Abstract
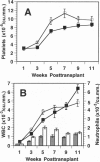
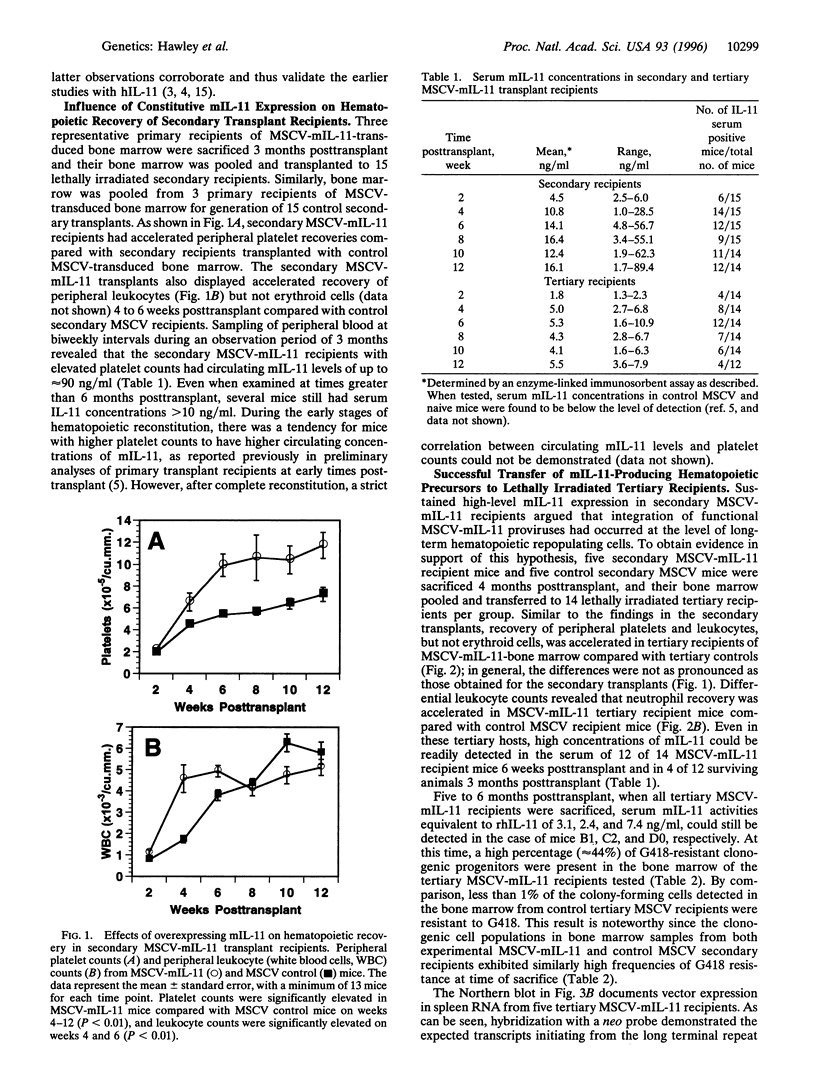
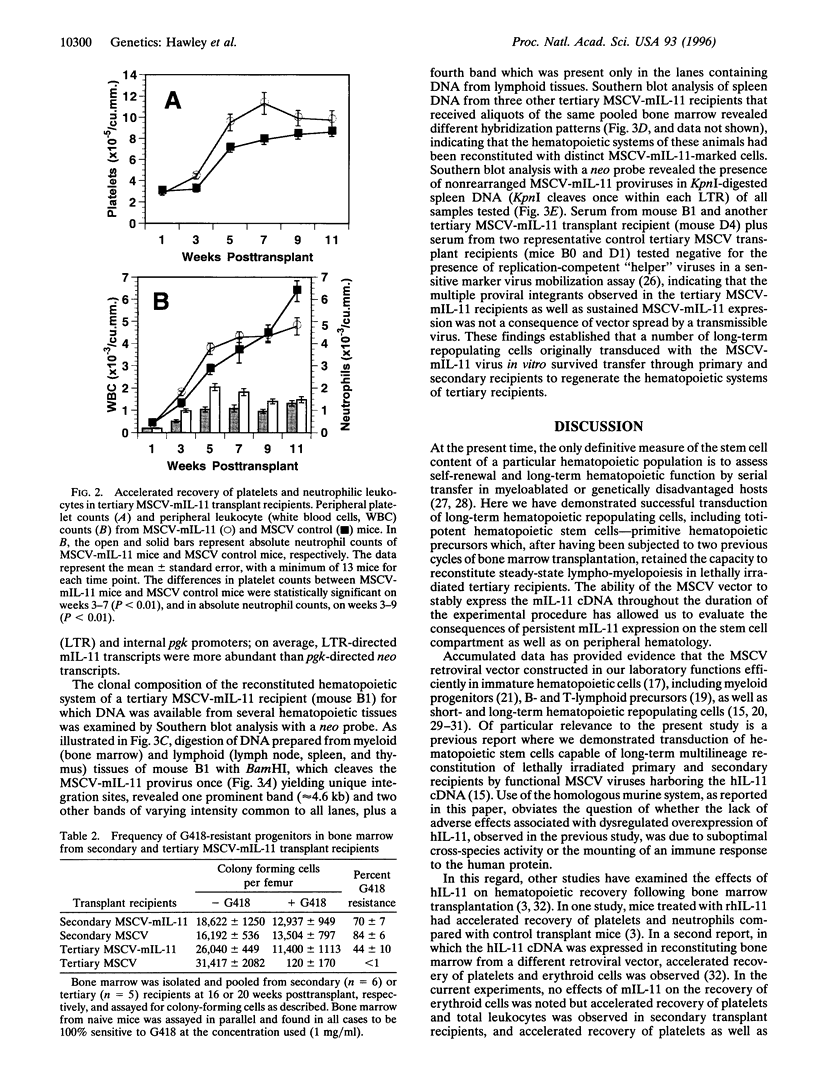
Based on transplantation studies with bone marrow cultured under various conditions, a role of interleukin 11 (IL-11) in the self-renewal and/or the differentiation commitment of hematopoietic stem cells has been indicated. To better evaluate the in vivo effects of IL-11 on stem/progenitor cell biology, lethally irradiated mice were serially transplanted with bone marrow cells transduced with a defective retrovirus, termed MSCV-mIL-11, carrying the murine IL-11 (mIL-11) cDNA and the bacterial neomycin phosphotransferase (neo) gene. High serum levels (i.e., > 1 ng/ml) of mIL-11 in all (20/20) primary and 86% (12/14) of secondary long-term reconstituted mice, as well as 86% (12/14) of tertiary recipients examined at 6 weeks posttransplant, demonstrated persistence of vector expression subsequent to transduction of bone marrow precursors functionally definable as totipotent hematopoietic stem cells. In agreement with results obtained with human IL-11 in other myeloablation models, ectopic mIL-11 expression accelerated recovery of platelets, neutrophils, and, to some extent, total leukocytes while preferentially increasing peripheral platelet counts in fully reconstituted mice. When analyzed 5 months posttransplant, tertiary MSCV-mIL-11 recipients had a significantly greater percentage of G418-resistant colony-forming cells in their bone marrow compared with control MSCV animals. Collectively, these data show that persistent stimulation of platelet production by IL-11 is not detrimental to stem cell repopulating ability; rather, they suggest that IL-11 expression in vivo may have resulted in enhanced maintenance of the most primitive hematopoietic stem cell compartment. The prolonged expression achieved by the MSCV retroviral vector, despite the presence of a selectable marker, contrasts with the frequent transcriptional extinction observed with other retroviral vectors carrying two genes. These findings have potentially important implications for clinical bone marrow transplantation and gene therapy of the hematopoietic system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley J. F., Luskey B. D., Williams D. A. Retroviral gene transfer of human adenosine deaminase in murine hematopoietic cells: effect of selectable marker sequences on long-term expression. Blood. 1991 Jul 15;78(2):310–317. [PubMed] [Google Scholar]
- Berger L. C., Hawley T. S., Lust J. A., Goldman S. J., Hawley R. G. Tyrosine phosphorylation of JAK-TYK kinases in malignant plasma cell lines growth-stimulated by interleukins 6 and 11. Biochem Biophys Res Commun. 1994 Jul 15;202(1):596–605. doi: 10.1006/bbrc.1994.1970. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Johnson G. R., Kelso A., Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med. 1987 Aug;4(4):229–250. [PubMed] [Google Scholar]
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Conneally E., Bardy P., Eaves C. J., Thomas T., Chappel S., Shpall E. J., Humphries R. K. Rapid and efficient selection of human hematopoietic cells expressing murine heat-stable antigen as an indicator of retroviral-mediated gene transfer. Blood. 1996 Jan 15;87(2):456–464. [PubMed] [Google Scholar]
- Correll P. H., Colilla S., Karlsson S. Retroviral vector design for long-term expression in murine hematopoietic cells in vivo. Blood. 1994 Sep 15;84(6):1812–1822. [PubMed] [Google Scholar]
- Du X. X., Neben T., Goldman S., Williams D. A. Effects of recombinant human interleukin-11 on hematopoietic reconstitution in transplant mice: acceleration of recovery of peripheral blood neutrophils and platelets. Blood. 1993 Jan 1;81(1):27–34. [PubMed] [Google Scholar]
- Du X. X., Scott D., Yang Z. X., Cooper R., Xiao X. L., Williams D. A. Interleukin-11 stimulates multilineage progenitors, but not stem cells, in murine and human long-term marrow cultures. Blood. 1995 Jul 1;86(1):128–134. [PubMed] [Google Scholar]
- Goldman S. J. Preclinical biology of interleukin 11: a multifunctional hematopoietic cytokine with potent thrombopoietic activity. Stem Cells. 1995 Sep;13(5):462–471. doi: 10.1002/stem.5530130503. [DOI] [PubMed] [Google Scholar]
- Hajihosseini M., Iavachev L., Price J. Evidence that retroviruses integrate into post-replication host DNA. EMBO J. 1993 Dec 15;12(13):4969–4974. doi: 10.1002/j.1460-2075.1993.tb06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. F., Hawley R. G., Hawley T. S., Crawford-Sharpe G. C. Increased frequency of both total and specific monoclonal antibody producing hybridomas using a fusion partner that constitutively expresses recombinant IL-6. J Immunol Methods. 1992 Apr 8;148(1-2):199–207. doi: 10.1016/0022-1759(92)90173-q. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982 Dec 1;156(6):1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Jordan C. T., Zhong R. K., Astle C. M. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993 Feb;21(2):206–219. [PubMed] [Google Scholar]
- Hawley R. G., Covarrubias L., Hawley T., Mintz B. Handicapped retroviral vectors efficiently transduce foreign genes into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2406–2410. doi: 10.1073/pnas.84.8.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Fong A. Z., Burns B. F., Hawley T. S. Transplantable myeloproliferative disease induced in mice by an interleukin 6 retrovirus. J Exp Med. 1992 Oct 1;176(4):1149–1163. doi: 10.1084/jem.176.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Fong A. Z., Lu M., Hawley T. S. The HOX11 homeobox-containing gene of human leukemia immortalizes murine hematopoietic precursors. Oncogene. 1994 Jan;9(1):1–12. [PubMed] [Google Scholar]
- Hawley R. G., Fong A. Z., Ngan B. Y., Hawley T. S. Hematopoietic transforming potential of activated ras in chimeric mice. Oncogene. 1995 Sep 21;11(6):1113–1123. [PubMed] [Google Scholar]
- Hawley R. G., Fong A. Z., Ngan B. Y., de Lanux V. M., Clark S. C., Hawley T. S. Progenitor cell hyperplasia with rare development of myeloid leukemia in interleukin 11 bone marrow chimeras. J Exp Med. 1993 Oct 1;178(4):1175–1188. doi: 10.1084/jem.178.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G. Hematopathology of interleukin 6-type cytokines. Stem Cells. 1994;12 (Suppl 1):155–171. [PubMed] [Google Scholar]
- Hawley R. G., Lieu F. H., Fong A. Z., Hawley T. S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994 Mar;1(2):136–138. [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- Kaleko M., Garcia J. V., Osborne W. R., Miller A. D. Expression of human adenosine deaminase in mice after transplantation of genetically-modified bone marrow. Blood. 1990 Apr 15;75(8):1733–1741. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990 May 1;171(5):1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lerner C., Harrison D. E. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990 Feb;18(2):114–118. [PubMed] [Google Scholar]
- Luskey B. D., Rosenblatt M., Zsebo K., Williams D. A. Stem cell factor, interleukin-3, and interleukin-6 promote retroviral-mediated gene transfer into murine hematopoietic stem cells. Blood. 1992 Jul 15;80(2):396–402. [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch P., Lamont C., Neben T. Y., Quinto C., Goldman S. J., Witsell A. Hematopoietic stem cells in the blood after stem cell factor and interleukin-11 administration: evidence for different mechanisms of mobilization. Blood. 1995 Dec 15;86(12):4674–4680. [PubMed] [Google Scholar]
- Musashi M., Clark S. C., Sudo T., Urdal D. L., Ogawa M. Synergistic interactions between interleukin-11 and interleukin-4 in support of proliferation of primitive hematopoietic progenitors of mice. Blood. 1991 Sep 15;78(6):1448–1451. [PubMed] [Google Scholar]
- Musashi M., Yang Y. C., Paul S. R., Clark S. C., Sudo T., Ogawa M. Direct and synergistic effects of interleukin 11 on murine hemopoiesis in culture. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):765–769. doi: 10.1073/pnas.88.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neben S., Donaldson D., Sieff C., Mauch P., Bodine D., Ferrara J., Yetz-Aldape J., Turner K. Synergistic effects of interleukin-11 with other growth factors on the expansion of murine hematopoietic progenitors and maintenance of stem cells in liquid culture. Exp Hematol. 1994 Apr;22(4):353–359. [PubMed] [Google Scholar]
- Neben T. Y., Loebelenz J., Hayes L., McCarthy K., Stoudemire J., Schaub R., Goldman S. J. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood. 1993 Feb 15;81(4):901–908. [PubMed] [Google Scholar]
- Ohashi T., Boggs S., Robbins P., Bahnson A., Patrene K., Wei F. S., Wei J. F., Li J., Lucht L., Fei Y. Efficient transfer and sustained high expression of the human glucocerebrosidase gene in mice and their functional macrophages following transplantation of bone marrow transduced by a retroviral vector. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T., Thacker J. D., Eaves C. J., Hogge D. E. Differential effects of microenvironmentally presented interleukin 3 versus soluble growth factor on primitive human hematopoietic cells. J Clin Invest. 1991 Aug;88(2):417–422. doi: 10.1172/JCI115320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. R., Bennett F., Calvetti J. A., Kelleher K., Wood C. R., O'Hara R. M., Jr, Leary A. C., Sibley B., Clark S. C., Williams D. A. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. R., Hayes L. L., Palmer R., Morris G. E., Neben T. Y., Loebelenz J., Pedneault G., Brooks J., Blue I., Moore M. A. Interleukin-11 expression in donor bone marrow cells improves hematological reconstitution in lethally irradiated recipient mice. Exp Hematol. 1994 Mar;22(3):295–301. [PubMed] [Google Scholar]
- Peters S. O., Kittler E. L., Ramshaw H. S., Quesenberry P. J. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996 Jan 1;87(1):30–37. [PubMed] [Google Scholar]
- Rivière I., Brose K., Mulligan R. C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau G., Thorsteinsdottir U., Eaves C. J., Lawrence H. J., Largman C., Lansdorp P. M., Humphries R. K. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995 Jul 15;9(14):1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Lyman S. D., Sudo T., Clark S. C., Ogawa M. Enhancement of murine hematopoiesis by synergistic interactions between steel factor (ligand for c-kit), interleukin-11, and other early acting factors in culture. Blood. 1992 Jun 1;79(11):2855–2860. [PubMed] [Google Scholar]
- Van Zant G., Scott-Micus K., Thompson B. P., Fleischman R. A., Perkins S. Stem cell quiescence/activation is reversible by serial transplantation and is independent of stromal cell genotype in mouse aggregation chimeras. Exp Hematol. 1992 May;20(4):470–475. [PubMed] [Google Scholar]
- Yan X. Q., Lacey D., Fletcher F., Hartley C., McElroy P., Sun Y., Xia M., Mu S., Saris C., Hill D. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995 Dec 1;86(11):4025–4033. [PubMed] [Google Scholar]