Abstract
We first determined the analytical specificity and ubiquity (i.e., the ability to detect all or most strains) of a Clostridium perfringens-specific real-time PCR (rtPCR) assay based on the cpa gene (cpa rtPCR) by using a bacterial strain panel composed of C. perfringens and non-C. perfringens Clostridium strains. All non-C. perfringens Clostridium strains tested negative, whereas all C. perfringens strains tested positive with the cpa rtPCR, for an analytical specificity and ubiquity of 100%. The cpa rtPCR assay was then used to confirm the identity of 116 putative C. perfringens isolates recovered after filtration of water samples and culture on mCP agar. Colonies presenting discordant results between the phenotype on mCP agar and cpa rtPCR were identified by sequencing the 16S rRNA and cpa genes. Four mCP−/rtPCR+ colonies were identified as C. perfringens, whereas 3 mCP+/rtPCR− colonies were identified as non-C. perfringens. The cpa rtPCR was negative with all 51 non-C. perfringens strains and positive with 64 of 65 C. perfringens strains. Finally, we compared mCP agar and a CRENAME (concentration and recovery of microbial particles, extraction of nucleic acids, and molecular enrichment) procedure plus cpa rtPCR (CRENAME + cpa rtPCR) for their abilities to detect C. perfringens spores in drinking water. CRENAME + cpa rtPCR detected as few as one C. perfringens CFU per 100 ml of drinking water sample in less than 5 h, whereas mCP agar took at least 25 h to deliver results. CRENAME + cpa rtPCR also allows the simultaneous and sensitive detection of Escherichia coli and C. perfringens from the same potable water sample. In itself, it could be used to assess the public health risk posed by drinking water potentially contaminated with pathogens more resistant to disinfection.
INTRODUCTION
The presence of Escherichia coli in drinking water indicates a recent fecal contamination, as well as a risk of waterborne gastrointestinal diseases (1). Under some circumstances, however, it has been shown that this microorganism inadequately indicates the presence of viruses and protozoan parasites of human health significance (2). For example, while chlorine in water rapidly inactivates E. coli, it leaves most disinfection-resistant pathogens almost unaffected for several hours (3). According to Payment and Franco (3), Clostridium perfringens is a suitable indicator of human enteric viruses, Giardia cysts, and Cryptosporidium oocysts in finished water and can also be used in the assessment of water treatment processes due to the resistance of Clostridium spores to chlorine. Furthermore, the presence of C. perfringens in water is also associated with fecal contamination, and it has been evaluated and utilized as an alternative indicator of fecal pollution (4–14). Thus, a simple and reliable culture-based method to isolate and enumerate C. perfringens, the membrane filtration method on mCP agar, has been elaborated and evaluated to monitor the presence of C. perfringens in water (15–20). In the European Union, the mCP agar method is the reference method used to assess the presence of C. perfringens in water intended for human consumption (21).
The simultaneous detection of E. coli and C. perfringens in drinking water could provide a better estimation of the public health risk, since both microorganisms indicate the presence of bacteria and more environmentally resistant or disinfection-resistant pathogens (22, 23). However, this cannot be accomplished by current culture-based techniques, and two independent tests would be required to assess the presence of these indicator microorganisms.
The application of rapid molecular testing to the microbiological quality of water is hampered by the scarcity of simple and robust solutions for concentrating and recovering very low numbers of microbial particles present in a relatively large water sample. In this study, we have determined the analytical specificity and ubiquity (i.e., the ability to detect all or most strains) of a Clostridium perfringens-specific real-time PCR (rtPCR) assay based on the cpa gene (encoding C. perfringens alpha-toxin) (cpa rtPCR) (24–26). We then used it to rapidly confirm the identity of putative C. perfringens isolates recovered after filtration and culture on mCP agar. Colonies presenting discordant results between the phenotype on mCP agar and cpa rtPCR were identified by sequencing the 16S rRNA and cpa genes. Finally, we compared mCP agar and a CRENAME (concentration and recovery of microbial particles, extraction of nucleic acids, and molecular enrichment) procedure plus cpa rtPCR (CRENAME + cpa rtPCR) for their abilities to detect C. perfringens spores in drinking water (27, 28).
MATERIALS AND METHODS
Analytical performance of C. perfringens cpa rtPCR assay.
The analytical specificity of the C. perfringens-specific (cpa-based) rtPCR assay was determined by testing genomic DNAs isolated from 54 clinical and environmental non-C. perfringens Clostridium species and other bacterial species phylogenetically related to C. perfringens (Table 1). The ubiquity of the cpa rtPCR assay (i.e., the ability to detect all or most C. perfringens strains) was assessed against 37 C. perfringens strains of environmental and nonenvironmental origins. Environmental C. perfringens isolates (n = 33), recovered from different water sources by the culture-based method on mCP agar, were obtained from the Centre d'Expertise en Analyze Environnementale du Québec (CEAEQ) (Québec City, Québec, Canada). Nonenvironmental C. perfringens isolates (n = 4) were obtained from the CHU de Québec (Québec City, Canada) (n = 2) and the Culture Collection, University of Gothenburg (CCUG) (Gothenburg, Sweden) (n = 2). The identification of these isolates was confirmed by 16S rRNA gene nucleotide sequencing analysis.
Table 1.
Bacterial panel (n = 54 strains) for determining the analytical specificity of the cpa rtPCR assaya
| Bacterial species | Strain | Origin |
|---|---|---|
| Anaerococcus lactolyticus | ATCC 51172 | Human |
| Anaerococcus prevotii | ATCC 9321 | Human |
| Anaerococcus tetradius | ATCC 35098 | Human |
| Butyrivibrio fibrisolens | ATCC 19171 | Animal |
| Clostridium acetobutyricum | CCRI-16781 | Plant |
| Clostridium baratii | CCRI-19299 | Water |
| Clostridium beijerinckii | ATCC 8260 | NA |
| Clostridium biofermentans | ATCC 638 | NA |
| Clostridium bolteae | CCRI-16220 | NA |
| Clostridium botulinum | CCRI-25 | NA |
| Clostridium cavendishii | CCRI-19303 | Water |
| Clostridium difficile | ATCC 9689 | NA |
| Clostridium frigidicarnis | CCRI-19297 | Water |
| Clostridium frigidicarnis | CCRI-19301 | Water |
| Clostridium hathewayi | CCRI-16222 | NA |
| Clostridium histolyticum | ATCC 19401 | Human |
| Clostridium innocuum | ATCC 14501 | Human |
| Clostridium novyi | ATCC 19402 | NA |
| Clostridium ramosum | ATCC 25582 | NA |
| Clostridium ruminantium | CCRI-19300 | Water |
| Clostridium saccharoperbutylacetonicum | CCRI-19321 | Water |
| Clostridium septicum | ATCC 12464 | NA |
| Clostridium sphenoides | ATCC 19403 | NA |
| Clostridium sporogenes | CCRI-11132 | NA |
| Clostridium symbosium | ATCC 14940 | NA |
| Clostridium tertium | ATCC 14573 | NA |
| Clostridium tetani | ATCC 19406 | NA |
| Clostridium xylanolyticum | CCRI-16727 | Plant |
| Eubacterium brachy | ATCC 33089 | Human |
| Eubacterium nodatum | ATCC 33099 | Human |
| Fusobacterium mortiferum | ATCC 25557 | NA |
| Fusobacterium neosphorum | ATCC 25286 | NA |
| Fusobacterium gonidiaformans | ATCC 25563 | NA |
| Fusobacterium periodonticum | ATCC 33693 | Human |
| Fusobacterium russi | ATCC 25533 | Animal |
| Fusobacterium ulcerans | CCRI-15825 | Human |
| Fusobacterium varium | ATCC 8501 | NA |
| Helcococcus kunzii | ATCC 51366 | Human |
| Lactobacillus acidophilus | ATCC 4356 | Human |
| Lactobacillus crispatus | ATCC 33820 | NA |
| Lactobacillus gasseri | ATCC 33323 | NA |
| Lactobacillus johnsonii | ATCC 33200 | Human |
| Lactobacillus reuteri | ATCC 23272 | Human |
| Leptotrichia buccalis | ATCC 14201 | Human |
| Peptococcus niger | ATCC 27731 | Human |
| Peptoniphilus asaccharolyticus | CCRI-162 | Human |
| Peptoniphilus indolicus | ATCC 29427 | Animal |
| Peptoniphilus lacrimalis | ATCC 51171 | Human |
| Peptostreptococcus anaerobius | CCRI-16219 | NA |
| Ruminococcus bromii | ATCC 27255 | Human |
| Ruminococcus gnavus | CCRI-16371 | Human |
| Ruminococcus productus | ATCC 27340 | Human |
| Streptococcus mitis | ATCC 49456 | NA |
| Tissierella praeacuta | ATCC 25539 | NA |
None of these strains tested positive with the cpa rtPCR assay. ATCC, American Type Culture Collection; CCRI, culture collection of Centre de Recherche en Infectiologie de l'Université Laval; NA, not available.
Phenotypic and molecular characterization of C. perfringens isolates and phylogenetic analyses.
CEAEQ provided C. perfringens-positive mCP agar plates resulting from the filtration of surface and river water samples (Tables 2 and 3). Only plates with well-isolated colonies were selected, and all colonies were recovered for further analysis; 147 colonies were initially found on mCP agar plates, but 31 did not grow after isolation (Fig. 1). The identities of the remaining 116 colonies isolated on mCP agar were confirmed using the cpa rtPCR assay, and the identifications of environmental strains presenting discordant results between culture and cpa rtPCR were confirmed by nucleotide sequencing of 16S rRNA and cpa genes using amplification and/or sequencing primers listed in Table 4 and experimental conditions described by Isabel et al. (29). Phylogenetic and molecular analyses of sequence contigs were conducted using MEGA version 5 (30). Phylogenetic trees were calculated by using the neighbor-joining method with the maximum composite likelihood substitution model. The topological accuracy of the tree was evaluated using 500 bootstrap replicates.
Table 2.
Phenotypes and cpa rtPCR profiles of putative C. perfringens isolates found on mCP agar from surface water samplesa
| Sample | Reference strain | Phenotype on mCP agar | C. perfringens cpa rtPCR result | 16S rRNA gene-based identification |
|---|---|---|---|---|
| 1 | CCRI-19605 | Pink | + | NA |
| CCRI-19606 | Pink | + | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-19608 | Pink | + | NA | |
| CCRI-19609 | Pink | − | Clostridium baratii | |
| CCRI-19610 | Pink | + | NA | |
| CCRI-19607 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| 2 | CCRI-19611 | Pink | + | NA |
| CCRI-19612 | Pink | + | NA | |
| CCRI-19613 | Pink | + | NA | |
| CCRI-19614 | Pink | + | NA | |
| CCRI-19615 | Pink | + | NA | |
| CCRI-19710 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-19711 | Colorless | − | NA | |
| CCRI-19616 | Colorless | NA | ||
| CCRI-19617 | Colorless | + | C. perfringens | |
| CCRI-19618 | Pink | + | NA | |
| 3 | CCRI-19619 | Pink | + | NA |
| CCRI-19620 | Pink | + | NA | |
| CCRI-19654 | Pink | + | NA | |
| CCRI-19621 | Pink | + | NA | |
| CCRI-19622 | Pink | − | Clostridium sardiniense | |
| CCRI-19623 | Pink | + | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-19655 | Colorless | − | NA | |
| CCRI-19656 | Colorless | − | NA | |
| 4 | CCRI-19624 | Pink | + | NA |
| CCRI-19625 | Pink | + | NA | |
| CCRI-19626 | Pink | + | NA | |
| CCRI-19627 | Pink | + | NA | |
| CCRI-19628 | Pink | + | NA | |
| CCRI-19630 | Pink | + | NA | |
| CCRI-19631 | Pink | + | NA | |
| CCRI-19632 | Pink | + | NA | |
| CCRI-19633 | Pink | + | NA | |
| CCRI-19634 | Pink | + | NA | |
| CCRI-19635 | Pink | + | NA | |
| CCRI-19636 | Pink | + | NA | |
| CCRI-19637 | Pink | + | NA | |
| CCRI-19638 | Pink | + | NA | |
| DNG | Pink | ND | NA | |
| CCRI-19639 | Pink | + | NA | |
| CCRI-19640 | Pink | + | NA | |
| CCRI-19657 | Pink | + | NA | |
| CCRI-19641 | Pink | + | NA | |
| CCRI-19658 | Pink | + | NA | |
| CCRI-19665 | Pink | + | NA | |
| CCRI-19642 | Pink | + | NA | |
| CCRI-19643 | Pink | + | NA | |
| DNG | Pink | ND | NA | |
| DNG | Pink | ND | NA | |
| CCRI-19666 | Pink | + | NA | |
| CCRI-19660 | Pink | + | NA | |
| CCRI-19644 | Pink | + | NA | |
| CCRI-19645 | Pink | + | NA | |
| CCRI-19646 | Pink | + | NA | |
| CCRI-19667 | Pink | + | NA | |
| CCRI-19647 | Pink | + | NA | |
| CCRI-19662 | Pink | + | NA | |
| CCRI-19720 | Pink | + | NA | |
| CCRI-19650 | Pink | + | NA | |
| CCRI-19723 | Pink | + | NA | |
| CCRI-19651 | Pink | + | NA | |
| CCRI-19652 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-19653 | Colorless | + | C. perfringens |
The identification of discordant strains (mCP+/rtPCR− or mCP−/rtPCR+) was done by 16S rRNA gene sequence analysis. CCRI, culture collection of Centre de Recherche en Infectiologie de l'Université Laval; NA, not applicable; DNG, did not grow upon isolation attempt; ND, not done.
Table 3.
Phenotypes and cpa rtPCR profiles of putative C. perfringens isolates found on mCP agar from river water samplesa
| Sample | Reference strain | Phenotype on mCP agar | C. perfringens cpa rtPCR result | 16S rRNA gene-based identification |
|---|---|---|---|---|
| 5 | CCRI-20251 | Pink | − | C. perfringens |
| CCRI-20253 | Pink | + | NA | |
| CCRI-20304 | Pink | + | NA | |
| CCRI-20305 | Pink | − | C. sardiniense | |
| CCRI-20306 | Pink | + | NA | |
| CCRI-20307 | Colorless | − | NA | |
| CCRI-20308 | Colorless | + | C. perfringens | |
| CCRI-20309 | Colorless | − | NA | |
| CCRI-20310 | Colorless | + | C. perfringens | |
| CCRI-20328 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20313 | Pink | + | NA | |
| CCRI-20314 | Pink | + | NA | |
| CCRI-20315 | Pink | + | NA | |
| CCRI-20316 | Pink | + | NA | |
| CCRI-20317 | Pink | + | NA | |
| CCRI-20318 | Pink | + | NA | |
| DNG | Pink | ND | NA | |
| DNG | Pink | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20339 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20324 | Colorless | − | NA | |
| CCRI-20325 | Colorless | − | NA | |
| CCRI-20326 | Colorless | − | NA | |
| CCRI-20327 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20329 | Colorless | − | NA | |
| CCRI-20330 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20335 | Colorless | − | NA | |
| CCRI-20336 | Colorless | − | NA | |
| CCRI-20337 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20329 | Colorless | − | NA | |
| CCRI-20342 | Colorless | − | NA | |
| CCRI-20343 | Colorless | − | NA | |
| CCRI-20344 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20349 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20353 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20356 | Colorless | − | NA | |
| DNG | Colorless | ND | NA | |
| CCRI-20358 | Pink | + | NA | |
| CCRI-20359 | Pink | + | NA | |
| CCRI-20360 | Colorless | − | NA | |
| CCRI-20361 | Colorless | − | NA | |
| CCRI-20362 | Colorless | − | NA | |
| CCRI-20363 | Colorless | − | NA | |
| CCRI-20364 | Colorless | − | NA | |
| CCRI-20365 | Colorless | − | NA | |
| CCRI-20366 | Colorless | − | NA | |
| CCRI-20367 | Colorless | − | NA | |
| CCRI-20368 | Colorless | − | NA | |
| CCRI-20369 | Colorless | − | NA | |
| CCRI-20370 | Colorless | − | NA | |
| CCRI-20371 | Colorless | − | NA | |
| CCRI-20372 | Colorless | − | NA | |
| CCRI-20373 | Colorless | − | NA | |
| CCRI-20374 | Colorless | − | NA | |
| CCRI-20375 | Colorless | − | NA | |
| CCRI-20376 | Colorless | − | NA | |
| CCRI-20377 | Colorless | − | NA | |
| CCRI-20378 | Colorless | − | NA | |
| CCRI-20379 | Colorless | − | NA | |
| CCRI-20380 | Colorless | − | NA |
The identification of discordant strains (mCP+/rtPCR− or mCP−/rtPCR+) was done by 16S rRNA gene sequence analysis. CCRI, culture collection of Centre de Recherche en Infectiologie de l'Université Laval; NA, not applicable; DNG, did not grow upon isolation attempt; ND, not done.
Fig 1.
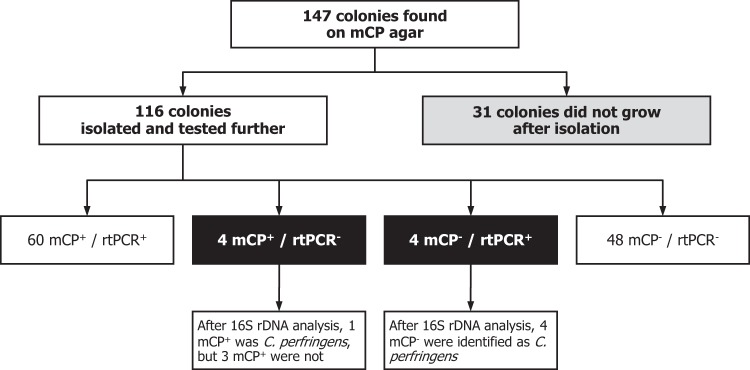
Schematic overview of the comparative study. The identities of discordant strains (mCP+/rtPCR− and mCP−/rtPCR+) were determined by 16S rRNA gene (rDNA) nucleotide sequence analysis.
Table 4.
Primers and probes used in this study
| rtPCR assay (target) | Primers and probe | Sequence (5′ → 3′)a | Reference |
|---|---|---|---|
| cpa (C. perfringens) | CpaTQ1 | CTAGATATGAATGGAAGAGGAAACTA | 25 |
| CpaTQ2 | TTAGCAGGATGATATGGAGTATCTATATCTC | ||
| CpaTQP | FAM-AAACAAGCTACATTCTATCTTGGAGAGGCTATGCACTATT-BHQ-1 | ||
| tuf (E. coli/Shigella) | TEcol553 | TGGGAAGCGAAAATCCTG | 28 |
| TEcol754 | CAGTACAGGTAGACTTCTG | ||
| TEcol573-T1-B1 | TET-AACTGGCTGGCTTCCTGG-BHQ-1 | ||
| atpD (B. atrophaeus subsp. globigii) | ABgl158 | CACTTCATTTAGGCGACGATACT | 31 |
| ABgl345a | TTGTCTGTGAATCGGATCTTTCTC | ||
| ABgl-T1-A1 | FAM-CGTCCCAATGTTACATTACCAA-CCGGCACT-(BHQ-1)-GAAATAGG | ||
| cpa (C. perfringens) | cpaCperseq298 | CCTGATACARATMATAATTTYWCAAAGG | This study |
| cpaCperseq961 | CATAAARTCATTTCCWGGGTTRTC | ||
| Small-subunit (16S) rRNA gene (bacteria) | SSU27 | AGAGTTTGATCMTGGCTCAG | 41 |
| SSU1492 | TACGGYTACCTTGTTACGACTT | ||
| SSU536b | GTGCCAGCMGCCGCGGTAATAC | ||
| SSU685b | TCTACGCATTTCACYGCTAC | ||
| SSU926b | AAACTYAAAKGAATTGACGG |
FAM, 6-carboxyfluorescein (fluorescent reporter dye); BHQ-1, Black Hole Quencher-1 (fluorescence quencher dye); TET, tetrachlorofluorescein (fluorescent reporter dye); R, A/G; M, A/C; W, A/T; Y, C/T; K, G/T.
Internal sequencing primer.
Components and parameters of the real-time PCR test. (i) Real-time PCR primers and probes.
The nucleotide sequences of rtPCR primers and probes used in this study to detect C. perfringens DNA and spores, as well as E. coli cells and Bacillus atrophaeus subsp. globigii spores used as a process control, are shown in Table 4. Oligonucleotide primers and probes were synthesized by Integrated DNA Technologies (Coralville, IA, USA).
(ii) Real-time PCR assay.
One microliter of bacterial suspension or of whole-genome amplification (WGA)-amplified products was transferred directly to a 24-μl rtPCR mixture containing 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 0.1% Triton X-100, 2.5 mM MgCl2, 0.4 μM primers, 0.2 μM probe, 200 μM each deoxyribonucleoside triphosphate (GE Healthcare Bio-Sciences Inc., Baie d'Urfé, Québec, Canada), 3.3 μg/μl of bovine serum albumin (BSA) (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada), 0.025 enzyme unit of Taq DNA polymerase (Promega, Madison, WI, USA), and TaqStart antibody (Clontech Laboratories, Mountain View, CA, USA). For each experiment, 1 μl of sterile water was added to the rtPCR mixture as a negative control. The C. perfringens and B. atrophaeus subsp. globigii rtPCR mixtures were subjected to thermal cycling in a Rotor Gene 3000 (Corbett Life Science, Sidney, Australia) under the following conditions: 3 min at 95°C and then 45 cycles (35 cycles for specificity and ubiquity analysis) consisting of a denaturation step of 15 s at 95°C and an annealing step of 60 s at 60°C. The E. coli/Shigella rtPCR mixture was subjected to thermal cycling for 1 min at 95°C and then to 35 cycles of 2 s at 95°C, 10 s at 58°C, and 20 s at 72°C.
Process control.
A process control consisting of approximately 60 spores/100 ml of B. atrophaeus subsp. globigii CCRI-9827 (equivalent to strain NRS1221 A) was added to all water samples prior to filtration. Spores were prepared as described by Picard et al. (31). The detection of B. atrophaeus subsp. globigii serves to monitor for the integrity of the sample preparation method and nucleic acid extraction and for the absence of inhibition of the molecular enrichment process by WGA, as well as target amplification by rtPCR.
Determination of the analytical limit of detection (LOD) of CRENAME + cpa rtPCR method. (i) Preparation of Clostridium perfringens spores.
A C. perfringens cell suspension was prepared by inoculating liquid thioglycolate medium with C. perfringens CCRI-16107 and incubating the culture under aerobic conditions for 24 h at 37°C, as described by Tórtora (32). In our hands, C. perfringens CCRI-16107 had better spore generation yields than the type strain (ATCC 13124T). Sporulating cultures of C. perfringens were prepared by inoculating 0.5 ml of an overnight culture into 10 ml of Duncan-Strong sporulation medium at pH 8.5 (33, 34) and by incubating the culture under aerobic conditions for 24 h at 37°C to induce the formation of spores. Finally, spore preparations were heated at 75°C for 20 min, rapidly cooled on ice, concentrated by low-speed centrifugation, purified by repeated washing with sterile distilled water until they were >99% free of sporulating cells, cell debris, and germinated spores (based on microscopic analysis), and stored at 4°C (35, 36).
(ii) Preparation of water samples spiked with C. perfringens spores.
To determine the analytical limit of detection (LOD) of the mCP agar and CRENAME + cpa rtPCR methods, spiked samples were made by inoculating water with predetermined numbers of C. perfringens spores, on the basis of a count obtained with a Petroff-Hauser chamber, to produce suspensions having approximately 300, 150, 75, 25, 12, 5, 2, 1, 0.5, and 0.1 CFU/100 ml (Table 5). The drinking water used for the concentration and recovery method experiments was ozonated spring water from Sainte-Marie-de-Blandford (Comté de Bécancour, Québec, Canada) (total dissolved mineral salt content, 60 ppm [40 mg/liter HCO3−, 11 mg/liter Ca2+, 1 mg/liter Cl−, 0.1 mg/liter F−, 2.7 mg/liter Mg2+, 1 mg/liter K+, 3 mg/liter Na+, and 8 mg/liter SO4−]). The bacterial spore count was verified by filtering 100 ml of each spiked water sample through a GN-6 membrane filter (47-mm diameter, 0.45-μm pore size; Pall Corporation, Mississauga, Ontario, Canada) with a standard platform manifold (Millipore Corporation, Billerica, MA, USA). The filter was then incubated on sheep blood agar plates for 24 ± 2 h at 35.0 ± 0.5°C in an anaerobic chamber prior to the determination of colony counts. Spiked samples were tested on mCP agar and by CRENAME + cpa rtPCR.
Table 5.
Determination of the analytical limit of detection of C. perfringens on mCP agar and by the CRENAME + cpa rtPCR procedure
|
C. perfringens count (CFU/100 ml) |
WGA rtPCR (presence/absence for each replicate) |
|||
|---|---|---|---|---|
| Estimated | On mCP agar | |||
| 300 | 329 | + | + | + |
| 150 | 154 | + | + | + |
| 75 | 74 | + | + | + |
| 25 | 24 | + | + | + |
| 12 | 12 | + | + | + |
| 5 | 4 | + | + | + |
| 2 | 2 | + | + | − |
| 1 | 1 | + | − | − |
| 0.5 | 0 | + | − | − |
| 0.1 | 0 | − | − | − |
| Negative control | 0 | − | − | − |
Membrane filtration.
Membrane filtration is the water sample concentration step used for both classical (mCP agar) and molecular (CRENAME + cpa rtPCR) microbiology methods. For the detection limit determination study, for example, each 400-ml sample spiked with C. perfringens spores was subdivided into four subsamples of 100 ml separately filtered on GN-6 membranes with a standard platform manifold. Tests to confirm the sterility of filter membranes and buffer used for rinsing the filtration apparatus were also performed. One filter was used for mCP agar method, while 3 filters (triplicates) were processed by the CRENAME water sample preparation procedure before subjecting the WGA-amplified nucleic acid sample to specific detection of C. perfringens by rtPCR. Since DNA is nonspecifically amplified by WGA, real-time PCR is used for amplicon detection, not for quantification. It is well known that the closed-tube format of rtPCR may significantly minimize cross-contamination.
To perform the mCP agar method, a membrane filter is placed onto mCP solid medium and incubated under anaerobic conditions at 44.5 ± 0.2°C for 24 ± 2 h (37). After incubation, filtration membranes containing straw yellow-colored colonies are transferred to pads saturated with ammonium hydroxide in a chemical fume hood. Following a 15-s exposure, colonies that turn dark pink to magenta are counted as C. perfringens. Quality control for each batch of mCP agar was performed, and filter, buffer, and rinse water blanks were included as sterility controls. For performing CRENAME on concentrated water samples, GN-6 filters were aseptically transferred to 3 individual 15-ml polypropylene tubes (Sarstedt, Newton, NC, USA) and processed as described by Maheux et al. (27, 28).
Simultaneous detection of C. perfringens and E. coli in drinking water.
To determine if CRENAME coupled to rtPCR can simultaneously detect C. perfringens spores and E. coli cells from the same drinking water sample, 30 different well water samples were collected in the Québec City area and blindly spiked by the CEAEQ with diluted sewage (10−1 in phosphate-buffered saline [PBS]) to produce 10 well water samples containing approximately 40 CFU/100 ml and 10 well water samples containing approximately 80 CFU/100 ml of C. perfringens. E. coli counts exceeded 200 CFU/100 ml. Ten well water samples were not spiked. These samples were filtered on GN-6 membranes and prepared by CRENAME before aliquots of WGA-amplified DNA were used as input in independent rtPCRs for the detection of cpa and tuf gene targets.
Statistical analysis.
Statistical analysis by logistic regression was done using the JMP v8.0 software (38).
RESULTS AND DISCUSSION
Analytical performance of the C. perfringens cpa rtPCR assay.
The C. perfringens-specific rtPCR assay used in this study, targeting the C. perfringens alpha-toxin gene (cpa) (25), was previously validated by Grant et al. (39) against a panel composed of 253 C. perfringens isolates, 19 isolates selected from 14 other Clostridium species, and Listeria monocytogenes and Bacillus sp. isolates. The cpa gene was (i) detected by rtPCR in all 253 C. perfringens cultures that had been identified as C. perfringens by standard microbiological tests and (ii) not amplified from the 14 Clostridium species other than C. perfringens; that work reported specificity and sensitivity rates of 100% for the cpa rtPCR assay.
In our study, the analytical specificity and the ubiquity of the cpa rtPCR assay were verified using a bacterial panel of clinical and environmental strains consisting of 54 non-C. perfringens species of the Clostridium genus and 37 C. perfringens strains (Table 1). None of the 54 non-C. perfringens strains tested positive with the cpa rtPCR assay, for an analytical specificity of 100%, whereas the cpa rtPCR assay tested positive with 37 of 37 C. perfringens strains, for an ubiquity of 100%.
Phenotypic and molecular characterization of C. perfringens isolates.
Of the 116 colonies recovered from mCP agar, 64 were positive (yellow initially but pink after exposure to ammonium hydroxide) and 52 were negative (colorless) (Tables 2 and 3 and Fig. 1). Both mCP agar and the cpa rtPCR assay were negative for 48 of 52 colorless colonies and positive for 60 of 64 pink colonies. After the identification of the colonies presenting discordant results between culture and molecular assay by 16S rRNA gene sequencing, 3 pink colonies were identified as Clostridium baratii, whereas 4 colorless colonies were identified as C. perfringens (Fig. 1 and 2). Thus, mCP agar was positive with 61 of 65 C. perfringens strains as well as 3 of 51 non-C. perfringens strains, and with these 116 colonies on mCP agar, the cpa rtPCR assay was negative with all 51 non-C. perfringens strains and positive with 64 of 65 C. perfringens strains.
Fig 2.
Phylogenetic tree based on a 1,384-bp portion of the 16S rRNA genes of discordant or atypical C. perfringens strains isolated during this study. Strains CCRI-19609, CCRI-19622, CCRI-20305, CCRI-19334, and CCRI-20251 were mCP+/rtPCR−. Strains CCRI-19617, CCRI-19653, CCRI-20308, and CCRI-20310 were mCP−/rtPCR+. For clarity, the original species identification of discordant strains has been conserved in the phylogenetic tree. The phylogenetic analysis was performed with the neighbor-joining method, and evolutionary distances were computed using the maximum composite likelihood model of the MEGA version 5.01 software. The topological accuracy of the tree was evaluated using 500 bootstrap replicates. Bootstrap values lower than 70% are not shown. GenBank accession numbers are given in parentheses.
The nucleotide sequence of the 16S rRNA gene of the only C. perfringens strain (CCRI-20251) that was not detected by the cpa rtPCR assay was 99.4% identical to that of the type strain (Fig. 2). No phenotypic test was performed on this discordant strain. However, we sequenced the cpa gene, and using a 635-bp region, we found that this gene is 96.4% identical to that of C. perfringens SWCP, a strain isolated from an avian source (40). The cpa gene of C. perfringens SWCP is 85.5% identical to its homolog of the C. perfringens type strain (Fig. 3). When we aligned the primers and probe designed by Amar et al. (25) to the cpa sequence of strain CCRI-20251, 6 mismatches were observed with forward and reverse primers. Mismatches were also observed with the internal TaqMan detection probe, thereby providing an explanation for the negative cpa rtPCR result with C. perfringens CCRI-20251. To date, it has been difficult to establish the impact of this strain on the sensitivity of the cpa rtPCR assay, since its prevalence in water remains unknown.
Fig 3.
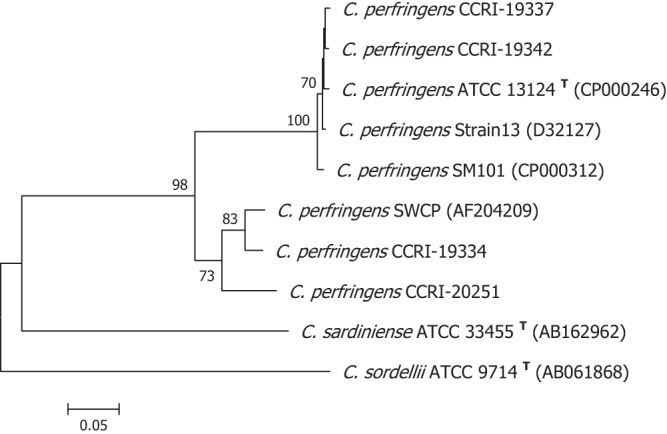
Phylogenetic tree based on a 632-bp portion of the cpa genes from C. perfringens strains. Atypical strain CCRI-20251 was identified as C. perfringens on mCP agar and by 16S rRNA gene analysis but was not detected by the cpa rtPCR assay. C. sardiniensis and C. sordellii alpha-toxin genes are represented as outgroups. The phylogenetic analysis was performed with the neighbor-joining method, and evolutionary distances were computed using the maximum composite likelihood model of the MEGA version 5.01 software. The topological accuracy of the tree was evaluated using 500 bootstrap replicates. Bootstrap values lower than 70% are not shown. GenBank accession numbers are given in parentheses.
Determination of the analytical LOD of the CRENAME + cpa rtPCR method.
The limit of detection (LOD) of the CRENAME-cpa rtPCR was evaluated by testing 100-ml well water samples spiked with different concentrations of C. perfringens CCRI-16107 spores. C. perfringens spores were always detected down to concentrations as low as 4 spores per 100 ml. Compared to colony count on mCP agar plates, the complete molecular microbiology method was able to detect as few as 1 spore per 100 ml (Table 5), which corresponds to the microbiological criterion for this indicator microorganism in Europe (21). No signal was observed with negative controls, and the process control tested positive for every sample. The LOD at 95% confidence for C. perfringens spores, as determined by logistic regression, was estimated to be 3.57 spores/100 ml.
Simultaneous detection of C. perfringens and E. coli in drinking water.
Enteric viruses, Cryptosporidium parvum oocysts, and Giardia intestinalis cysts can survive for several months in water. On one hand, the high persistence of Cryptosporidium and Giardia (oo)cysts limits the usefulness of the fecal contamination indicator E. coli, traditionally used to determine the microbial safety of water, while on the other hand, the rapid die-off of E. coli and fecal enterococci makes these parameters less suitable as indicators of the presence of waterborne pathogens such as enteric viruses, C. parvum oocysts, and G. intestinalis cysts (3, 14, 23). Since C. perfringens (spores) can survive in water for periods of time similar to those for the latter pathogens, their detection could provide a better indication of their presence in water than current fecal contamination indicators and thus a more adequate estimation of the public health risk (3, 14), but this issue is still controversial (13).
In consideration of these facts and taking into account that the determination of a single indicator is unlikely to be strategically appropriate in all instances, the recognition of C. perfringens as an indicator microorganism to predict the presence of waterborne pathogens that are more persistent and disinfection resistant than E. coli should stimulate the development and implementation of methods enabling multiparametric detection of fecal contamination indicators and microbial pathogens from a single water sample, to avoid equivocal results due to the stochastic distribution of microbial particles at very low concentrations (∼1 CFU/100 ml). Multiple analysis is not compatible with current classical microbiology approaches for testing drinking water, since a 100-ml sample cannot be tested on many different media, but we believe that molecular microbiology tests, in the form of multiplex rtPCRs or microarrays, could be tailored to detect many indicators and pathogens in a time- and cost-effective manner.
The ability of the CRENAME + rtPCR to simultaneously detect C. perfringens cpa and E. coli tuf genes in drinking water was verified by testing 30 drinking well water samples blindly spiked by CEAEQ with diluted sewage to produce suspensions having approximately 80, 40, and 0 CFU/100 ml of C. perfringens (10 samples with each concentration; E. coli counts exceeded 200 CFU/100 ml when sewage was added to drinking water samples). Before spiking, all well water samples tested negative for the presence of C. perfringens and E. coli, whereas 20 water samples tested positive after spiking with diluted sewage, corresponding to the 20 well water samples spiked by the CEAEQ. The process control, B. atrophaeus subsp. globigii, was detected in all instances, and the cycle thresholds (CTs) obtained with C. perfringens-positive samples were similar (data not shown) indicating that inhibitory substances present in well water were either (bio)chemically equivalent or not in sufficiently high concentrations to inhibit the WGA or rtPCR process.
Conclusions.
As CRENAME provides a nucleic acid sample (WGA-amplified DNA) that can be interrogated repetitively, technologies such as multiplex rtPCR or microarrays could be used for the simultaneous detection of many fecal contamination indicators, including C. perfringens, and pathogens from the same water sample, thereby providing a novel, time-efficient solution to better assess the microbial quality of drinking water. As C. perfringens is recognized to provide an indication of more persistent and disinfection-resistant waterborne pathogens such as enteric viruses and protozoan (oo)cysts, a CRENAME multiplex rtPCR test detecting E. coli and C. perfringens could be developed to yield a better assessment of the public health risk posed by drinking water potentially contaminated with pathogens.
ACKNOWLEDGMENTS
We thank Éric Jubinville and Chantal Fauvel for technical support.
This research was supported by grant PA-15586 from the Canadian Institutes of Health Research (CIHR) and by grant FCI-5251 from the Canada Foundation for Innovation (CFI). Andrée F. Maheux was supported by a scholarship from Nasivvik (Center for Inuit Health and Changing Environment, Canadian Institutes for Health Research).
Footnotes
Published ahead of print 27 September 2013
REFERENCES
- 1.Chao KK, Chao CC, Chao WL. 2003. Suitability of the traditional microbial indicators and their enumerating methods in the assessment of fecal pollution of subtropical freshwater environments. J. Microbiol. Immunol. Infect. 36:288–293 [PubMed] [Google Scholar]
- 2.Payment P, Armon R. 1989. Virus removal by drinking water treatment processes. Crit. Rev. Environ. Control 19:15–31 [Google Scholar]
- 3.Payment P, Franco E. 1993. Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl. Environ. Microbiol. 59:2418–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill RT, Knight IT, Anikis MS, Colwell RR. 1993. Benthic distribution of sewage-sludge indicated by Clostridium perfringens at a deep-ocean dump site. Appl. Environ. Microbiol. 59:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul JH, Rose JB, Jiang S, Kellogg C, Shinn EA. 1995. Occurrence of fecal indicator bacteria in surface waters and the subsurface aquifer in Key Largo, Florida. Appl. Environ. Microbiol. 61:2235–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards DD, McFeters GA, Venkatesan MI. 1998. Distribution of Clostridium perfringens and fecal sterols in a benthic coastal marine environment influenced by the sewage outfall from McMurdo Station, Antarctica. Appl. Environ. Microbiol. 64:2596–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujioka RS. 2001. Monitoring costal marine waters for spore-forming bacteria of faecal and soil origin to determine point from non-point source pollution. Water Sci. Technol. 44:181–188 [PubMed] [Google Scholar]
- 8.Lipp EK, Farrah SA, Rose JB. 2001. Assessment and impact of microbial fecal pollution and human enteric pathogens in a coastal community. Mar. Pollut. Bull. 42:286–293 [DOI] [PubMed] [Google Scholar]
- 9.Byamukama D, Mach RL, Kansiime F, Manafi M, Farnleitner AH. 2005. Discrimination efficacy of fecal pollution detection in different aquatic habitats of a high-altitude tropical country, using presumptive coliforms, Escherichia coli, and Clostridium perfringens spores. Appl. Environ. Microbiol. 71:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookes JD, Hipsey MR, Burch MD, Regel RH, Linden LG, Ferguson CM, Antenucci JP. 2005. Relative value of surrogate indicators for detecting pathogens in lakes and reservoirs Environ. Sci. Technol. 39:8614–8621 [DOI] [PubMed] [Google Scholar]
- 11.Wiedenmann A, Kruger P, Dietz K, Lopez-Pila JM, Szewzyk R, Botzenhart K. 2006. A randomized controlled trial assessing infectious disease risks from bathing in fresh recreational waters in relation to the concentration of Escherichia coli, intestinal enterococci, Clostridium perfringens, and somatic coliphages. Environ. Health Perspect. 114:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payment P, Locas A. 2011. Pathogens in water: value and limits of correlation with microbial indicators. Ground Water 49:4–11 [DOI] [PubMed] [Google Scholar]
- 13.Füschlin HP, Kötzscj S, Egli T. 2012. Cryptosporidium spp. in drinking water. Samples from rural sites in Switzerland. Swiss Med. Wkly. 10.4414/smw.2012.13683 [DOI] [PubMed] [Google Scholar]
- 14.Manafi M, Waldherr K, Kundi M. 2013. Evaluation of CP Chromo Select agar for the enumeration of Clostridium perfringens from water. Int. J. Food Microbiol. 10.1016/j.ijfoodmicro.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 15.Bisson JW, Cabelli CJ. 1979. Membrane filter enumeration method for Clostridium perfringens. Appl. Environ. Microbiol. 37:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armon R, Payment P. 1988. A modified m-CP medium for enumerating Clostridium perfringens from water samples Can. J. Microbiol. 34:78–79 [DOI] [PubMed] [Google Scholar]
- 17.Adcock PW, Saint CP. 2001. Rapid confirmation of Clostridium perfringens by using chromogenic and fluorogenic substrates. Appl. Environ. Microbiol. 67:4382–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araujo M, Sueiro RA, Gomez MJ, Garrido MJ. 2001. Evaluation of fluorogenic TSC agar for recovering Clostridium perfringens in groundwater samples. Water Sci. Technol. 43:201–204 [PubMed] [Google Scholar]
- 19.Wohlsen T, Bayliss J, Gray B, Bates J, Katouli M. 2006. Evaluation of an alternative method for the enumeration and confirmation of Clostridium perfringens from treated and untreated sewages. Lett. Appl. Microbiol. 42:438–444 [DOI] [PubMed] [Google Scholar]
- 20.Mueller-Spitz SR, Stewart LB, McLellan SL. 2010. Reliability of mCP method for identification of Clostridium perfringens from faecal polluted aquatic environments. J. Appl. Microbiol. 108:1994–2002 [DOI] [PubMed] [Google Scholar]
- 21.Council of the European Union 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Commun. L330:32–54 [Google Scholar]
- 22.World Health Organization 2001. Water quality: guidelines, standards and health. Assessment of risk and risk management for water-related infectious diseases. IWA Publishing, London, United Kingdom, ISBN: 1 900222 28 0 [Google Scholar]
- 23.Wu J, Long SC, Das D, Dorner SM. 2011. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J. Water Health 9:265–278 [DOI] [PubMed] [Google Scholar]
- 24.Tansuphasiri U. 2001. Development of duplex PCR assay for rapid detection of enterotoxigenic isolates of Clostridium perfringens. Southeast Asian J. Trop. Med. Public Health 32:105–113 [PubMed] [Google Scholar]
- 25.Amar CF, East CL, Grant KA, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. 2005. Detection of viral, bacterial, and parasitological RNA or DNA of nine intestinal pathogens in fecal samples archived as part of the English infectious intestinal disease study: assessment of the stability of target nucleic acid. Diagn. Mol. Pathol. 14:90–96 [DOI] [PubMed] [Google Scholar]
- 26.Tantawiwat S, Tansuphasirl U, Wongwit W, Wongchotigul V, Kitayaporn D. 2005. Development of multiplex PCR for the detection of total coliform bacteria for Escherichia coli and Clostridium perfringens in drinking water. Southeast Asian J. Trop. Med. Public Health 36:162–169 [PubMed] [Google Scholar]
- 27.Maheux AF, Bissonnette L, Boissinot M, Bernier J-LT, Huppé V, Bérubé È, Boudreau DK, Picard FJ, Huletsky A, Bergeron MG. 2011. Method for rapid and sensitive detection of Enterococcus sp. and Enterococcus faecalis/faecium cells in potable water samples. Water Res. 45:2342–2354 [DOI] [PubMed] [Google Scholar]
- 28.Maheux AF, Bissonnette L, Boissinot M, Bernier J-LT, Huppé V, Picard FJ, Bérubé È, Bergeron MG. 2011. Rapid concentration and molecular enrichment approach for sensitive detection of Escherichia coli and Shigella species in potable water samples. Appl. Environ. Microbiol. 77:6199–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isabel S, Leblanc É, Boissinot M, Boudreau DK, Grondin M, Picard FJ, Martel ÉA, Parham N, Chain PSG, Bader DE, Mulvey MR, Bryden L, Roy PH, Ouellette M, Bergeron MG. 2008. Divergence among genes encoding the elongation factor Tu of Yersinia species. J. Bacteriol. 190:7548–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard FJ, Gagnon M, Bernier MR, Parham NJ, Bastien M, Boissinot M, Peytavi R, Bergeron MG. 2009. Internal control for nucleic acid testing based on the use of purified Bacillus atrophaeus subsp. globigii spores. J. Clin. Microbiol. 47:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tórtora JC. 1984. Alternative medium for Clostridium perfringens sporulation. Appl. Environ. Microbiol. 47:1172–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan CL, Strong DH. 1968. Improved medium for sporulation of Clostridium perfringens. Appl. Microbiol. 16:82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craven SE. 1990. The effect of the pH of the sporulation environment on the heat resistance of Clostridium perfringens spores. Curr. Microbiol. 22:233–237 [Google Scholar]
- 35.Labbé RG, Rey DK. 1979. Raffinose increases sporulation and enterotoxin production by Clostridium perfringens type A. Appl. Environ. Microbiol. 37:1196–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong AE, Beumer RR, Rombouts FM. 2002. Optimizing sporulation of Clostridium perfringens J. Food Prot. 65:1457–1462 [DOI] [PubMed] [Google Scholar]
- 37.Fout GS, Schaefer III FW, Messer JW, Dahling DR, Stetler RE. 1996. ICR microbiology laboratory manual. EPA/600/R-95/178. Office of Research and Development, US Environmental Protection Agency, Washington, DC [Google Scholar]
- 38.SAS Institute Inc 1989–2007. JMP, version 8. SAS Institute Inc., Cary, NC [Google Scholar]
- 39.Grant KA, Kenyon S, Nwafor I, Plowman J, Ohai C, Halford-Maw R, Peck MW, McLauchlin J. 2008. The identification and characterization of Clostridium perfringens by real-time PCR, location of enterotoxin gene, and heat resistance. Foodborne Pathog. Dis. 5:629–639 [DOI] [PubMed] [Google Scholar]
- 40.Justin N, Walker N, Bullifent HL, Songer G, Bueschel DM, Jost H, Naylor C, Miller J, Moss DS, Titball RW, Basak AJ. 2002. The first strain of Clostridium perfringens isolated from an avian source has an alpha-toxin with divergent structural and kinetic properties. Biochemistry 41:6253–6262 [DOI] [PubMed] [Google Scholar]
- 41.Sistek V, Boissinot M, Boudreau DK, Huletsky A, Picard FJ, Bergeron MG. 2012. Development of a real-time PCR assay for the specific detection and identification of Streptococcus pseudopneumoniae using the recA gene. Clin. Microbiol. Infect. 18:1089–1096 [DOI] [PubMed] [Google Scholar]



