Abstract
Acetate-mediated growth inhibition of Escherichia coli has been found to be a consequence of the accumulation of homocysteine, the substrate of the cobalamin-independent methionine synthase (MetE) that catalyzes the final step of methionine biosynthesis. To improve the acetate resistance of E. coli, we randomly mutated the MetE enzyme and isolated a mutant enzyme, designated MetE-214 (V39A, R46C, T106I, and K713E), that conferred accelerated growth in the E. coli K-12 WE strain in the presence of acetate. Additionally, replacement of cysteine 645, which is a unique site of oxidation in the MetE protein, with alanine improved acetate tolerance, and introduction of the C645A mutation into the MetE-214 mutant enzyme resulted in the highest growth rate in acetate-treated E. coli cells among three mutant MetE proteins. E. coli WE strains harboring acetate-tolerant MetE mutants were less inhibited by homocysteine in l-isoleucine-enriched medium. Furthermore, the acetate-tolerant MetE mutants stimulated the growth of the host strain at elevated temperatures (44 and 45°C). Unexpectedly, the mutant MetE enzymes displayed a reduced melting temperature (Tm) but an enhanced in vivo stability. Thus, we demonstrate improved E. coli growth in the presence of acetate or at elevated temperatures solely due to mutations in the MetE enzyme. Furthermore, when an E. coli WE strain carrying the MetE mutant was combined with a previously found MetA (homoserine o-succinyltransferase) mutant enzyme, the MetA/MetE strain was found to grow at 45°C, a nonpermissive growth temperature for E. coli in defined medium, with a similar growth rate as if it were supplemented by l-methionine.
INTRODUCTION
Inhibition of bacterial growth and metabolism by weak organic acids is a well-known phenomenon that has been exploited for food preservation for hundreds of years (1). Undissociated weak acids can permeate the cell membrane, and once inside, they can dissociate to release anions and protons, resulting in a decrease in the intracellular pH and growth inhibition (2, 3). The anion accumulation inside the cell affects cell turgor pressure (3). Through facilitation of anion accumulation, the external pH also has a strong effect on the toxicity of weak acids. Moreover, Takahashi et al. (4) demonstrated an enhanced toxic effect of acetic acid on Escherichia coli at a lower extracellular pH level, resulting in a decreased growth rate and biomass yield.
The mechanism underlying this weak acid toxicity has not been easy to elucidate. Formic and propionic acids were found to inhibit macromolecular synthesis, particularly DNA biosynthesis (5). Weak acids have been shown to reduce the intracellular concentration of some amino acids, including glutamate, aspartate, lysine, arginine, glutamine, and methionine (3, 6). Interestingly, supplementation with exogenous methionine abrogates most of the inhibitory effects of acetate on E. coli growth (6, 7), and a similar effect has been observed when cultures are treated with either benzoate or propionate (6). Furthermore, an increased level of intracellular methionine in metK mutants almost completely protects E. coli cells against the inhibitory effect of acetate, suggesting a critical role for methionine in overcoming growth limitation or inhibition in acetate-treated cells (6). Roe et al. (6) demonstrated that the increased accumulation of l-homocysteine (HCY), the substrate of the MetE enzyme, in acetate-treated cells seriously inhibits E. coli growth, and they have proposed that MetE is a key enzyme associated with acetate-induced growth inhibition.
The MetE enzyme is a cobalamin-independent methionine synthase (EC 2.1.1.14), encoded by the metE gene, that catalyzes the final step in methionine biosynthesis in E. coli under aerobic conditions (8). Under anaerobic conditions, this reaction is driven by the MetH enzyme, a cobalamin-dependent methionine synthase (EC 2.1.1.13) encoded by the metH gene (8). Both enzymes transfer a methyl group to HCY to form methionine (8). However, the methyl donors involved in these reactions are different: 5-methyltetrahydropteroyl-tri-l-glutamate is the donor for the MetE enzyme, whereas 5-methyltetrahydrofolate is the donor for the MetH enzyme (9). The MetE enzyme catalyzes the direct transfer of the methyl group to HCY in what appears to be a catalytically less ideal solution than the use of cobalamin as a cofactor by MetH (9), as cobalamin is one of the most potent nucleophiles known (10), in contrast to the thiol of the MetE enzyme functioning as an intermediate methyl acceptor (8). The MetE enzyme is approximately 50 times less active than MetH (11), which may explain why MetE is quite abundant in E. coli cells growing aerobically in glucose minimal medium, where it accounts for approximately 3 to 5% of the total cellular protein content (12, 13).
Recent studies have shown that the MetE protein is sensitive to two types of stress conditions: elevated temperature (14) and oxidative stress (15, 16). Roe et al. (6) suggested that acetate may inhibit MetE enzyme activity, and they attempted (unsuccessfully) to protect E. coli cells through overexpression of the metE gene. Mogk et al. (14) showed that MetE is a major aggregation-prone enzyme in E. coli cells at an elevated temperature (45°C). Thus, the MetE protein could limit methionine availability under stress conditions (heat, acid, and oxidation), which leads to a slowing down of many cellular biosynthetic processes (protein, RNA, and DNA biosynthesis) and total growth arrest (17). Thermolabile MetA, another aggregation-prone protein in the methionine biosynthesis pathway (18), has been proposed as a “metabolic fuse” (19) that senses stress conditions destabilizing cellular proteins and, consequently, blocks protein synthesis via the inherent instability of MetA. The advantage of having a metabolic fuse is to spare cell energy under nonpermissive growth conditions, where the cells need to spend most of their energy for maintenance and protein quality control to survive under stress conditions (17). These observations suggested that stabilization of the inherently instable MetE protein might increase the growth temperature of E. coli and provide novel biotechnological applications for developing a microbial cell factory (20).
In the present study, we employed random mutagenesis to obtain acetate-tolerant MetE mutants. The MetE-214 mutant, which exhibited faster growth in the presence of sodium acetate, contained multiple amino acid substitutions, including V39A, R46C, T106I, and K713E. Alanine replacement of the surface-exposed cysteine 645, which has been identified as a unique site of oxidation in the MetE protein (15, 21), also improved acetate tolerance. However, the highest acetate resistance was demonstrated by the MetE-214A mutant, in which the multiple substitutions from MetE-214 and the single-site C645A substitution were combined. We found an increased tolerance to propionate but not to benzoate in E. coli WE strains harboring the mutated MetE enzymes. The acetate-tolerant MetE mutants were found to stimulate the growth of the host strain at elevated temperatures (44 and 45°C). Unexpectedly, the mutated MetE proteins displayed reduced melting temperatures (Tm) but were more stable in vivo. We propose that MetE, similar to MetA (the first enzyme in the methionine biosynthetic pathway), may serve as a “metabolic fuse” for the detection of unfavorable environmental conditions (19) and that MetE and MetA may be reengineered to improve E. coli growth under stressful conditions, including the presence of acetate and high temperatures.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The strains and plasmids employed in this study are listed in Table 1. E. coli strains were grown in minimal M9 medium (22) supplemented with glucose (0.2%) or in rich LB medium (Difco, San Jose, CA). Antibiotics were used at the following concentrations: ampicillin at 100 μg ml−1 and kanamycin at 25 μg ml−1. l-Methionine and l-isoleucine were added to the medium to a final concentration of 50 μg ml−1. Bacterial cultures were grown in 25 ml of M9 glucose medium in 125-ml Erlenmeyer flasks. Seed cultures were grown overnight at 30°C and then diluted to an optical density at 600 nm (OD600) of 0.1, after which the cells were incubated at 32, 37, or 42°C with shaking. Growth was measured by monitoring the optical density at 600 nm at 1-h intervals.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant descriptiona | Source or reference |
|---|---|---|
| Escherichia coli strains | ||
| DH5α | F− supE44 hsdR17 recA1 gyrA96 endA1 thi-1 relA1 deoR λ− | 22 |
| JW3805 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− rph-1 ΔmetE774::kan Δ(rhaD-rhaB)568 hsdR514 | Keio Collection (National Institute of Genetics, Japan) |
| WE | JW3973 carrying the metAW3110 gene | 24 |
| WEΔmetE | WE ΔmetE::kan | This study |
| WE-214 | WE carrying mutated metE-214 gene | This study |
| WE-CA | WE carrying metE gene with C645A substitution | This study |
| WE-214A | WE carrying metE-214 gene with C645A substitution | This study |
| A39 | WE carrying metE gene with V39A substitution | This study |
| C46 | WE carrying metE gene with R46C substitution | This study |
| I106 | WE carrying metE gene with T106I substitution | This study |
| E713 | WE carrying metE gene with K713E substitution | This study |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen (Billerica, MA) |
| Plasmids | ||
| pKD46 | λ Red (gam bet exo) araC rep101(Ts), Apr | 23 |
| pET22b/MetE | Expression vector contains wild-type metE gene, Apr | This study |
| pET22b/MetE214 | Expression vector contains mutated metE-214 gene, Apr | This study |
| pET22b/MetCA | Expression vector contains metE gene with C645A substitution, Apr | This study |
| pET22b/MetE214A | Expression vector contains mutated metE-214 gene with C645A substitution, Apr | This study |
Apr, ampicillin resistance; kan, kanamycin resistance gene; metAW3110, metA gene from E. coli strain W3110.
To examine the effects of weak organic acids on the growth of different E. coli strains, cells were cultivated in M9 glucose medium (pH 6.0) supplemented with sodium acetate (20 mM), sodium benzoate (4 mM), or sodium propionate (10 mM) in a TVS126MB automatic growth-measuring incubator (Advantec MFS Inc., Tokyo, Japan). The specific growth rate (μ, in h−1) was calculated through linear regression analysis of the ln(X/X0) data using Sigma Plot software, where the initial OD600 (X0) was 0.15 at the zero time point, and X represents the OD600 value, measured every 10 min in an exponentially growing culture for 1 h. All cultures were repeated twice.
Library construction through error-prone PCR and selection of acetic acid-tolerant metE mutants.
The metE gene, together with its promoter region, was amplified from genomic DNA from E. coli strain W3110 with the primers metE1 (CCGCAACGCTTGTAGCG) and metE2 (GATGGCTGGCAGCGTATG), gel purified, and employed as a template for error-prone PCR. Random mutagenesis was conducted with the GeneMorph II random mutagenesis kit (Stratagene, La Jolla, CA), using the primers metE1 and metE2 to obtain the maximal number of nucleotide substitutions per kb, according to the manufacturer's manual. The PCR product was purified with the QIAquick PCR purification kit (Qiagen, Germantown, MD), amplified one additional time using the primers MutMetE1 (CCTTCCCTAAATTCAAAATCCATAGGATTTACATATAATTAGAGGAAGAAAAAATG) and MutMetE2 (GCTGGAATGGTTTAAGCAGTATGGTGGGAAGAAGTCGCTGTAATGAGAAAAGACCGGGTGGTATTAC) and Vent polymerase, and subsequently digested with DpnI. The amplified sequence was then transfected into freshly prepared E. coli WE ΔmetE(pKD46) cells via electroporation, as described previously (23). The WEΔmetE strain was obtained through P1vir transduction of a kanamycin resistance construct from the JW3805 ΔmetE donor strain into the WE strain (24). The transformed cells were finally incubated at 37°C in M9 minimal medium plates supplemented with glucose to select clones containing a functional metE gene. The metE mutants were cultivated in 100 μl of M9 glucose medium adjusted to pH 6.0 in a BioScreen C incubator (Labsystems, Helsinki, Finland) in the presence of sodium acetate (20 mM) at 37°C for 49 h, with shaking at 15-min intervals. The growth curve for each mutant was compared to the control strain harboring the nonmutated metE gene to select acetate-tolerant clones. The candidate clones were then flask cultivated in 25 ml of M9 glucose medium (pH 6.0) supplemented with 10 mM sodium acetate at 37°C to determine the fastest-growing clones. Finally, the metE gene was amplified from the genomic DNA of the acetate-tolerant mutant and sequenced.
Construction of single-site MetE mutants.
The V39A, R46C, T106I, C645A, and K713E single-site mutants were constructed through overlap extension PCR by using the QuikChange II-E site-directed mutagenesis kit (Stratagene, La Jolla, CA) with the primers MetEA1 forward (GAAGAACTGCTGGCGGCAGGGCGTGAATTGCGTG), MetEC1 forward (CGTGAATTGCGTGCTTGTCACTGGGATCAACAAAAG). MetEI1 forward (CGTGGACGTGCGCCGATTGGCGAACCTGCGG), MetEC2 forward (CACACTCACATGTGTTATGCGGAGTTCAACGACATC), MetEE1 forward (CTGAAGAAAGCGGCAGAACGCATTCCGGCAGAGCG), and the reverse primers, completing the corresponding forward primers. The changes in the sequences are underlined. The mutated metE sequences were introduced into the E. coli WEΔmetE chromosome by using the λ Red recombination system (23).
Cloning, expression, and protein purification.
The wild-type and mutated metE sequences were cloned into the NdeI/HindIII restriction sites of the pET22b plasmid in frame with a C-terminal six-histidine tag by using the primers MetE3 (CGCCTCCATATGCCGATTCGTGTGCCG) and MetE4 (CGCCTCAAGCTTCCCCCGACGCAAGTTCTG). The plasmid DNA was purified from ampicillin-resistant clones and sequenced to verify that the correct genes had been cloned. Competent E. coli BL21(DE3) cells were then transformed with the constructed plasmids. A single colony of the E. coli BL21(DE3) strain harboring each plasmid was cultivated overnight at 30°C in 75 ml of LB medium with ampicillin. Two liters of 2× YT medium containing ampicillin and 0.5 mM zinc sulfate (25) was inoculated with an overnight culture, incubated at 30°C to an OD600 of 0.6, induced with isopropyl-β-d-thiogalactopyranoside (1 mM final concentration), and cooled to 18°C. Following overnight induction, the cells were harvested via centrifugation, and the pellets were resuspended in ice-cold buffer (50 mM Tris-HCl, 300 mM NaCl, 10 mM MgCl2, 5 mM imidazole; pH 7.5) at a ratio of 3 ml of buffer g−1 of wet cells. The cells were lysed by incubation with 1 mg ml−1 lysozyme, Halt protease inhibitor cocktail (Pierce, Rockford, IL), and DNase I at 4°C for 30 min with stirring, followed by sonication 10 times for 1 min at 30-s intervals with a Branson sonifier (model 450). The cell debris was removed via centrifugation at 13,000 × g for 30 min. The proteins were purified from the supernatants by using Ni-nitrilotriacetic acid–agarose (Qiagen, Germantown, MD). Six milliliters of agarose slurry was incubated with 20 ml of supernatant overnight at 4°C with rocking. The unbound proteins were removed through gravity filtration, and the agarose was washed with 12 ml of buffer (50 mM Tris-HCl, 300 mM NaCl, 100 mM imidazole; pH 7.5). The proteins were then released from the agarose via elution with 20 ml of buffer (50 mM Tris-HCl, 300 mM NaCl, 250 mM imidazole; pH 7.5), and the eluate was dialyzed against two changes of dialysis buffer (50 mM K-phosphate buffer, 150 mM NaCl; pH 7.6) and then concentrated with an Amicon Ultra-15 centrifugal device over a 30,000 NMWL membrane (Millipore, Billerica, MA). The presence of pure protein in all samples was confirmed via SDS-PAGE.
Differential scanning calorimetry.
The thermal stabilities of the MetE proteins were measured calorimetrically over a temperature interval of 15 to 90°C at a scan rate of 90°C/h. A VP differential scanning calorimeter (VP-DSC; MicroCal, LLC, Northampton, MA) was employed for these measurements, with 20 μM protein in 50 mM K-phosphate buffer (pH 7.5). Three scans were obtained from independent protein preparations.
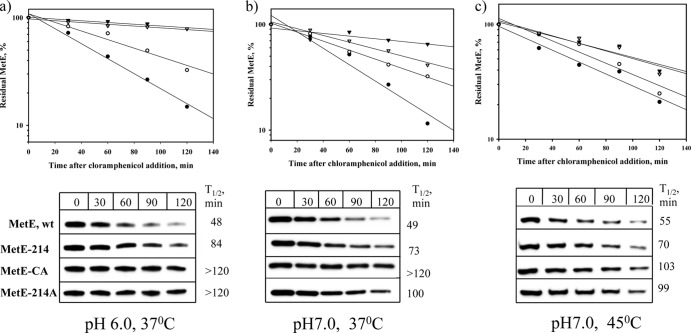
In vivo MetE stability analysis.
The WE, WE-214, WE-CA, and WE-214A strains were grown in M9 glucose medium with pH values of 6.0 and 7.0 at 37°C to exponential phase (OD600 of 0.3) and then were treated with 200 μg chloramphenicol ml−1. One-half of the pH 7.0 culture was shifted to 45°C. Samples (2 ml) were collected before and after the addition of chloramphenicol every 30 min for 2 h, centrifuged at 14,000 rpm for 5 min at 4°C, and resuspended in 50 μl of distilled water, after which 25-μl aliquots were mixed with 25 μl of 2× sample buffer, and the remaining 25 μl was used for the quantification of total protein with a Bio-Rad protein assay kit. A 3-μg sample of total protein was loaded into a 4 to 15% Criterion TGX precast gel (Bio-Rad, Hercules, CA), followed by Western blotting. A rabbit anti-MetE antibody (a generous gift from Axel Mogk) was employed as the primary antibody, and horseradish peroxidase-conjugated anti-rabbit IgG (Pierce, Rockford, IL) was employed as the secondary antibody. The immunoblots were developed using the SuperSignal West Pico chemiluminescent substrate kit (Pierce, Rockford, IL), scanned with a Fujifilm LAS-3000 image reader, and analyzed with WCIF ImageJ software.
Tryptic digestion of the MetE mutants.
Samples of the mutated MetE enzymes (1 nmol) were digested with 0.01% (wt/vol) trypsin at 37°C in a total volume of 200 μl of 20 mM potassium phosphate buffer (pH 7.2) (25). Samples were collected at the indicated time points and analyzed via SDS-PAGE. Band intensities were quantified using WCIF ImageJ software.
RESULTS
Directed evolution of MetE increases the acetate tolerance of E. coli cells.
It has been shown that the slowed growth of E. coli observed in the presence of acetate is mainly due to a malfunction of the MetE enzyme that results in accumulation of HCY, a substrate of MetE that is toxic to cells at high concentrations (6). However, overexpression of the metE gene does not protect cells against growth inhibition by acetate, revealing that the observed growth inhibition is not due to insufficient MetE enzyme activity but rather to a malfunction of MetE (6). To improve the acetate tolerance of E. coli cells, we performed random mutagenesis of the metE gene, followed by insertion of the mutant metE into the chromosome of the E. coli WEΔmetE::kan strain, as described in Materials and Methods. A mutant library consisting of 870 clones was constructed and tested for growth in the presence of sodium acetate (20 mM) by using a BioScreen C incubator. A 20 mM concentration of sodium acetate normally inhibits the growth of the E. coli WE strain in a BioScreen C incubator. One clone grew faster than the other mutants in acetate-enriched medium and was designated the WE-214 strain (Fig. 1). Sequencing analysis revealed the presence of four amino acid substitutions in the metE-214 mutant: V39A, R46C, T106I, and K713E.
Fig 1.
Effect of the mutated MetE-214 protein on E. coli growth in the presence of sodium acetate. Strains WE and WE-214 were flask cultivated in M9 glucose medium (pH 6.0) supplemented with sodium acetate (10 mM). Symbols: WE, filled circles; WE-214, open circles. The averages of two independent experiments are presented.
Previously, Hondorp and Matthews (15, 21) showed that replacement of MetE cysteine 645 with alanine completely eliminated methionine auxotrophy in oxidatively stressed cells. We hypothesized that cysteine 645 might represent a site of weak organic acid sensitivity and that replacement of this residue with alanine would further enhance the acetate tolerance of the MetE protein. Thus, we constructed the metE-CA (C645A) and metE-214A (V39A, R46C, T106I, C645A, K713E) mutant genes and inserted them into the chromosome of the WEΔmetE::kan strain. The WE-214, WE-CA, and WE-214A strains and the control WE strain harboring wild-type metE were cultured in M9 glucose medium (pH 6.0) supplemented with sodium acetate (20 mM), sodium benzoate (4 mM), or sodium propionate (10 mM) in an automatic growth-measuring incubator at 37°C. As shown in Fig. 2 and Table 2, the mutants grew faster than the control strain in the presence of sodium acetate or sodium propionate. The WE-214A mutant that harbored all of the amino acid substitutions present in MetE-214 as well as C645A demonstrated the highest resistance among the three tested mutants (Fig. 2; Table 2). Supplementation of the culture medium with sodium benzoate did not reveal any differences between the control and mutant strains (Table 2). Apparently, this compound has another target in the methionine biosynthesis pathway, as the addition of methionine has been found to relieve the inhibitory effect of benzoate on E. coli growth (6). Price-Carter et al. (19) proposed that, in Salmonella enterica, benzoate inhibits the first enzyme in the methionine biosynthesis pathway (MetA).
Fig 2.
Effects of the mutated MetE enzymes on E. coli growth in the presence of weak organic acids. The WE (filled circles), WE-214 (open circles), WE-CA (filled triangles), and WE-214A (open triangles) strains were incubated in M9 glucose medium (pH 6.0) supplemented with 20 mM sodium acetate (a) or 10 mM sodium propionate (b) in an automatic growth-measuring incubator at 37°C for 28 h. The averages of two independent experiments are presented.
Table 2.
Effects of mutated MetE proteins on growth of E. coli strain WE in the presence of weak organic acids
| Mediuma | Specific growth rateb (μ [h−1]) |
|||
|---|---|---|---|---|
| WE | WE-214 | WE-CA | WE-214A | |
| M9 glucose (control) | 0.57 ± 0.006 | 0.58 ± 0.004 | 0.57 ± 0.01 | 0.58 ± 0.02 |
| +Na-acetate | 0.162 ± 0.01 | 0.181 ± 0.001▲ | 0.178 ± 0.01▲ | 0.192 ± 0.001▲ |
| +Na-benzoate | 0.138 ± 0.01 | 0.131 ± 0.007 | 0.136 ± 0.006 | 0.138 ± 0.01 |
| +Na-propionate | 0.147 ± 0.005 | 0.186 ± 0.007▲ | 0.182 ± 0.004▲ | 0.196 ± 0.014▲ |
| +Na-aceate, +l-methionine (control) | 0.27 ± 0.004 | 0.27 ± 0.016 | 0.27 ± 0.001 | 0.28 ± 0.002 |
Strains were grown in M9 glucose medium (pH 6.0) supplemented with sodium acetate (20 mM), sodium benzoate (4 mM), or sodium propionate (10 mM) in an automatic growth-measuring incubator at 37°C for 28 h, with two repetitions. l-Methionine was added to the medium to a final concentration of 50 μg/ml.
The specific growth rate was calculated through linear regression analysis of ln(X/X0) data with Sigma Plot software, where the initial OD600 (X0) was 0.1 to 0.15 at the zero time point, and X represents the OD600 value measured every 10 min in an exponentially growing culture over 1 h. Values are means ± standard errors. ▲, the increase in the specific growth rate was ≥10% of the rate in the control strains.
Acetate-resistant MetE enzymes are more tolerant of homocysteine.
Roe et al. (6) showed that supplementation of minimal medium with sodium acetate leads to intracellular accumulation of HCY, which is a substrate for the MetE enzyme, and excess HCY has been shown to inhibit E. coli growth (6, 26, 27). We hypothesized that the acetate-resistant MetE mutants may be tolerant of an increased concentration of HCY. Thus, the MetE mutants and the control strain were cultivated in minimal medium supplemented with 8 mM dl-HCY (Table 3). Under these conditions, the growth of all strains was inhibited approximately 50% in comparison with their growth in HCY-free medium, and we did not detect any differences in growth rates between the mutant and control strains (Table 3). Supplementation of the HCY-enriched medium with l-methionine slightly relieved the growth inhibition of all the tested strains (Table 3). Previously, Tuite et al. (26) demonstrated that the E. coli growth defect observed in the presence of HCY is caused by the inhibition of threonine deaminase, the first enzyme in the isoleucine biosynthesis pathway, and this inhibition leads to isoleucine auxotrophy. To exclude the toxic effect of HCY on threonine deaminase, we added l-isoleucine in the HCY-enriched medium. l-Isoleucine relieved the E. coli growth defect caused by HCY to a greater extent than l-methionine (Fig. 3; Table 3), which is consistent with the previous findings of Sikora and Jakubowski (27). Mutants expressing the acetate-resistant MetEs grew approximately 10% faster than the control strain in the presence of l-isoleucine in the HCY-enriched medium (Fig. 3; Table 3). Thus, we have demonstrated that stabilized MetE mutants that confer an increased acetate tolerance in E. coli also become more resistant to HCY. Simultaneous supplementation of the culture medium with l-methionine and l-isoleucine resulted in a higher growth rate but did not completely restore E. coli growth in the presence of HCY (Table 3), which suggests a more complex effect of HCY on E. coli cell physiology. Sikora and Jakubowski (27) showed that the HCY-induced inhibition of E. coli growth is accompanied by a significantly increased accumulation of HCY-thiolactone. The conversion of HCY to HCY-thiolactone occurs through an ATP-consuming reaction that unproductively reduces cellular energy (27). In addition, HCY-thiolactone modifies E. coli proteins (N-homocysteinylation) in a way that alters or impairs their functions (27). Both of these negative consequences of increased HCY-thiolactone accumulation, reduced cellular energy and impaired protein function, could inhibit E. coli growth.
Table 3.
Effects of mutated MetE proteins on growth of E. coli strain WE in the presence of HCY
| Mediuma | Specific growth rateb (μ [h−1]) |
|||
|---|---|---|---|---|
| WE | WE-214 | WE-CA | WE-214A | |
| M9 glucose (control) | 0.57 ± 0.006 | 0.58 ± 0.004 | 0.57 ± 0.01 | 0.58 ± 0.02 |
| +HCY | 0.29 ± 0.01 | 0.28 ± 0.005 | 0.28 ± 0.02 | 0.28 ± 0.01 |
| +HCY +l-Met | 0.33 ± 0.008 | 0.32 ± 0.008 | 0.32 ± 0.003 | 0.3 ± 0.004 |
| +HCY, +l-Ile | 0.38 ± 0.03 | 0.42 ± 0.008▲ | 0.42 ± 0.003▲ | 0.43 ± 0.01▲ |
| +HCY, +l-Met, +l-Ile | 0.48 ± 0.006 | 0.49 ± 0.007 | 0.49 ± 0.012 | 0.5 ± 0.007 |
Strains were grown in M9 glucose medium (pH 6.0), with or without HCY supplementation (8 mM), at 37°C in an automatic growth-measuring incubator for 24 h with two repetitions. l-Methionine and l-isoleucine were added to a final concentration of 50 μg/ml.
The specific growth rate was calculated through linear regression analysis of ln(X/X0) data with Sigma Plot software, where the initial OD600 (X0) was 0.15 at the zero time point and X represents the OD600 value measured every 10 min in an exponentially growing culture during 1 h. Values are means ± standard errors. ▲, the increase in the specific growth rate was ≥10% of the rate in the control strains.
Fig 3.
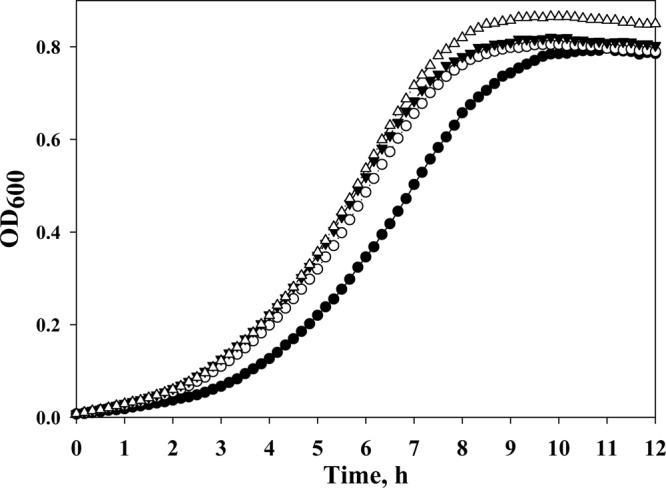
Acetate-tolerant MetE enzymes accelerate E. coli growth in the presence of HCY and l-isoleucine. The WE (filled circles), WE-214 (open circles), WE-CA (filled triangles), and WE-214A (open triangles) strains were incubated in M9 glucose medium (pH 6.0) supplemented with 8 mM HCY and l-isoleucine (50 μg ml−1) in an automatic growth-measuring incubator at 37°C for 24 h. The averages of two independent experiments are presented.
Acetate-tolerant MetE enzymes displayed reduced thermal transition midpoint values but higher in vivo stability.
To determine whether the accelerated growth of the metE mutants in the presence of acetate correlated with an increase in MetE thermal stability (unfolding free energy), the melting temperature (Tm) of the proteins was measured through DSC. The wild-type and mutant MetE enzymes, containing a C-terminal six-histidine tag, were purified as described in Materials and Methods. The Tm of the native MetE enzyme was 54.7°C ± 0.25°C (Table 4), and the single C645A substitution slightly increased the Tm to 55.9°C ± 0.08°C, which contrasted with changes for the multiple mutants MetE-214 and MetE-214A, whose Tm values were 51.3°C ± 0.1°C and 48.5°C ± 0.4°C, respectively (Table 4).
Table 4.
Differential scanning calorimetric data for the wild-type and mutant MetE enzymes
| Enzyme | Tm (°C)a |
|---|---|
| MetE, wild type | 54.7 ± 0.25 |
| MetE-214 | 51.3 ± 0.1 |
| MetE-C645A | 55.9 ± 0.08 |
| MetE-214A | 48.5 ± 0.4 |
All measurements were performed in triplicate. Values are means ± standard errors.
Because the acetate-tolerant MetE mutants demonstrated reduced Tm values, we analyzed the stability of the engineered and native MetE enzymes in vivo after protein synthesis had been blocked by the addition of chloramphenicol. Residual MetE proteins were quantified via Western blotting of cells grown in M9 glucose medium with a pH value of 6.0 or 7.0, and also at 45°C under neutral conditions as described in Materials and Methods. As shown in Fig. 4, the mutants demonstrated an increased in vivo stability under both weakly acidic and neutral conditions, displaying half-lives (t1/2) of 84 min (MetE-214) and more than 120 min (MetE-CA and MetE-214A) at pH 6.0 and 73, >120, and 100 min at pH 7.0 (for MetE-214, MetE-CA, and MetE-214A, respectively). The half-lives of the native MetE protein were 48 min at pH 6.0 and 49 min at pH 7.0. Interestingly, the acetate-tolerant MetE mutants were more stable at a lower pH (Fig. 4a and b). At an elevated temperature (45°C), the MetE mutants were also degraded more slowly than the native enzyme, with half-lives of 70, 103, and 99 min (for MetE-214, MetE-CA, and MetE-214A, respectively), versus 55 min for the wild-type MetE (Fig. 4c). The half-lives of the native and mutated MetE enzymes at 45°C were similar to those half-lives found at 37°C, most likely because their Tm values were higher than 45°C.
Fig 4.
In vivo stability of the MetE mutants. Cells of the WE (filled circles), WE-214 (open circles), WE-CA (filled triangles), and WE-214A (open triangles) strains growing exponentially (OD600 of 0.3) at 37°C in M9 glucose medium with pH values of 6.0 (a) or 7.0 (b) were treated with 200 μg chloramphenicol ml−1, and then one-half of the pH 7.0 culture was shifted to 45°C (c). Samples were collected at the indicated time points and analyzed via Western blotting, as described in Materials and Methods. The densitometry results were normalized by setting the MetE level prior to chloramphenicol addition equal to 100%.
The acetate-tolerant MetE proteins confer thermal stability in E. coli.
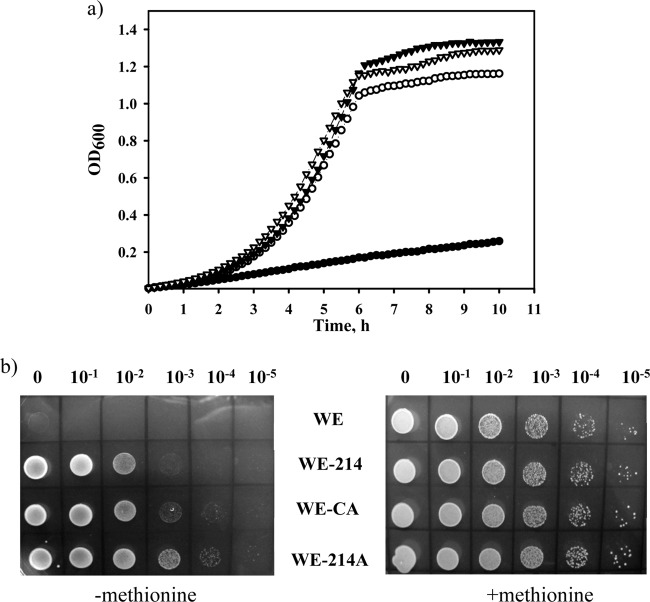
Previously, we found that stabilized mutants of the MetA protein demonstrate an increased tolerance to acetic acid (24). Here, we hypothesized that the acetate-tolerant MetE mutants might improve the thermal stability of E. coli cells. The MetE mutants and the control strain were cultivated at 44°C in minimal M9 glucose medium (Fig. 5a). All of the mutants grew slightly more than four times faster than the control WE strain. The specific growth rate (μ) of the WE strain was 0.17 h−1, while those of the WE-214, WE-CA, and WE-214A mutant strains were 0.69, 0.7, and 0.72 h−1, respectively. The enhanced thermal stability of the acetate-tolerant MetE mutants was confirmed through incubation of serially diluted cultures on solid M9 glucose plates at 44°C (Fig. 5b). The viability of the mutant strains was increased by at least 2 to 3 orders of magnitude above that of the wild-type strain (Fig. 5b, left panel), although the same maximal viability was not observed in the presence of methionine (Fig. 5b, right panel). This result can be explained by the presence of another thermolabile protein, MetA, in the methionine biosynthetic pathway. Supplementation of the culture medium with l-methionine stimulated the growth of the wild-type and mutant strains at 44°C to the same extent, thus abolishing the differences observed among these strains (Fig. 5b, right panel).
Fig 5.
Thermal stability of the MetE mutants. (a) The WE (filled circles), WE-214 (open circles), WE-CA (filled triangles), and WE-214A (open triangles) strains were grown in M9 glucose medium in an automatic growth-measuring incubator at 44°C. The averages of two independent experiments are presented. (b) Serial dilutions of cultures growing logarithmically at 37°C in M9 glucose medium (OD600 of 0.5) were spotted onto M9 glucose or M9 glucose supplemented with l-methionine (50 μg ml−1) agar plates. The cells were then incubated for 24 h at 44°C.
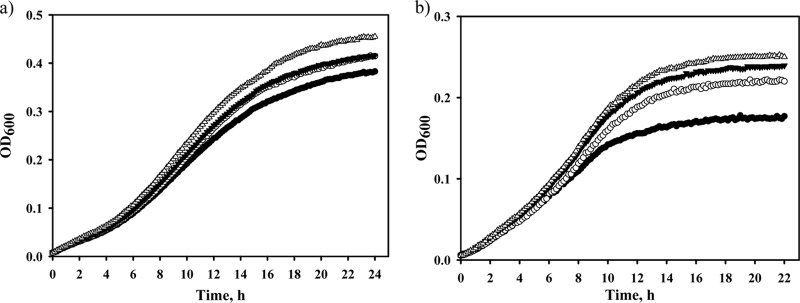
To determine the individual contribution of each amino acid residue to improved acetate tolerance and thermal stability, single amino acid substitutions corresponding to those found in the MetE-214 were introduced into the wild-type MetE protein by site-directed mutagenesis. The single-site metE mutants were inserted into the WEΔmetE::kan chromosome to yield strains A39, C46, I106, and E713, which were then tested for growth in M9 glucose medium (pH 6.0) supplemented with sodium acetate (20 mM) at 37°C or in M9 glucose medium (pH 7.0) at 44°C (Fig. 6). Two amino acid substitutions, R46C and T106I, conferred the highest growth rates to the WE strain in the presence of acetate (specific growth rates of 0.178 and 0.185 h−1 for the C46 and I106 mutations, respectively, versus 0.164 h−1 for the control strain, WE) (Fig. 6a). The other mutations, V39A and K713E, also stimulated the A39 and E713 mutant strain growth under acidic conditions but to a lesser extent (specific growth rates of 0.174 and 0.175 h−1, respectively) (Fig. 6a). All the single-mutated MetEs stimulated growth of the E. coli strains at 44°C (specific growth rates of 0.6, 0.62, 0.62, and 0.67 h−1 for the A39, C46, I106, and E713 strains, respectively, versus 0.17 h−1 for the control strain, WE) (Fig. 6b). The E713 (K713E) mutant was the fastest-growing mutant among those tested for growth at 45°C (specific growth rate of 0.425 h−1) (data not shown). Other single-site mutants, C46 and I106, also demonstrated an accelerated growth at 45°C (specific growth rates of 0.3 and 0.32 h−1, respectively) (data not shown). The control WE strain did not grow at 45°C. As seen in the data presented here, all of the amino acid residues replaced in the MetE-214 mutant enzyme are involved in MetE stability, but to variable degrees.
Fig 6.
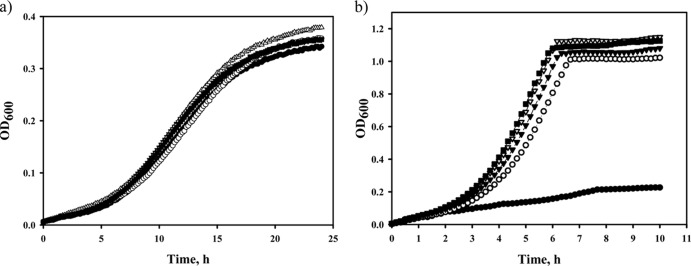
Effects of single mutated MetE enzymes on acetate tolerance or thermal stability of E. coli strain WE. The WE (filled circles), A39 (open circles), C46 (filled triangles), I106 (open triangles), and E713 (filled rectangles) strains were incubated in M9 glucose medium (pH 6.0) supplemented with 20 mM sodium acetate at 37°C for 28 h (a) or in M9 glucose medium (pH 7.0) at 44°C for 10 h (b) in an automatic growth-measuring incubator. The averages of two independent experiments are presented.
Stabilized MetA and MetE enzymes synergistically increase the growth rate of E. coli in the presence of acetate and at an elevated temperature.
To test the combined effect of the stabilized MetA and MetE enzymes on the growth of the WE strain in sodium acetate medium and at the higher temperature of 45°C, the metA-Y229 gene, encoding the thermal-tolerant Y229 MetA mutant (28), was inserted into the chromosome of the WE-214A strain instead of the wild-type metA gene. We previously found that stabilized MetA mutants display enhanced acetate tolerance (24). The Y229-214A double mutant MetA-MetE strain and the single mutant MetA (Y229) and MetE (WE-214A) strains were cultivated in minimal M9 glucose medium in the presence of sodium acetate (20 mM) or at 45°C, and they were compared with the control WE strain harboring the wild-type metA and metE genes (Fig. 7a and b). The growth rate of the double mutant Y229-214A was higher than the growth rates of the control WE strain and the single mutants tested for growth in sodium acetate-enriched medium (specific growth rates of 0.205 h−1 for the Y229-214A mutant versus 0.162, 0.181, and 0.183 h−1 for the WE, WE-214A, and Y229 strains, respectively) (Fig. 7a). However, the mutant Y229-214A grew more slowly in the presence of l-methionine (specific growth rate of 0.27 h−1 [data not shown]). This result might reflect the presence of other acetate-sensitive regions in the MetA/MetE enzymes, or it might mean that another protein in the methionine biosynthetic pathway is involved in acetate tolerance.
Fig 7.
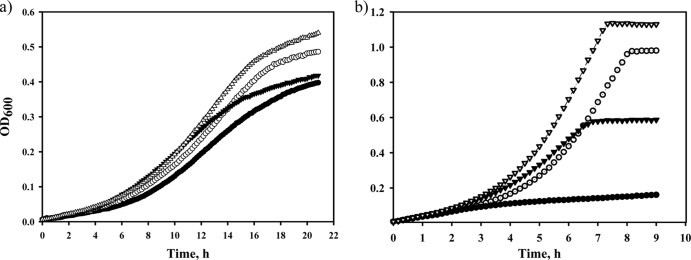
Combined effects of mutated MetA and MetE proteins on E. coli growth in the presence of acetate or at elevated temperature. The WE (filled circles), Y229 (open circles), WE-214A (filled triangles), and Y229-214A (open triangles) strains were grown in M9 glucose medium (pH 6.0) supplemented with sodium acetate (20 mM) at 37°C (a) or in M9 glucose medium at 45°C (b) in an automatic growth-measuring incubator. The averages of two independent experiments are presented.
The Y229-214A double mutant grew faster than the Y229 and WE-214A single mutants at the elevated temperature of 45°C, with specific growth rates of 0.53, 0.47, and 0.31 h−1, respectively (Fig. 7b). Moreover, the specific growth rate of the Y229-214A stabilized double mutant was the same in the presence of l-methionine (0.53 and 0.55, respectively) (data not shown), and the Y229, WE-214A and control WE strains demonstrated similar specific growth rates in l-methionine-supplemented medium (0.56, 0.54, and 0.54, respectively) (data not shown). The control WE strain did not grow at 45°C (Fig. 7b).
We also tested the viability of these strains at 45°C. Serially diluted cultures were incubated on M9 glucose plates, with or without l-methionine (see Fig. S1a and b in the supplemental material). As shown in Fig. S1a, the Y229-214A double mutant strain was more viable than the stabilized single mutants Y229 and WE-214A by 1 and 2 orders of magnitude, respectively. The survival of the Y229-214 double mutant was only slightly lower than the survival observed in the presence of l-methionine (see Fig. S1b). Thus, the stabilization of two thermolabile enzymes in the methionine biosynthesis pathway, MetA and MetE, synergistically increased the thermal tolerance of the E. coli WE strain, bringing it close to the maximum tolerance achieved with l-methionine supplementation. We hypothesize that this further improvement of the thermal stability of the MetA/MetE proteins contributes to completely overcoming the methionine auxotrophy that occurs at elevated temperatures and thereby accelerates E. coli growth.
DISCUSSION
Earlier observations made by Han et al. (7) and Roe et al. (6) demonstrated that l-methionine relieves the growth inhibition of E. coli cells caused by weak organic acids, including acetic acid. Roe et al. (6) showed that there was an increased level of HCY, which is a substrate for the MetE protein, in acetate-treated cells and that E. coli growth was inhibited in HCY-enriched medium. However, overproduction of MetE did not protect these E. coli cells against growth inhibition by acetate (6), most likely due to a significant decline of the soluble MetE protein level in response to acetate challenge. We found that the amount of soluble wild-type MetE protein was reduced by 40% after 2 h of cultivation of the WE strain in minimal M9 glucose medium supplemented with 20 mM sodium acetate (see Fig. S2a in the supplemental material). The level of the soluble native MetE protein in the overexpressed BL21(DE3) cells decreased by 25% compared to untreated cells after 2 h of acetate treatment (see Fig. S2b). Another way to increase the acetate resistance of E. coli cells is random mutagenesis of metE, followed by selection of clones growing more rapidly in the presence of sodium acetate. Through this process, we obtained the MetE-214 mutant variant (with the V39A, R46C, T106I, and K713E mutations) that displayed acetate tolerance. We found that all of the single-site mutations corresponding to those found in the MetE-214 mutant increased both acetic and thermal tolerance of the native MetE, but to varied degrees. The C645A substitution, which was found to confer resistance to oxidative stress in the MetE protein (15, 21), also increased the protein's tolerance to acetate.
It is necessary to note that despite the increased acetate tolerance of these MetE mutants, we did not achieve the same extent of growth inhibition relief as observed in the presence of l-methionine (Table 2), which may be explained by two factors. First, the substitution of other amino acid residues can also increase the acetate tolerance of the MetE enzyme. Second, different proteins in the methionine biosynthesis pathway (e.g., the MetA enzyme) appear to be involved in acetate resistance. Arnold et al. (29) found that the expression of the metA gene was increased 3- to 4-fold in acetate-treated E. coli cells, and we previously observed an increased tolerance to acetic acid in stabilized MetA mutants (24).
Roe et al. (6) hypothesized that the hampered E. coli growth observed in the presence of acetate is a consequence of a drastically increased pool of HCY due to inhibition of the biosynthetic step downstream of the HCY metabolic intermediate, corresponding to the MetE enzyme. In the present study, we demonstrated that the acetate-tolerant MetE enzymes conferred greater resistance to HCY in E. coli cells, but only in the presence of l-isoleucine. This finding is consistent with the previous observation made by Tuite et al. (26) that the primary target of HCY is the first enzyme in the isoleucine biosynthetic pathway, i.e., threonine deaminase. We may therefore assume that when branched-chain amino acid biosynthesis is protected by l-isoleucine addition, the stabilized MetE enzymes metabolize HCY to methionine more effectively than the wild-type MetE enzyme. Consequently, the E. coli cells can overcome two negative effects: the decreased intracellular levels of methionine and the accumulation of the toxic methionine precursor HCY.
MetE catalyzes the final step in de novo methionine biosynthesis in E. coli cells grown under aerobic conditions (8). The MetE protein has been found to account for approximately 3 to 5% of the total cellular protein content when E. coli is grown in glucose minimal medium (12, 13). This high abundance of MetE protein may be explained by its approximately 50-times-lower activity than the MetH enzyme, which drives the same reaction, but under anaerobic conditions (11). A higher cellular content of the MetE protein is correlated with an increased susceptibility to unfavorable environments, such as heat (14), oxidative stress (15, 16, 21), and acidic conditions (6). Mogk et al. (14) determined that MetE is a major aggregation-prone protein at an elevated temperature (45°C), which is significantly below the melting temperature of the purified protein (55°C) (15) (Table 4). It was surprising to find that the acetate- and temperature-tolerant MetE enzymes exhibited melting temperatures below that determined for the wild-type protein (Table 4). The Tm is a good indicator of thermal stability (30). Apparently, we are faced with another type of stabilization here, specifically, kinetic stabilization, where specific conformational changes lead to a high unfolding barrier that ultimately results in very slow unfolding rates (31). We previously obtained similar results for stabilized MetA enzymes (28).
None of the amino acids changed in the acetate-tolerant MetE-214 mutant was located at the conserved active sites (11, 25, 32, 33) (see Fig. S3 in the supplemental material), so we hypothesize that these mutations lead to kinetic stabilization of the MetE protein. Hondorp and Matthews noticed that cysteine 645, identified as a unique site of oxidation in the MetE protein (15, 21), is conserved only within the Enterobacteriaceae (21). The MetE enzymes from the acidophilic and thermophilic strains do not possess a homologous cysteine 645 (see Fig. S3). On the other hand, the MetE enzymes from other species, which have been found to be sensitive to oxidation, do not contain a homologous cysteine 645 (34, 35). Obviously, the MetE amino acid residues involved in the response to environmental stress are quite diverse and species specific.
Hondorp and Matthews have shown that glutathione (GSSC) oxidation of MetE causes a conformational change compared to the reduced protein (15). The oxidized MetE protein was found to be more stable in response to tryptic digestion than the reduced form (15). In the present study, all of the MetE mutants were much more susceptible to tryptic digestion than the wild-type protein (Fig. 8), which suggests that the conformational changes that occur in the mutated MetE proteins prevent them from unfolding and being inactivated in a stressful environment. This assumption may explain the increased acetate tolerance of the MetE-214, MetE-CA, and MetE-214A mutant enzymes that was observed when the intracellular concentration of acetate was much higher than the external concentration at moderately acidic pH values (3). In support of this notion, the mutated MetE proteins, which were more stable in vivo than the wild-type enzyme under either an acidic or a neutral pH, demonstrated a higher level of in vivo stability, specifically at low pH (Fig. 4). The increased thermal tolerance of the acetate-resistant MetE mutants may also be a consequence of the conformational changes in the thermolabile MetE protein (14).
Fig 8.
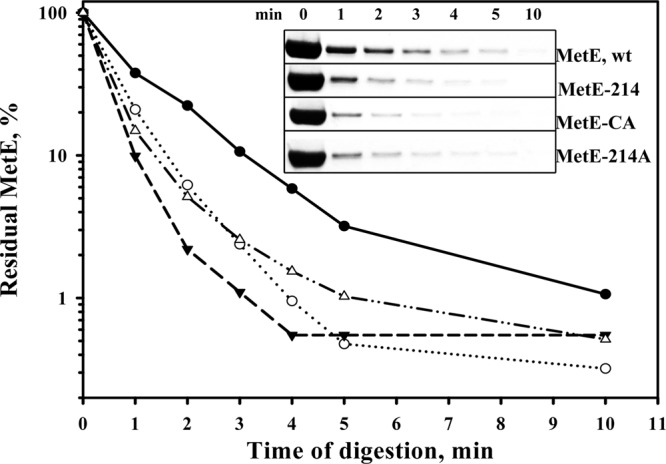
Tryptic digestion of the MetE proteins. The MetE enzymes (1 nmol) were digested with 0.01% (wt/vol) trypsin at 37°C in a total volume of 200 μl of 20 mM potassium phosphate buffer (pH 7.2). Samples were collected at the indicated time points and analyzed via SDS-PAGE. Band intensities were quantified using WCIF ImageJ software. Symbols: MetE wild type (filled circles), MetE-214 (open circles), MetE-CA (filled triangles), and MetE-214A (open triangles).
In the present study, we demonstrated increased thermal tolerance of E. coli strains expressing acetate-resistant MetE enzymes. It is quite notable that E. coli cells acquire cross-protection from acetate and heat from mutations in MetE at the single protein level. A higher heat resistance of acid-adapted E. coli strains has previously been shown using a complex medium (36). One of the major nutrient sources in this complex medium was the pool of amino acids, which included l-methionine, peptides, and proteins derived from organic nitrogen substrates, such as soybean flour, yeast extract, and corn steep liquor (37). The presence of l-methionine in the culture medium represses genes involved in methionine biosynthesis, as shown by Greene (38), suggesting the existence of another mechanism of thermotolerance in the complex medium. E. coli cells have been found to grow significantly more slowly after a temperature upshift without l-methionine supplementation (39). This phenomenon was linked to the inherent instability of MetA, the first enzyme in the methionine biosynthetic pathway (17, 39). In heat-treated E. coli cells, Mogk et al. (14) identified MetE as another thermolabile protein in the methionine biosynthesis pathway. Here, we demonstrated that the stabilized MetE enzymes alone were responsible for the accelerated growth and higher survival observed in E. coli cells at elevated temperatures. The simultaneous presence of both the stabilized MetA and stabilized MetE enzymes improved the survival of heat-stressed E. coli cells to the levels observed under l-methionine supplementation (see Fig. S1 in the supplemental material). We therefore believe that the introduction of stabilized MetA and MetE proteins is sufficient to enable the growth of E. coli at higher temperatures. Moreover, further stabilization of the E. coli MetA/MetE enzymes may completely overcome methionine auxotrophy and provide an increased growth rate and enhanced survival at higher temperatures. This MetA/MetE reengineering may increase the temperature window for E. coli cells, permitting them to grow and form products at higher temperatures, and thus widen the biotechnological applications for E. coli as a cell factory.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the 21C Frontier Program of Microbial Genomics and Applications (grant MGC2100834) of the Ministry of Education, Science and Technology (MEST) of the Republic of Korea, and by a KRIBB Basic Research Grant.
Footnotes
Published ahead of print 11 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01952-13.
REFERENCES
- 1.Eklund T. 1980. Inhibition of growth and uptake processes in bacteria by some food preservatives. J. Appl. Bacteriol. 48:423–432 [DOI] [PubMed] [Google Scholar]
- 2.Booth IR. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49:359–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roe AJ, McLaggan D, Davidson I, O'Byrne C, Booth IR. 1998. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J. Bacteriol. 180:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi CM, Takahashi DF, Carvalhal ML, Alterthum F. 1999. Effects of acetate on the growth and fermentation performance of Escherichia coli KO11. Appl. Biochem. Biotechnol. 81:193–203 [DOI] [PubMed] [Google Scholar]
- 5.Cherrington CA, Hinton M, Chopra I. 1990. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J. Appl. Bacteriol. 68:69–74 [DOI] [PubMed] [Google Scholar]
- 6.Roe AJ, O'Byrne C, McLaggan D, Booth IR. 2002. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology 148:2215–2222 [DOI] [PubMed] [Google Scholar]
- 7.Han K, Hong J, Lim HC. 1993. Relieving effects of glycine and methionine from acetic acid inhibition in Escherichia coli fermentation. Biotechnol. Bioeng. 41:316–324 [DOI] [PubMed] [Google Scholar]
- 8.Hondorp ER, Matthews RG. 28 April 2006, posting date Chapter 3.6.1.7, Methionine. In Böck A, Curtiss R, III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D. (ed). EcoSal —Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. 10.1128/ecosal.3.6.1.7 [DOI] [Google Scholar]
- 9.Figge RM. 2006. Methionine biosynthesis in Escherichia coli and Corynebacterium glutamicum, p 164–189 In Wendisch VF. (ed), Amino acid biosynthesis: pathways, regulation and metabolic engineering. Springer-Verlag, Berlin, Germany [Google Scholar]
- 10.Brown KL. 1982. Synthesis of organo cobalt complexes, p 245–294 In Dolphin D. (ed), B12, vol 1 Wiley Interscience, New York, NY [Google Scholar]
- 11.González JC, Peariso K, Penner-Hahn JE, Matthews RG. 1996. Cobalamin-independent methionine synthase from Escherichia coli: a zinc metalloenzyme. Biochemistry 35:12228–12234 [DOI] [PubMed] [Google Scholar]
- 12.Pedersen S, Bloch PL, Reeh S, Neidhardt FC. 1978. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell 14:179–190 [DOI] [PubMed] [Google Scholar]
- 13.VanBogelen RA, Abshire KZ, Pertsemlidis A, Clark RL, Neidhardt FC. 1996. Gene-protein database of Escherichia coli K-12, p 2067–2117 In Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 14.Mogk A, Tomoyasu T, Goloubinoff P, Rüdiger S, Röder , Langen DH, Bukau B. 1999. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18:6934–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hondorp ER, Matthews RG. 2004. Oxidative stress inactivates cobalamin-independent methionine synthase (MetE) in Escherichia coli. PLoS Biol. 2:1738–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leichert LA, Jakob U. 2004. Protein thiol modifications visualized in vivo. PLoS Biol. 2:1723–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz C, Rasouly A, Gur E, Shenhar Y, Biran D, Ron EZ. 2009. Temperature-dependent proteolysis as a control element in Escherichia coli metabolism. Res. Microbiol. 160:684–686 [DOI] [PubMed] [Google Scholar]
- 18.Gur E, Biran D, Gazit E, Ron EZ. 2002. In vivo aggregation of a single enzyme limits growth of Escherichia coli at elevated temperature. Mol. Microbiol. 46:1391–1397 [DOI] [PubMed] [Google Scholar]
- 19.Price-Carter M, Fazzio TG, Vallbona EI, Roth JR. 2005. Polyphosphate kinase protects Salmonella enterica from weak organic acid stress. J. Bacteriol. 187:3088–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner P, Mamo G, Karlsson EN. 2007. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 6:9. 10.1186/1475-2859-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hondorp ER, Matthews RG. 2009. Oxidation of cysteine 645 of cobalamin-independent methionine synthase causes a methionine limitation in Escherichia coli. J. Bacteriol. 191:3407–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 23.Datsenko K, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mordukhova EA, Lee H-S, Pan J-G. 2008. Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl. Environ. Microbiol. 74:7660–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González JC, Banerjee RV, Huang S, Sumner JS, Matthews RG. 1992. Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli: two solutions to the same chemical problem. Biochemistry 31:6045–6056 [DOI] [PubMed] [Google Scholar]
- 26.Tuite NL, Fraser KR, O'Byrne CP. 2005. Homocysteine toxicity in Escherichia coli is caused by a perturbation of branched-chain amino acid biosynthesis. J. Bacteriol. 187:4362–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sikora M, Jakubowski H. 2009. Homocysteine editing and growth inhibition in Escherichia coli. Microbiology 155:1858–1865 [DOI] [PubMed] [Google Scholar]
- 28.Mordukhova EA, Kim D, Pan J-G. 2013. Stabilized homoserine o-succinyltransferases (MetA) or l-methionine partially recover the growth defect in Escherichia coli lacking ATP-dependent proteases or the DnaK chaperone. BMC Microbiol. 13:179. 10.1186/1471-2180-13-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold CN, McElhanon J, Lee A, Leonhart R, Siegele DA. 2001. Global analysis of Escherichia coli expression during the acetate-induced acid tolerance response. J. Bacteiol. 183:2178–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Tsai C-J, Nissinov R. 2000. Factors enhancing protein thermostability. Protein Eng. 13:179–191 [DOI] [PubMed] [Google Scholar]
- 31.Manning M, Colon W. 2004. Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward β-sheet structure. Biochemistry 43:11248–11254 [DOI] [PubMed] [Google Scholar]
- 32.Zhou ZS, Peariso K, Penner-Hahn JE, Matthews RG. 1999. Identification of the zinc ligands in cobalamin-independent methionine synthase (MetE) from Escherichia coli. Biochemistry 38:15915–15926 [DOI] [PubMed] [Google Scholar]
- 33.Pejchal R, Ludwig ML. 2005. Cobalamin-independent methionine synthase (MetE): a face-to-face double barrel that evolved by gene duplication. PLoS Biol. 3(2):e31. 10.1371/journal.pbio.0030031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochgräfe F, Mostertz J, Albrecht D, Hecker M. 2005. Fluorescence thiol modification assay: oxidatively modified in Bacillus subtilis. Mol. Microbiol. 58:409–425 [DOI] [PubMed] [Google Scholar]
- 35.Wolf C, Hochgräfe F, Kusch H, Albrecht D, Hecker M, Engelmann S. 2008. Proteomic analysis of antioxidant strategies of Staphylococcus aureus: diverse responses to different oxidants. Proteomics 8:3139–3153 [DOI] [PubMed] [Google Scholar]
- 36.Ryu J-H, Beuchat LR. 1999. Changes in heat tolerance of Escherichia coli O157:H7 after exposure to acidic environments. Food Microbiol. 16:317–324 [Google Scholar]
- 37.Bapat PM, Das D, Sohoni SV, Wangikar PP. 2006. Hierarchical amino acid utilization and its influence on fermentation dynamics: rifamycin B fermentation using Amycolatopsis mediterranei S699, a case study. Microb. Cell Fact. 5:32. 10.1186/1475-2859-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene RC. 1996. Biosynthesis of methionine, p 542–560 In Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 39.Ron EZ, Davis BD. 1971. Growth rate of Escherichia coli at elevated temperatures: limitation by methionine. J. Bacteriol. 107:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.