicted to catalyze the next transformation step, oxidation of succinate semialdehyde to succinate (22).
Although the proposed pathway for THF metabolism in CB1190 based upon gene expression analyses (Fig. 1) shares many similarities with the pathways proposed for P. tetrahydrofuranoxydans K1 (22) and Graphium sp. ATCC 58400 (7), one notable difference between the CB1190 and K1 pathways is the transcriptional evidence for the tautomeric transformation of 2-hydroxytetrahydrofuran to 4-hydroxybutyraldehyde in CB1190, similar to that proposed in the fungus. The exclusion of this transformation step in the THF pathway in strain K1 was based on transcriptional results indicating that aldH was not expressed during growth on THF (22). However, aldH on pPSED02 near the thm cluster in CB1190 (Psed_6981) was upregulated during growth on THF, suggesting that tautomeric conversion of 2-hydroxytetrahydrofuran likely occurs during THF degradation, as proposed in Fig. 1.
The protein product of thmADBC oxidizes dioxane and THF.
In order to verify the THF and dioxane transformation activity of the enzyme encoded by thmADBC, the gene cluster from CB1190 was inserted into pTip-QC2 and used to transform R. jostii RHA1. RHA1 was chosen as a host for expression of thmADBC for several reasons: (i) it belongs to the same Actinomycetales order as CB1190 (25), (ii) its wild type is unable to oxidize or otherwise transform dioxane or THF (data not shown), and (iii) it expresses an analogous multicomponent propane monooxygenase prmABCD (34), which has a GC content (64%) similar to that of thmADBC of CB1190 (60%). Whole cells of RHA1(pTip-CB1190-thfmo), following growth on nutrient broth and induction by thiostrepton, demonstrated nearly complete transformation of 4 mM THF within 3 days (Fig. 2A). The amount of THF loss in the abiotic and RHA1(pTip-QC2) controls was minimal and could be attributed to the volatility of THF (Henry's constant, Hc, of 7.05 × 10−3 atm · m3/mol) (38). RHA1(pTip-CB1190-thfmo) suspensions degraded 2.5 mM dioxane within 3 days (Fig. 2B), while dioxane loss in the abiotic and RHA1(pTip-QC2) controls was negligible. The dioxane degradation pathway intermediate HEAA accumulated stoichiometrically with dioxane disappearance. No dioxane transformation activity was detected in cell extracts prepared by bead beating or sonication of RHA1(pTip-CB1190-thfmo) cells, even with the addition of the MO electron donor NADH.
Fig 2.
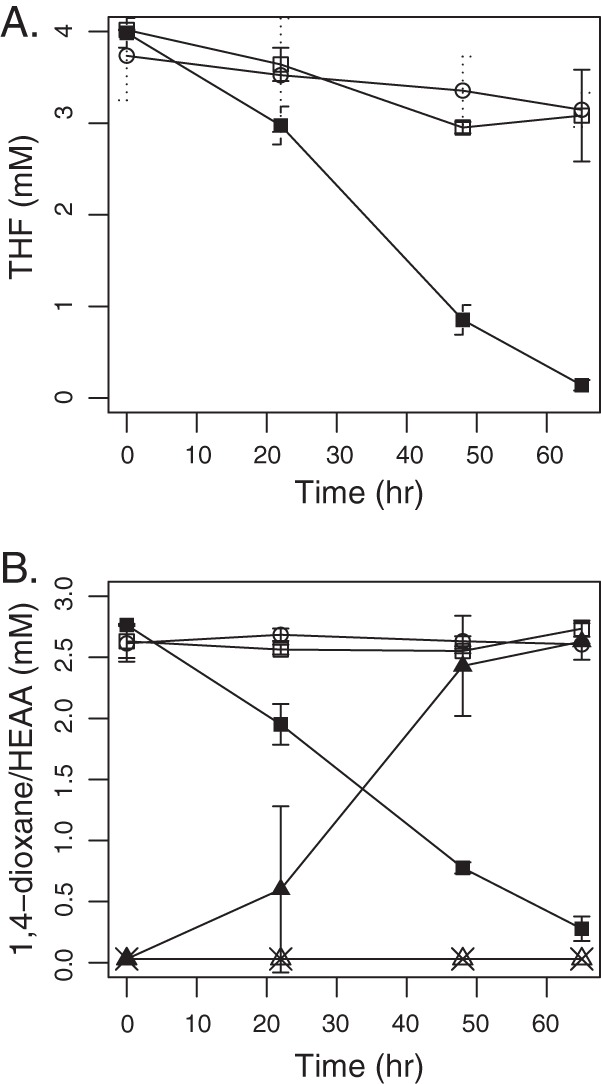
Functional activity of heterologous CB1190 THF MO expression clones. (A) THF removal by RHA1 containing plasmid pTip-CB1190-thfmo (■), the empty vector pTip-QC2 (control) (□), and abiotic samples (○). (B) Removal of dioxane and accumulation of HEAA by plasmid pTip-CB1190-thfmo clones, (■, dioxane; ▲, HEAA), empty vector pTip-QC2 clones (□, dioxane; △, HEAA), and abiotic samples (○, dioxane; ×, HEAA). Error bars indicate standard deviations. All conditions were run in triplicate.
The thmADBC gene cluster was also cloned from P. tetrahydrofuranoxydans K1 and expressed in RHA1 cells using the pTip-QC1 vector, RHA1(pTip-K1-thfmo). As with RHA1(pTip-CB1190-thfmo), RHA1(pTip-K1-thfmo) transformed both THF and dioxane (see Fig. S2 in the supplemental material), and HEAA was produced in stoichiometric amounts from dioxane (data not shown). RHA1(pTip-K1-thfmo) and RHA1(pTip-CB1190-thfmo) removed nearly equivalent (∼1 mM) amounts of dioxane and THF over 77 h (see Fig. S2).
The demonstration of THF and dioxane oxidation activity in the two RHA1 clones is significant for two reasons: (i) it confirms the role of the CB1190 THF MO in the initial oxidation of these two cyclic ethers, and (ii) it demonstrates that RHA1 can be used as a heterologous host for expression of multicomponent MOs. In addition to this study, functional genetic evidence for the involvement of THF MO genes (thm) has been demonstrated in Pseudonocardia sp. strain ENV478, in which decreased translation of the thmB gene, caused by antisense RNA, resulted in loss of its ability to degrade THF and oxidize 1,4-dioxane (23). To date, we have been unable to express active THF MO from CB1190 in E. coli, as well as active propane MO enzymes encoded by the prmABCD gene clusters from the actinomycetes RHA1 (34) and Gordonia sp. strain TY-5 (39). In support of our finding that the expression of multicomponent MOs is easier in actinomycete hosts, a recent study by Furuya et al. (40) reported the heterologous expression of mimABCD gene clusters from Mycobacterium smegmatis strain MC2 155 and Mycobacterium goodii strain 12523. These gene clusters encode binuclear monooxygenases involved in the oxidization of propane and phenol in the host Rhodococcus opacus B-4.
HEAA induces, but is not transformed by, the CB1190 THF MO.
It was previously proposed that the MO enzyme that catalyzes the first oxidation step in the dioxane degradation pathway may also hydroxylate HEAA, leading to the formation of a number of 2-carbon intermediates (16). Our previous transcriptomic study showed that the thm gene cluster was the only MO-encoding cluster of CB1190 to be upregulated during growth on dioxane (17). However, those transcriptomic results did not clarify whether the same MO catalyzes the hydroxylation of both dioxane and HEAA, necessitating additional functional studies to clarify the nature of HEAA transformation in CB1190.
HEAA supported the growth of CB1190, with complete removal of 1.5 mM HEAA within 11 days by a culture inoculated with a 1:500 dilution of dioxane-grown cells (Fig. 3A). RT-qPCR analysis was used to compare the transcriptional level of thmA in HEAA- and pyruvate-grown cells. Normalized to the housekeeping genes tpi (Psed_3417) and rpoD (Psed_3051), thmA was transcribed at a level 41-fold higher in HEAA-grown cells than in pyruvate-grown cells. We previously showed that dioxane, but not the dioxane degradation metabolite glycolate, induced thmA 15-fold relative to pyruvate (17). Although gene expression results appear to indicate that the THF MO is involved in HEAA transformation, the accumulation of HEAA in RHA1(pTip-CB1190-thfmo) clones degrading dioxane (see Fig. S2B in the supplemental material) demonstrates the inability of the THF MO to degrade HEAA.
Fig 3.
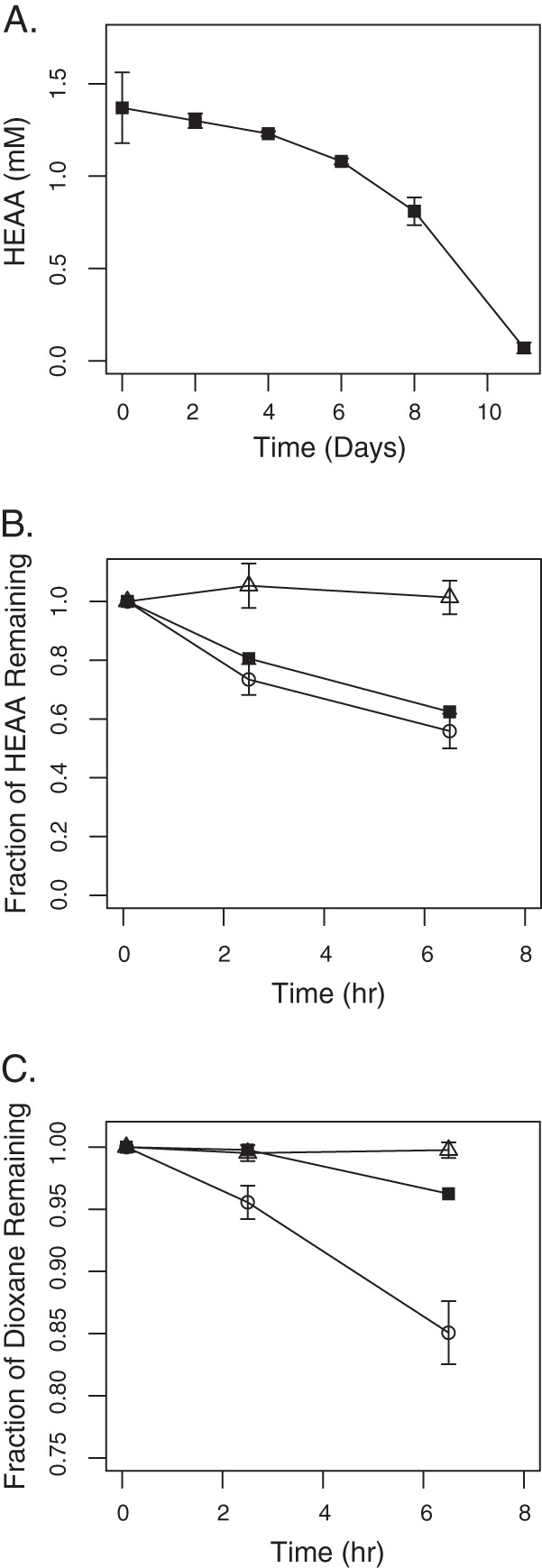
HEAA and dioxane degradation by CB1190. (A) Disappearance of HEAA during growth of CB1190 on HEAA as the sole carbon and energy source. Effect of acetylene exposure on HEAA (B) and dioxane (C) degradation by CB1190. All experiments with acetylene-exposed cells (■), non-acetylene-exposed cells (○), and abiotic controls (△) were performed in triplicate. Error bars indicate standard deviations.
In addition to the RHA1(pTip-CB1190-thfmo) clones, the RHA1(pTip-K1-thfmo) clones also demonstrated HEAA generation and accumulation when exposed to dioxane (Fig. 2B; see also Fig. S2). Even after prolonged incubation (48 h), these clones were not able to degrade HEAA (data not shown). These results support the hypothesis that the THF MO encoded by the thmADBC gene cluster is not responsible for the transformation of HEAA.
Brief exposure of acetylene gas can cause irreversible inhibition of specific types of MO enzyme activity (41–45) and may specifically inhibit the oxidation of dioxane (11, 16). In order to test the hypothesis that an acetylene-sensitive MO is involved in the transformation of HEAA by CB1190 (16), resting cells of dioxane-grown CB1190 were exposed to acetylene, and then degradation of dioxane and HEAA was monitored. During the first couple of hours, cells preexposed to acetylene removed negligible dioxane (Fig. 3B) but degraded 0.5 mM HEAA (Fig. 3C), which was similar to results with the positive control (cells not exposed to acetylene). After 2 h, some dioxane removal was observed in acetylene-exposed cells, likely due to de novo THF MO synthesis. These results contradict the hypothesis that an acetylene-sensitive MO enzyme is responsible for HEAA degradation. Further, we had previously observed that HEAA is a transient metabolite generated during dioxane degradation, appearing and disappearing within a short span of time (1 to 4 h) rather than accumulating while dioxane is still detectable (16). Together with the data shown in Fig. 2B and 3C, this indicates that dioxane and HEAA transformations are performed simultaneously rather than stepwise by CB1190, supporting the hypothesis that two different enzymes are responsible for the two oxidation reactions.
Potential alternative mechanisms for HEAA transformation in CB1190.
The thm gene cluster is present in strains CB1190 (18), K1 (22), and ENV478 (18, 22, 23). Heterologous expression demonstrated that in both CB1190 and K1, this gene cluster encodes a monooxygenase that transforms both THF and dioxane, while similar activity for this gene cluster was suggested by gene knockdown techniques in ENV478 (23). Unlike CB1190, K1 and ENV478 degrade dioxane cometabolically. However, ENV478 accumulates HEAA as a dead-end product of dioxane degradation (10) while K1 does not accumulate HEAA (16). Thus, it is plausible that both CB1190 and K1 express enzymes that catalyze the transformation of HEAA that ENV478 lacks. Given K1's ability to mineralize dioxane (16), its inability to metabolize dioxane for supporting growth remains unresolved.
In addition to MOs, a variety of non-MO enzymes have been reported to catalyze ether bond cleavage, including dioxygenases, ether hydrolases, carbon-oxygen lyases, peroxidases, laccases, and etherases (46). Further studies with CB1190, K1, and other strains capable of growth on dioxane are needed to identify the enzymes involved in HEAA transformation during dioxane biodegradation.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by the Strategic Environmental Research and Development Program (SERDP ER-1417) and NIEHS Superfund Basic Research Program grant ES04705-19.
Plasmids pTip-QC2 and pTip-QC1 were obtained from the National Institute of Advanced Industrial Science and Technology (Japan).
Footnotes
Published ahead of print 4 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02418-13.
REFERENCES
- 1.Bernhardt D, Diekmann H. 1991. Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Appl. Microbiol. Biotechnol. 36:120–123 [DOI] [PubMed] [Google Scholar]
- 2.Burback BL, Perry JJ. 1993. Biodegradation and biotransformation of groundwater pollutant mixtures by Mycobacterium vaccae. Appl. Environ. Microbiol. 59:1025–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parales RE, Adamus JE, White N, May HD. 1994. Degradation of 1,4-dioxane by an actinomycete in pure culture. Appl. Environ. Microbiol. 60:4527–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy D, Anagnostu G, Chaphalkar P. 1994. Biodegradation of dioxane and diglyme in industrial waste. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 29:129–147 [Google Scholar]
- 5.Patt TE, Abebe HM. March 1995. Microbial degradation of chemical pollutants. US patent 5399495
- 6.Raj CBC, Ramkumar N, Siraj AHJ, Chidambaram S. 1997. Biodegradation of acetic, benzoic, isophthalic, toluic and terephthalic acids using a mixed culture: effluents of PTA production. Process. Saf. Environ. Prot. 75:245–256 [Google Scholar]
- 7.Skinner K, Cuiffetti L, Hyman M. 2009. Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 75:5514–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zenker MJ, Borden RC, Barlaz MA. 2000. Mineralization of 1,4-dioxane in the presence of a structural analog. Biodegradation 11:239–246 [DOI] [PubMed] [Google Scholar]
- 9.Nakamiya K, Hashimoto S, Ito H, Edmonds JS, Morita M. 2005. Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl. Environ. Microbiol. 71:1254–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vainberg S, McClay K, Masuda H, Root D, Condee C, Zylstra GJ, Steffan RJ. 2006. Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Appl. Environ. Microbiol. 72:5218–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahendra S, Alvarez-Cohen L. 2006. Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ. Sci. Technol. 40:5435–5442 [DOI] [PubMed] [Google Scholar]
- 12.Kim YM, Jeon JR, Murugesan K, Kim EJ, Chang YS. 2009. Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation 20:511–519 [DOI] [PubMed] [Google Scholar]
- 13.Shen W, Chen H, Pan S. 2008. Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms. Bioresour. Technol. 99:2483–2487 [DOI] [PubMed] [Google Scholar]
- 14.Mahendra S, Alvarez-Cohen L. 2005. Pseudonocardia dioxanivorans sp. nov., a novel actinomycete that grows on 1,4-dioxane. Int. J. Syst. Evol. Microbiol. 55:593–598 [DOI] [PubMed] [Google Scholar]
- 15.Sei K, Kakinoki T, Inoue D, Soda S, Fujita M, Ike M. 2010. Evaluation of the biodegradation potential of 1,4-dioxane in river, soil and activated sludge samples. Biodegradation 21:585–591 [DOI] [PubMed] [Google Scholar]
- 16.Mahendra S, Petzold CJ, Baidoo EE, Keasling JD, Alvarez-Cohen L. 2007. Identification of the intermediates of in vivo oxidation of 1,4-dioxane by monooxygenase-containing bacteria. Environ. Sci. Technol. 41:7330–7336 [DOI] [PubMed] [Google Scholar]
- 17.Grostern A, Sales CM, Zhuang WQ, Erbilgin O, Alvarez-Cohen L. 2012. Glyoxylate metabolism is a key feature of the metabolic degradation of 1,4-dioxane by Pseudonocardia dioxanivorans strain CB1190. Appl. Environ. Microbiol. 78:3298–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sales CM, Mahendra S, Grostern A, Parales RE, Goodwin LA, Woyke T, Nolan M, Lapidus A, Chertkov O, Ovchinnikova G, Sczyrba A, Alvarez-Cohen L. 2011. Genome sequence of the 1,4-dioxane-degrading Pseudonocardia dioxanivorans strain CB1190. J. Bacteriol. 193:4549–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daye KJ, Groff JC, Kirpekar AC, Mazumder R. 2003. High efficiency degradation of tetrahydrofuran (THF) using a membrane bioreactor: identification of THF-degrading cultures of Pseudonocardia sp. strain M1 and Rhodococcus ruber isolate M2. J. Ind. Microbiol. Biotechnol. 30:705–714 [DOI] [PubMed] [Google Scholar]
- 20.Kampfer P, Kohlweyer U, Thiemer B, Andreesen JR. 2006. Pseudonocardia tetrahydrofuranoxydans sp. nov. Int. J. Syst. Evol. Microbiol. 56:1535–1538 [DOI] [PubMed] [Google Scholar]
- 21.Kohlweyer U, Thiemer B, Schrader T, Andreesen JR. 2000. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 186:301–306 [DOI] [PubMed] [Google Scholar]
- 22.Thiemer B, Andreesen J, Schräder T. 2003. Cloning and characterization of a gene cluster involved in tetrahydrofuran degradation in Pseudonocardia sp. strain K1. Arch. Microbiol. 179:266–277 [DOI] [PubMed] [Google Scholar]
- 23.Masuda H, McClay K, Steffan R, Zylstra G. 2012. Biodegradation of tetrahydrofuran and 1,4-dioxane by soluble diiron monooxygenase in Pseudonocardia sp. strain ENV478. J. Mol. Microbiol. Biotechnol. 22:312–316 [DOI] [PubMed] [Google Scholar]
- 24.Yao Y, Lv Z, Min H, Jiao H. 2009. Isolation, identification and characterization of a novel Rhodococcus sp. strain in biodegradation of tetrahydrofuran and its medium optimization using sequential statistics-based experimental designs. Bioresour. Technol. 100:2762–2769 [DOI] [PubMed] [Google Scholar]
- 25.McLeod MP, Warren RL, Hsiao WW, Araki N, Myhre M, Fernandes C, Miyazawa D, Wong W, Lillquist AL, Wang D, Dosanjh M, Hara H, Petrescu A, Morin RD, Yang G, Stott JM, Schein JE, Shin H, Smailus D, Siddiqui AS, Marra MA, Jones SJ, Holt R, Brinkman FS, Miyauchi K, Fukuda M, Davies JE, Mohn WW, Eltis LD. 2006. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. U. S. A. 103:15582–15587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson DR, Brodie EL, Hubbard AE, Andersen GL, Zinder SH, Alvarez-Cohen L. 2008. Temporal transcriptomic microarray analysis of “Dehalococcoides ethenogenes” strain 195 during the transition into stationary phase. Appl. Environ. Microbiol. 74:2864–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentleman R. 2005. Bioinformatics and computational biology solutions using R and Bioconductor. Springer, New York, NY [Google Scholar]
- 28.Gautier L, Cope L, Bolstad B, Irizarry R. 2004. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315 [DOI] [PubMed] [Google Scholar]
- 29.Smyth GK. 2004. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article3. 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57:289–300 [Google Scholar]
- 31.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:research0034. 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pridmore RD. 1987. New and versatile cloning vectors with kanamycin-resistance marker. Gene 56:309–312 [DOI] [PubMed] [Google Scholar]
- 33.Nakashima N, Tamura T. 2004. Isolation and characterization of a rolling-circle-type plasmid from Rhodococcus erythropolis and application of the plasmid to multiple-recombinant-protein expression. Appl. Environ. Microbiol. 70:5557–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp JO, Sales CM, LeBlanc JC, Liu J, Wood TK, Eltis LD, Mohn WW, Alvarez-Cohen L. 2007. An inducible propane monooxygenase is responsible for N-nitrosodimethylamine degradation by Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 73:6930–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman E, Nelson T, Fales H, Levin D. 1988. Isolation and characterization of a hydroxyacid-oxoacid transhydrogenase from rat kidney mitochondria. J. Biol. Chem. 263:16872–16879 [PubMed] [Google Scholar]
- 36.Bergmeyer H, Gawehn K, Klotzsch H, Krebs H, Williamson D. 1967. Purification and properties of crystalline 3-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Biochem. J. 102:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Söhling B, Gottschalk G. 1996. Molecular analysis of the anaerobic succinate degradation pathway in Clostridium kluyveri. J. Bacteriol. 178:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr T, Stickney J, Diguiseppi B. 2010. Environmental investigation and remediation: 1,4-dioxane and other solvent stabilizers. CRC Press, Boca Raton, FL [Google Scholar]
- 39.Kotani T, Yamamoto T, Yurimoto H, Sakai Y, Kato N. 2003. Propane monooxygenase and NAD+-dependent secondary alcohol dehydrogenase in propane metabolism by Gordonia sp. strain TY-5. J. Bacteriol. 185:7120–7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuya T, Hayashi M, Semba H, Kino K. 2012. The mycobacterial binuclear iron monooxygenases require a specific chaperonin-like protein for functional expression in a heterologous host. FEBS J. 280:817–826 [DOI] [PubMed] [Google Scholar]
- 41.Colby J, Dalton H, Whittenbury R. 1975. An improved assay for bacterial methane mono-oxygenase: some properties of the enzyme from Methylomonas methanica. Biochem. J. 151:459–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stirling DI, Dalton H. 1977. Effect of metal-binding and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch. Microbiol. 114:71–76 [DOI] [PubMed] [Google Scholar]
- 43.Prior SD, Dalton H. 1985. Acetylene as a suicide substrate and active-site probe for methane monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 29:105–109 [Google Scholar]
- 44.Hamamura N, Storfa RT, Semprini L, Arp DJ. 1999. Diversity in butane monooxygenases among butane-grown bacteria. Appl. Environ. Microbiol. 65:4586–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp JO, Wood TK, Alvarez-Cohen L. 2005. Aerobic biodegradation of N-nitrosodimethylamine (NDMA) by axenic bacterial strains. Biotechnol. Bioeng. 89:608–618 [DOI] [PubMed] [Google Scholar]
- 46.White GF, Russell NJ, Tidswell EC. 1996. Bacterial scission of ether bonds. Microbiol. Rev. 60:216–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.