Abstract
The bacterial ecology during rye and wheat sourdough preparation was described by 16S rRNA gene pyrosequencing. Viable plate counts of presumptive lactic acid bacteria, the ratio between lactic acid bacteria and yeasts, the rate of acidification, a permutation analysis based on biochemical and microbial features, the number of operational taxonomic units (OTUs), and diversity indices all together demonstrated the maturity of the sourdoughs during 5 to 7 days of propagation. Flours were mainly contaminated by metabolically active genera (Acinetobacter, Pantoea, Pseudomonas, Comamonas, Enterobacter, Erwinia, and Sphingomonas) belonging to the phylum Proteobacteria or Bacteroidetes (genus Chryseobacterium). Their relative abundances varied with the flour. Soon after 1 day of propagation, this population was almost completely inhibited except for the Enterobacteriaceae. Although members of the phylum Firmicutes were present at very low or intermediate relative abundances in the flours, they became dominant soon after 1 day of propagation. Lactic acid bacteria were almost exclusively representative of the Firmicutes by this time. Weissella spp. were already dominant in rye flour and stably persisted, though they were later flanked by the Lactobacillus sakei group. There was a succession of species during 10 days of propagation of wheat sourdoughs. The fluctuation between dominating and subdominating populations of L. sakei group, Leuconostoc spp., Weissella spp., and Lactococcus lactis was demonstrated. Other subdominant species such as Lactobacillus plantarum were detectable throughout propagation. As shown by PCR-denaturing gradient gel electrophoresis (PCR-DGGE) analysis, Saccharomyces cerevisiae dominated throughout the sourdough propagation. Notwithstanding variations due to environmental and technology determinants, the results of this study represent a clear example of how the microbial ecology evolves during sourdough preparation.
INTRODUCTION
Sourdough is “a mixture of flour and water, spontaneously fermented by lactic acid bacteria and yeasts, which after several refreshments are responsible for its capacity to leaven the dough, while contemporarily and unavoidably acidifying it” (1). The use of sourdough as the natural starter for bread making is one of the oldest biotechnology processes in food fermentation (2). Nowadays, sourdough is largely used for the manufacture of wheat and rye breads, crackers, pizza, various sweet baked goods, and gluten-free products (1, 3). Differently from other chemical or biological agents (e.g., baker's yeast), acidification, proteolysis, and activation of a number of enzymes as well as the synthesis of microbial metabolites during sourdough fermentation cause changes of the dough and baked good matrix and positively influence their sensory, nutritional, and functional features (3, 4).
The microbial composition of mature sourdoughs from various European countries has been investigated in numerous studies (5–12), which revealed large lactic acid bacterium diversities (for reviews, see references 1, 3, 13, and 14). Indeed, a high number of species belonging to the Leuconostoc, Pediococcus, Enterococcus, Weissella, and, especially, Lactobacillus genera were identified. Yeasts, especially Saccharomyces spp. and Candida spp., also occur in sourdoughs (3, 15). Usually, the diversity of lactic acid bacteria is larger than that of the yeast microbiota, since Saccharomyces cerevisiae very frequently was the only dominating species in sourdough (3). The literature also agreed that almost all the features that are attributed to sourdough are mainly the consequence of the metabolism of lactic acid bacteria (1, 3, 16). The complexity and stability of the sourdough microbiota depend on a number of determinants, which include environmental microbiota (e.g., microbiota of flour and other ingredients and house microbiota) and their potential metabolic activities (e.g., cofactor regeneration capability and energy synthesis from various sources), and technology parameters (e.g., chemical and enzyme composition of the flour, leavening temperature, pH and redox potential, dough yield, and number and length of sourdough refreshments) (3, 16–19). Because of the complexity of determinants, the temporary stability of the sourdough microbiota is still debated. Usually, mature sourdoughs show almost constant technology performance and lactic acid bacterium biota beyond 108 CFU g−1, mainly consisting of facultative and obligate heterofermentative species. Nevertheless, both the persistence of dominant biotypes and the unpredictable succession of species and biotypes during long-time propagation have been described (15, 17). These findings may allow the conclusion that all mature sourdoughs are stable in terms of loads of lactic acid bacteria and technology features (e.g., acidification rate) but that only some stably harbor the same species and biotypes over time.
Although an abundant literature dealt with the characterization of the mature sourdough microbiota (5, 6, 8–12), the microbial dynamics leading from dough to mature sourdough were never thoroughly elucidated. The few studies that were done demonstrated that during sourdough preparation Gram-positive bacteria outgrew Gram-negative bacteria (20) and that the sourdough stability is reached through a three-phase evolution (21, 22). An in-depth study on the microbial community dynamics during sourdough preparation may provide new information regarding (i) the influence of the flour as a source of microbial diversity and the representativeness of lactic acid bacteria within the autochthonous population, (ii) the microbial dynamics that occur prior to getting a mature sourdough, and (iii) the temporary or stable presence of dominant and subdominant populations of lactic acid bacteria. Unveiling the dynamic changes of this microbial community, coupled with biochemical, technology, and environmental determinants, will reveal how lactic acid bacteria may adapt and dominate such food ecosystems and increase the understanding of the microbial ecology associated with such important food fermentation. High-throughput sequencing and metagenomics offer a possibility for a more in-depth analysis of food microbiota (23), and the recent literature shows how the structure and evolution of the microbiota during food fermentation may be highlighted through a deep sequencing approach (24, 25).
First, this study used a 16S rRNA gene-based high-throughput sequencing approach, targeting DNA and RNA, to describe the microbial ecology dynamics during sourdough preparation.
MATERIALS AND METHODS
Flours.
Rye (Secale cereale) (R) and wheat (Triticum durum or Triticum aestivum) (D or A, respectively) flours were used. Rye and wheat flours were purchased from Rieper SpA (Vandoies, Bolzano, Italy) and Molini Tandoi SpA (Corato, BT, Italy), respectively. The gross compositions for R, D, and A were, respectively, as follows: moisture, 15.5% ± 0.43%, 13.5% ± 0.67%, and 15.5% ± 0.21%; protein (N × 5.7), 6.4% ± 0.03%, 12.5% ± 0.01%, and 9% ± 0.02% of dry matter (d.m.); total carbohydrates, 73.2% ± 0.4%, 73.0% ± 0.4%, and 76.1% ± 0.2% of d.m.; dietary fiber, 6.5% ± 0.04%, 3.3% ± 0.01%, and 1.5% ± 0.03% of d.m.; fat 1.0% ± 0.02%, 0.7% ± 0.01%, and 1.0% ± 0.02% of d.m. Three different batches of each flour were pooled and used to prepare the respective sourdoughs.
Dough preparation and sourdough propagation.
Dough was prepared and sourdough was propagated according to traditional protocols (10), without using starter cultures or baker's yeast. Dough preparation was as follows: R, D, or A flour (187.5 g) and tap water (112.5 ml) were used to produce 300 g of dough (dough yield [dough weight × 100/flour weight], 160) with a continuous high-speed mixer (60 × g, dough mixing time of 5 min) (Chopin & Co., Boulogne, Seine, France). This preparation yields dough prior to fermentation and before becoming sourdough. Daily, each sourdough was subjected to fermentation (propagation) at 25°C for 5 h. The only exception was the first fermentation, which lasted 8 h according to traditional protocols (10). Between each daily fermentation, sourdoughs were stored at 10°C for ca. 16 h. Sourdough propagation was according to the backslopping (refreshment) procedure, where the sourdough from the day before was used as the starter (25% [wt/wt] of inoculum) to ferment a new mixture of flour (140.62 g) and tap water (84.38 ml), having a dough yield of 160. Sourdoughs were daily propagated for 11 days, and samples were taken after 0 (dough), 1, 2, 5, and 10 (sourdough) days of propagation. Sourdoughs were cooled down to 4°C and analyzed within 2 h after collection. Preparation and propagation were carried out in triplicate.
Determination of pH, titratable acidity, organic acids, and free amino acids and enumeration of cultivable bacteria and yeasts.
The values of pH were determined by a pH meter. Total titratable acidity (TTA) was measured on 10 g of sample, which was homogenized with 90 ml of distilled water for 3 min in a bag mixer (400P; Interscience, St Nom, France), and expressed as the amount (ml) of 0.1 M NaOH needed to achieve the pH of 8.3. Lactic and acetic acids in the water-soluble extract of the sample were determined by high-performance liquid chromatography (HPLC) using an ÄKTA purifier system (GE Healthcare Bio-Sciences, Uppsala, Sweden) (26). The quotient of fermentation (FQ) was determined as the molar ratio between d,l-lactic and acetic acids. The concentration of free amino acids (FAA) of the water-soluble extract was determined using the Biochrom 30 amino acid analyzer (Biochrom Ltd., Cambridge Science Park, England), as previously described (10).
Ten grams of sample was homogenized with 90 ml of sterile peptone-water (0.1% [wt/vol] peptone, 0.85% [wt/vol] NaCl) solution. Lactic acid bacteria were counted at 30°C for 48 h under anaerobiosis using sourdough bacterium (SDB) agar medium supplemented with cycloheximide (0.1 g liter−1). Usually, SDB agar medium gave the highest recovery of lactic acid bacteria among the media routinely used for sourdough analysis (10). The number of yeasts was estimated at 30°C for 48 h on Sabouraud dextrose agar (SDA) (Oxoid, Basingstoke, Hampshire, United Kingdom) medium supplemented with chloramphenicol (0.1 g liter−1). Enterobacteriaceae were counted using eosin-methylene blue (EMB) agar medium (Oxoid). Plates were incubated under anaerobic conditions at 37°C for 48 h.
Total bacterial genomic DNA and RNA extraction.
Ninety milliliters of potassium phosphate (50 mM, pH 7.0) buffer was added to 10 g of sample and homogenized for 5 min, and DNA extraction was carried out as previously described (10). Total RNA was extracted using the RiboPure bacterial kit (Ambion RNA, Life Technologies Co., Carlsbad, CA), according to the manufacturer's instructions. The purified RNA (100 ng; final volume, 20 μl) was incubated at 42°C for 2 min in 2 μl of 7× genomic DNA (gDNA) wipeout buffer (QuantiTect reverse transcription kit; Qiagen srl., Milan, Italy) and RNase-free water (final volume, 14 μl). The cDNA was obtained by the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer's instructions.
PCR and DGGE analysis.
Because of the very low diversity of yeasts in mature sourdoughs (10), only denaturing gradient gel electrophoresis (DGGE) analysis was carried out to describe the dynamics of yeasts during sourdough preparation. DNA (40 ng) was amplified with primers NL1 and LS2, corresponding to the D1-D2 region of the 26S rRNA gene to identify the yeast community. The PCR core program was carried out as described elsewhere (27). Amplicon separation by DGGE (DCode system; Bio-Rad Laboratories, Milan, Italy) and band purification were performed as previously described (28). DNA sequencing reactions were carried out by MWG Biotech AG (Ebersberg, Germany). Sequences were compared using the GenBank database and the BLAST program (29).
Amplicon library preparation and pyrosequencing.
Both DNA and cDNA were used to study the bacterial diversity by pyrosequencing the amplified V1-V3 region (amplicon size, 520 bp) (25). PCRs were carried out using DNA or cDNA as the template as previously described (25). PCR products were purified twice with the Agencourt AMPure kit (Beckman Coulter, Milan, Italy) and then quantified using the QuantiFluor system (Promega, Milan, Italy) prior to further processing. Amplicons were used for pyrosequencing on a GS Junior platform (454 Life Sciences, Roche Diagnostics, Milan, Italy) according to the manufacturer's instructions by using titanium chemistry.
Bioinformatics.
A first filtering of the results was performed using 454 amplicon signal processing; then sequences were analyzed by using QIIME 1.5.0 software (30). After the split library script performed by QIIME, the reads were excluded from the analysis if they had an average quality score lower than 25, if they were shorter than 300 bp, and if there were ambiguous base calls. Sequences that passed the quality filter were denoised, and singletons were excluded. Operational taxonomic units (OTUs) were defined by a 97% similarity; the taxonomy assignment and alpha and beta diversity analyses were performed through QIIME as previously described (31).
Weighted UniFrac distance matrices were used for principal coordinate analysis (PCoA) analysis. The OTU taxonomy table generated by QIIME was used to produce pseudo-heat maps by using the software TMeV v. 4.8 (32).
Weighted UniFrac distance matrices and OTU tables were used to perform statistical tests. An OTU network was generated by QIIME, and a bipartite graph was constructed in which each node represented either a sourdough sample or a bacterial OTU. Connections were drawn between samples and OTUs, with edge weights defined as the number of sequences from each OTU that occurred in each sample. Networks were visualized using Cytoscape 2.5.2 (33).
Statistical analyses.
Data (at least three replicates) of pH, TTA, organic acids, FAA, FQ, and cell density of presumptive lactic acid bacteria, yeasts, and Enterobacteriaceae were subjected to one-way analysis of variance (ANOVA), and pair comparison of treatment means was achieved by Tukey's procedure at a P of 0.05 using the statistical software Statistica 7.0 for Windows. The above data were subjected to permutation analysis using PermutMatrix (10).
Nucleotide sequence accession number.
The pyrosequencing data are available in the Sequence Read Archive database of the National Center of Biotechnology Information under accession no. SRP019996.
RESULTS
Cell counts and acidification during sourdough propagation.
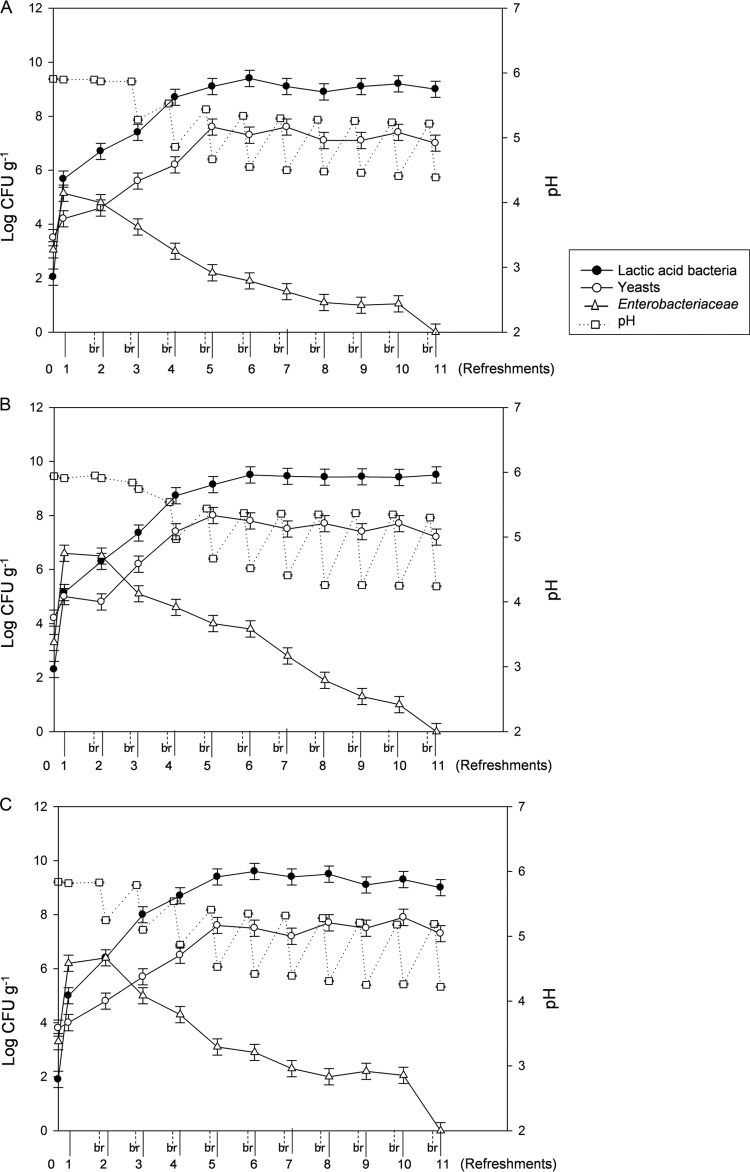
After 8 h of fermentation at 25°C (first fermentation, 1 day), cell numbers of presumptive lactic acid bacteria significantly (P < 0.05) increased in day 1 rye (R1), T. durum (D1), and T. aestivum (A1) sourdoughs (Fig. 1). After 5 days of propagation, cell densities of presumptive lactic acid bacteria reached values ranging from ca. 9.1 to ca. 9.4 log CFU g−1 for R5, D5, and A5. These cell densities stayed almost constant during subsequent propagation. Doughs contained a significantly (P < 0.05) higher (ca. 1.5 to 2.0 log units) initial number of yeasts than of presumptive lactic acid bacteria (Fig. 1). Nevertheless, yeast numbers decreased during propagation. The ratio between lactic acid bacteria and yeasts stabilized to ca. 100:1 after 5 days of propagation for rye and T. aestivum wheat sourdoughs and after 6 days for T. durum wheat sourdough. Enterobacteriaceae were counted in all doughs, and their number significantly (P < 0.05) increased after 1 or 2 days, but they progressively disappeared at the end of sourdough propagation (Fig. 1).
Fig 1.
Cell numbers of presumptive lactic acid bacteria, yeasts, and Enterobacteriaceae, and kinetics of acidification of rye (A), Triticum durum (B), and Triticum aestivum (C) sourdoughs. Sourdoughs were daily propagated for 11 days; numbers on the x axis indicate days of propagation. Day 0 represents dough prior to fermentation and before becoming sourdough. Data are the means of three independent experiments ± standard deviations (n = 3) analyzed in duplicate. br, before refreshment.
During sourdough propagation, the median values of ΔpH ranged from 0.78 (R sourdough) to 0.90 (A sourdough). Values of ΔpH became higher than ca. 0.5 after 3, 4, or 2 days of propagation for R, A, and D sourdoughs, respectively. The maximum values (ΔpH 0.77, 0.95, and 0.93), which remained almost constant, were found after 5, 7, and 6 days of propagation for R, A, and D sourdoughs, respectively (Fig. 1).
Biochemical characteristics of sourdoughs.
The values of pH, TTA, organic acids, FAA, and FQ during dough preparation and sourdough propagation are listed in Table S1 in the supplemental material. The permutation analysis, based on biochemical characteristics and microbial cell densities, is shown in Fig. S1 in the supplemental material. Doughs and sourdoughs were distributed into two major clusters (A and B). Irrespective of the type of flour, cluster A included sourdoughs after 5 and 10 days of propagation. These sourdoughs showed the highest cell numbers of lactic acid bacteria and yeasts, almost the lowest number of Enterobacteriaceae cells, very low values of pH, and almost the highest TTA. Cluster B mainly included doughs and sourdoughs after only 1 and 2 days of propagation. Although the number of refreshments mainly affected clustering, the type of flour also had an influence. After 5 and 10 days of propagation (cluster A), R sourdoughs differed from D and A sourdoughs because of the lower concentration of lactic acid, the higher concentration of acetic acid, and, consequently, the lower FQ.
Yeast community by PCR-DGGE analysis.
DGGE profiles of amplicons from the D1-D2 region of 26S rRNA gene were similar between sourdoughs and during propagation (Table 1). Sequencing the bands revealed the presence of Saccharomyces cerevisiae, Candida humilis/Kazachstania barnettii, Wickerhamomyces anomalus, and Saccharomyces bayanus/Kazachstania sp. S. cerevisiae and S. bayanus/Kazachstania sp. were persistent in all samples, while C. humilis/K. barnettii and W. anomalus were found after 10 days of propagation in R and A sourdoughs (Table 1).
Table 1.
Sequencing results from excised PCR-DGGE-DNA bands
| Sample(s)a | Closest relative (identity [%])b | Nearest GenBank accession no. |
|---|---|---|
| D0, D1, D2, D5, D7, D10 | Triticum sp. (100) | AY049041.1 |
| R0, R1, R2, R5, R7, R10, D0, D1, D2, D5, D7, D10, A0, A1, A2, A5, A7, A10 | Saccharomyces cerevisiae (99) | JX141338.1 |
| R0 | Cryptococcus sp./Holtermanniella sp. (99) | KC442264.1/KC433527.1 |
| R0, R1, R2, R5, R7, R10, D0, D1, D2, D5, D7, D10, A0, A1, A2, A5, A7, A10 | Saccharomyces bayanus/Kazachstania sp. (99) | AY048156.1/FJ437053.1 |
| R7, A1, A2, A5, A7 | S. cerevisiae (99) | JQ672609.1 |
| R10, D10, A10 | Candida humilis/Kazachstania barnettii (100) | KC481701.1/FN393991.1 |
| R10, A10 | Wickerhamomyces anomalus (99) | JX183967.1 |
Rye, Triticum durum, and Triticum aestivum dough (after mixing and before fermentation) (R0, D0, and A0) and sourdough after 1 (R1, D1, A1), 2 (R2, D2, A2), 5 (R5, D5, A5), 7 (R7, D7, A7), and 10 (R10, D10, A10) days of propagation.
Species showing the highest identity. The percentage of identity was that determined by performing multiple sequence alignments in BLAST.
Pyrosequencing data analysis and alpha diversity.
A total of 150,292 raw sequence reads of 16S rRNA gene amplicons after 454 amplicon signal processing were obtained. After the further filtering protocols, the number of sequence reads decreased to 140,952, with an average of 4,933 reads/sample and an average length of 477 bp calculated after primer removal.
The number of OTUs, the Chao1 and Shannon indices, and Good's estimated sample coverage (ESC) are reported in Table 2. The highest diversity was found for doughs (R0, D0, and A0) and sourdoughs after 1 day of fermentation (R1, D1, and A1). Satisfactory coverage was reached for all the samples, as shown by the ESC (Table 2) and by the rarefaction curves (see Fig. S2 in the supplemental material). In particular, about 2,000 reads were enough to reach a good coverage of the microbial complexity for sourdoughs after 5 and 10 days of propagation. However, at least 4,000 reads were necessary for doughs (see Fig. S2). In other words, microbial diversity was markedly simplified after 5 days of propagation and remained almost constant at 10 days.
Table 2.
Number of sequences analyzed, observed diversity, and estimated sample coverage for 16S rRNA amplification from rye, Triticum durum, and Triticum aestivum dough and sourdougha
| Sample | No. of OTUsb | Chao1 richness | Shannon diversity index | ESC (%) |
|---|---|---|---|---|
| R0 | 151 | 274.33 | 3.13 | 99 |
| R1 | 300 | 839.55 | 4.15 | 95 |
| R2 | 136 | 244.50 | 2.51 | 99 |
| R5 | 34 | 64.60 | 0.49 | 99 |
| R10 | 108 | 156.75 | 3.10 | 99 |
| D0 | 158 | 225.03 | 2.37 | 99 |
| D1 | 159 | 489.00 | 4.18 | 96 |
| D2 | 76 | 128.80 | 3.05 | 99 |
| D5 | 96 | 178.09 | 3.06 | 99 |
| D10 | 67 | 81.25 | 2.98 | 99 |
| A0 | 180 | 347.44 | 2.47 | 99 |
| A1 | 155 | 334.11 | 3.93 | 97 |
| A2 | 64 | 180.25 | 3.29 | 98 |
| A5 | 81 | 155.38 | 3.02 | 99 |
| A10 | 70 | 145.43 | 2.89 | 99 |
| R0 RNA | 310 | 510.47 | 4.58 | 97 |
| R1 RNA | 101 | 193.81 | 0.74 | 99 |
| R2 RNA | 73 | 106.83 | 0.71 | 99 |
| R5 RNA | 36 | 55.43 | 0.22 | 99 |
| R10 RNA | 64 | 106.86 | 2.39 | 99 |
| D0 RNA | 261 | 424.28 | 4.57 | 98 |
| D1 RNA | 101 | 203.55 | 3.58 | 99 |
| D2 RNA | 83 | 108.20 | 2.77 | 99 |
| D5 RNA | 106 | 165.13 | 3.29 | 99 |
| D10 RNA | 77 | 123.50 | 3.09 | 99 |
| A0 RNA | 292 | 513.81 | 4.67 | 98 |
| A1 RNA | 67 | 125.50 | 2.88 | 99 |
| A2 RNA | 89 | 124.06 | 2.84 | 99 |
| A5 RNA | 69 | 88.25 | 2.67 | 99 |
| A10 RNA | 93 | 159.60 | 2.97 | 99 |
Sample designations are as defined for Table 1. Chao1 richness, Shannon diversity, and estimated sample coverage (ESC) were calculated with QIIME at the 3% distance level.
OTUs, operational taxonomic units.
Structure and changes of the microbiota during propagation.
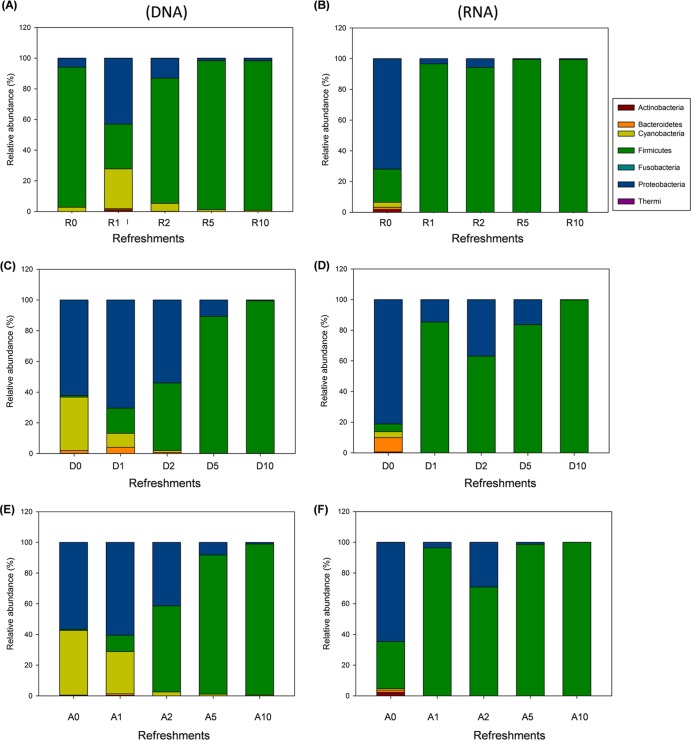
The bacterial sequences from DNA and RNA assigned to bacterial phyla and their relative abundances (%) varied depending on flour, number of propagations, and template nucleic acid (Fig. 2). Proteobacteria, Cyanobacteria, and Firmicutes were mainly found in the doughs and showed differences in abundance between DNA and RNA samples. RNA from doughs also included that of Actinobacteria and Bacteroidetes. As shown by both DNA and RNA analyses, only Firmicutes dominated at 5 and 10 days of propagation.
Fig 2.
Relative abundance of bacterial phyla in DNA (A, C, and E) and RNA (B, D, and F) samples directly extracted from rye (R), Triticum durum (D), and Triticum aestivum (A) doughs (prior to fermentation and before becoming sourdough) (R0, D0, and A0) and sourdoughs after 1 (R1, D1, A1), 2 (R2, D2, A2), 5 (R5, D5, A5), and 10 (R10, D10, A10) days of propagation.
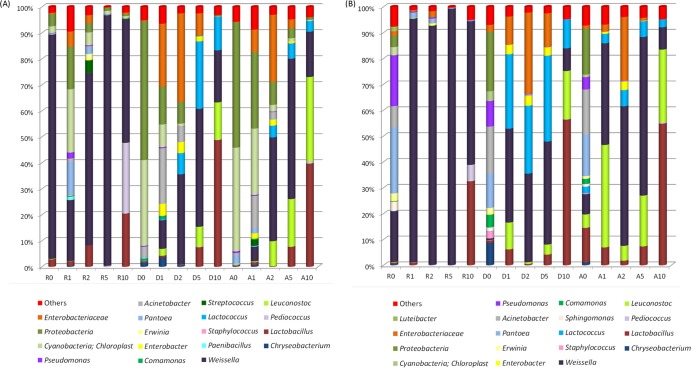
Most of the OTUs classified at genus level were similarly distributed between DNA and RNA samples (Fig. 3). The only exceptions were doughs and sourdoughs after 1 day of fermentation, in which the RNA represented a higher number of genera and relative abundance than the DNA. Pantoea (25.5%), Pseudomonas (19.7%), Weissella (19.7%), and Acinetobacter (8.3%) were the main genera found in R0 (Fig. 3B). At low incidence, Comamonas, Sphingomonas, Staphylococcus, Erwinia, Chryseobacterium and Luteibacter were also found. Soon after the first fermentation (R1), the bacterial profile markedly changed and became dominated by Weissella (94.3%), with very low incidence of the other genera. This dominance remained almost constant during propagation. At 10 days, Weissella (55.6%) still dominated, even in the presence of Lactobacillus (32.5%) and Pediococcus (6.3%). Almost the same diversity but in a different proportion was found in durum wheat sourdoughs. D0 included Acinetobacter (18.2%), Pantoea (13.3%), Pseudomonas (9.9%), Chryseobacterium (8.7%), Comamonas (4.9%), Staphylococcus (2.7%), Erwinia (2.4%), and Sphingomonas (1.5%). After the first fermentation (D1), the distribution of OTUs mainly consisted of lactic acid bacteria such as Weissella (36.3%), Lactococcus (28.8%), Leuconostoc (10.3%), and Lactobacillus (6.2%). There was an initial increase of Enterobacter and the Enterobacteriaceae family, but this disappeared after 5 days of propagation. At 10 days, Lactobacillus dominated (56.4%), followed by Leuconostoc (18.7%), Lactococcus (11.1%), and Weissella (8.8%). T. aestivum wheat sourdough showed similar microbial community dynamics. The only exception was the presence of lactic acid bacteria already in the dough (A0).
Fig 3.
Incidence of OTUs based on 16S rRNA gene pyrosequencing analysis of all DNA (A) and RNA (B) samples directly extracted from rye (R), Triticum durum (D), and Triticum aestivum (A) doughs (prior to fermentation and before becoming sourdough) (R0, D0, and A0) and sourdoughs after 1 (R1, D1, A1), 2 (R2, D2, A2), 5 (R5, D5, A5), and 10 (R10, D10, A10) days of propagation. Only OTUs with an incidence above 1% in at least one sample are shown.
Abundance of OTUs from RNA samples (Firmicutes only) is displayed in Fig. 4, with taxonomic details up to species level when such assignment was possible. Aerococcus spp., the Lactobacillus sakei group, the Lactobacillus plantarum group, Pediococcus pentosaceus, Weissella spp., Paenibacillus spp., Staphylococcus spp., and Lactococcus lactis were common to all doughs. During propagation, contaminants such as Aerococcus spp., Paenibacillus spp., and Staphylococcus spp. rapidly disappeared. Although with variable abundance, Weissella spp. dominated all three sourdoughs from the early stage of propagation, except for late fermentation of wheat sourdoughs, where the L. sakei group and Leuconostoc spp. were more abundant. Although it dominated only after 10 days of propagation, the L. sakei group occurred in all fermentations. P. pentosaceus was constantly found in rye, while it was more abundant in the late stages of propagation of wheat sourdoughs. Leuconostoc spp. and Lc. lactis were mainly found in wheat sourdoughs, especially in D sourdough for the latter species. Lactobacillus sanfranciscensis was the second dominant species of lactic acid bacteria in D dough (ca. 6%). However, it was not found in the later stages of propagation, i.e., 5 days and up. The L. plantarum group was initially found in all three doughs, ranging from ca. 0.2% to 0.8%. The abundance of the L. plantarum group increased only at the very late stage of fermentation.
Fig 4.
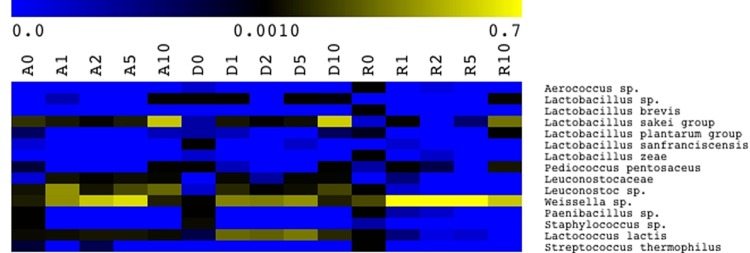
Pseudo-heat map depicting bacterial diversity and relative abundance in RNA samples directly extracted from rye (R), Triticum durum (D), and Triticum aestivum (A) doughs (prior to fermentation and before becoming sourdough) (R0, D0, and A0) and sourdoughs after 1 (R1, D1, A1), 2 (R2, D2, A2), 5 (R5, D5, A5), and 10 (R10, D10, A10) days of propagation. The color key defines the percentages of OTUs in the samples.
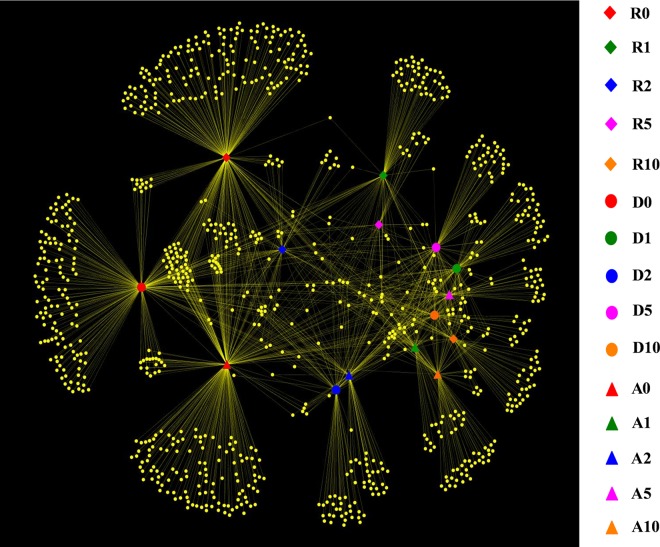
Network-based analyses were used to map sourdough microbial community composition (RNA data) onto time of propagation and type of flour (Fig. 5). The separation of OTUs and samples depended on the time of propagation. Sourdoughs tended to be more similar with increasing time of propagation, and samples, apart from the flour, showed a higher number of shared OTUs in the late stages of propagation. This seemed to highlight a cooccurring microbial community. Similar results were found by analyzing the DNA-based data set (results not shown).
Fig 5.
Simplified illustration of possible sourdough-microbe networks based on RNA data. Network diagrams are color- and symbol-coded by time of propagation and type of flour. Samples include rye (R), Triticum durum (D), and Triticum aestivum (A) doughs (prior to fermentation and before becoming sourdough) (R0, D0, and A0) and sourdoughs after 1 (R1, D1, A1), 2 (R2, D2, A2), 5 (R5, D5, A5), and 10 (R10, D10, A10) days of propagation.
The principal coordinate analysis (PCoA) based on the weighted UniFrac distance matrix is shown in Fig. S3 in the supplemental material. The two principal coordinates explained 81.7% of the total variability of the data. The distribution of sourdoughs on the plot mainly depended on time of propagation. PCoA mostly differentiated dough samples, which were characterized by higher variability and were spread in the right part of the plot. As the time of propagation increased, samples grouped together regardless of the type of flour, although rye sourdoughs tended to form a separate subgroup.
DISCUSSION
Rye and wheat (T. durum and T. aestivum) doughs and sourdoughs were prepared and propagated (backslopped) under laboratory conditions, which markedly restricted environmental contaminations. First, the phylogenetic composition of the bacterial communities during propagation was described through high-throughput sequencing of DNA and RNA.
Although there were slight variations between flours, complementary evidence suggested that sourdoughs achieved maturity during 5 to 7 days of propagation. Maturity refers to a sourdough that has reached constant technology properties (e.g., acidification, leavening capacity). At this time, presumptive lactic acid bacteria reached stable values above 9.0 log CFU g−1, the ratio between lactic acid bacteria and yeasts stabilized to ca. 100:1, and the rate of acidification became constant (ΔpH 0.77 to 0.95). Permutation analysis, based on biochemical characteristics and microbial cell densities, separated two major clusters, which included doughs and sourdoughs after 1 and 2 days of propagation and mature sourdoughs (after 5 and 10 days, respectively). The number of OTUs and alpha diversity analysis indicated that microbial diversity was markedly simplified after 5 days of propagation and remained constant. PCoA, based on the weighted UniFrac distance matrix of the number of OTUs, differentiated doughs and mainly grouped sourdoughs from 5 and 10 days regardless of the type of flour. Previously, other studies (8, 21) showed that sourdoughs became mature within 1 week, with slight variations that depended on environmental and technology determinants.
What happens prior to getting a mature sourdough? The only information available concerns the outgrowth of Gram-positive versus Gram-negative bacteria (20) and the dominance of lactic acid bacteria through a three-phase evolution (21, 22). For food ecosystems, where the microbial diversity of the metabolically active microbiota has relevant applicative repercussions, high-throughput sequencing from RNA data may provide a more complete description of the microbiota (23). Therefore, mainly RNA data will be further discussed. Flours and doughs, before fermentation, were contaminated by metabolically active phyla, which likely represented the outcome of environmental contamination. Usually, Actinobacteria and Bacteroidetes dominate the wheat root endosphere (34), Cyanobacteria are present in freshwater environments (35), and Proteobacteria are found in wastewater, forage feed, and soils (36). Genera belonging to Proteobacteria (e.g., Acinetobacter, Pantoea, Pseudomonas, Comamonas, Enterobacter, Erwinia, and Sphingomonas) and Bacteroidetes (e.g., Chryseobacterium) phyla were mainly identified. Although with some quantitative variations, these genera formed the common non-Firmicutes bacterial population of rye and wheat flours. Soon after 1 day of propagation (8 h at 25°C), this population was almost completely inhibited. The only exception was represented by the Enterobacteriaceae family (e.g., Enterobacter genus). These contaminants even increased during early propagations and were found in wheat sourdoughs until 5 days. Enterobacteriaceae grew, probably contributed to the synthesis of organic acids, and survived because of a certain tolerance of acid stress. Their inhibition required several days of propagation and almost coincided with the formation of the mature sourdough.
By definition (1), the continuous sourdough refreshments aim at selecting lactic acid bacteria and yeasts. A very abundant literature well depicted the mature sourdough microbiota, especially through culture-dependent approaches (5–12). Nevertheless, some issues are still relevant. Excluding environmental contaminations, how representative are the lactic acid bacteria within the flour autochthonous population? Culture-dependent approaches showed that lactic acid bacteria were present at low levels (ca. 2.0 log CFU g−1) in T. durum grains, and their population mainly consisted of Enterococcus, Lactobacillus, Lactococcus, Weissella, and Pediococcus genera (37). Almost the same genera inhabited maize and rye flours (11). High-throughput sequencing of RNA showed that the abundance of Firmicutes varied from ca. 30 (T. aestivum) to 5% (T. durum), with Firmicutes being quantitatively the second phylum after Proteobacteria in rye and T. aestivum flours and the third after Proteobacteria and Bacteroidetes in durum wheat flour. The Firmicutes were mainly represented by Staphylococcus in durum wheat flour and Weissella in rye flour, and multiple genera (Staphylococcus, Lactobacillus, Leuconostoc, Weissella, and Lactococcus) inhabited T. aestivum flour. Soon after 1 day of propagation (8 h at 25°C), Firmicutes became dominant also in durum wheat sourdough (relative abundance of ca. 85%). In spite of their initial low numbers, genera of lactic acid bacteria almost exclusively represented the Firmicutes. This study showed that just one fermentation was needed to completely turn the microbial diversity from mainly Proteobacteria to Firmicutes. It is also noteworthy that this turning of the microbial diversity occurred when a percentage of fermented dough that was lower than the percentage of flour was used. The evolution of yeasts was simpler. As shown by plate count, flours harbored yeasts at higher orders of magnitude than lactic acid bacteria. Their number progressively increased during propagation. As shown by PCR-DGGE analysis, S. cerevisiae occurred in all sourdoughs (10, 16, 38). The stable persistence of S. cerevisiae was mainly attributed to environmental contamination (10), but contamination from flour might also have occurred (3, 39).
A further question is the following: once lactic acid bacteria outgrow other bacteria, are genera/species always persistent during sourdough propagation? The answer might not be univocal. As shown by the pseudo-heat map depicting bacterial diversity within Firmicutes, a Weissella sp. was already the dominant species in rye flour. Subsequently, it stably dominated during propagation, though it was flanked by the L. sakei group and P. pentosaceus. A few studies demonstrated sourdough stability during long-time propagation (17). The stable persistence of lactic acid bacterium species was attributed to environmental adaptation (19) and, especially, to the synthesis of antimicrobial compounds (e.g., reutericyclin by Lactobacillus reuteri) (17). A very selective environment and, probably, different and/or diversely intense flour enzyme activities might explain the stabilization of rye compared to wheat sourdough (21). However, there was a succession of species during 10 days of propagation of wheat sourdough, according to a three-phase evolution. First, the abundance of the L. sakei group, Leuconostoc spp., Weissella spp., and Lc. lactis increased. The transitory second phase (2 to 5 days) led to a decrease of the L. sakei group and Leuconostoc spp., while Weissella spp. and Lc. lactis slightly increased. The third phase (5 to 10 days) had the opposite trend, with Leuconostoc spp. and the L. sakei group dominating. A similar evolution was described for spontaneously fermented wheat and spelt sourdoughs (21, 22). Other studies (15, 21, 22, 40) showed that the microbial diversity of sourdoughs was always fluctuating, with species and/or biotypes succeeding or alternating, though stability of the ratio between lactic acid bacteria and yeasts and of other technology parameters (e.g., acidification rate) was achieved. As shown by high-throughput sequencing of RNA, this seemed the case for the wheat sourdoughs of this study. Furthermore, L. sanfranciscensis, a key sourdough lactic acid bacterium (1), was only sporadically detected with low relative abundance. Also the L. plantarum group was found with very low incidence (0.2 to 0.8%) during late sourdough propagation. This lactic acid bacterium was previously shown to have good technology performance in sourdough (41, 42), although it apparently dominates sourdoughs during refreshments at artisan bakeries but not under laboratory conditions (15). Lactobacillus zeae and Streptococcus thermophilus were also part of this subdominant population of rye and wheat sourdoughs. Although in some cases not completely resolving at the species level, high-throughput sequencing also offered the advantage of describing subpopulations, which are hardly highlighted through culture-dependent approaches due to inherent limitations.
Overall, although high-throughput sequencing does not allow the species-specific composition of sourdough, which in turn influences the quality of bread, to be highlighted in depth, this study elucidated for the first time the microbial dynamics underlying the above microbial composition.
The OTU network analysis provided a novel and immediate interpretation of the dynamics during sourdough preparation (Fig. 5). The initial complexity of the microbiota clearly distinguishes different types of flours. However, as soon as the fermentation starts, the microbial complexity is rapidly simplified and the sourdoughs become more similar to each other, based on the presence and abundance of lactic acid bacteria driving the fermentation. In addition, core microbiota are defined, which are shared between sourdoughs at the end of fermentation. Notwithstanding variations due to environmental and technology determinants, the results of this study represent a clear example of how the microbial ecology evolves during sourdough preparation.
Supplementary Material
Footnotes
Published ahead of print 4 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02955-13.
REFERENCES
- 1.Gobbetti M. 1998. Interactions between lactic acid bacteria and yeasts in sourdoughs. Trends Food Sci. Technol. 9:267–274 [Google Scholar]
- 2.Röcken W, Voysey PA. 1995. Sourdough fermentation in bread making. J. Appl. Bacteriol. 79:S38–S48 [Google Scholar]
- 3.De Vuyst L, Vrancken G, Ravyts F, Rimaux T, Weckx S. 2009. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 26:666–675 [DOI] [PubMed] [Google Scholar]
- 4.Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M. 16 May 2013. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 10.1016/j.fm.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 5.Gobbetti M, Corsetti A, Rossi J. 1994. The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of amino acids. World J. Microbiol. Biotechnol. 10:275–279 [DOI] [PubMed] [Google Scholar]
- 6.De Vuyst L, Schrijvers V, Paramithiotis S, Hoste B, Vancanneyt M, Swings J, Kalantzopoulos G, Tsakalidou E, Messens W. 2002. The biodiversity of lactic acid bacteria in Greek traditional wheat sourdoughs is reflected in both composition and metabolite formation. Appl. Environ. Microbiol. 68:6059–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsetti A, De Angelis M, Dellaglio F, Paparella A, Fox PF, Settanni L, Gobbetti M. 2003. Characterization of sourdough lactic acid bacteria based on genotypic and cell-wall protein analysis. J. Appl. Microbiol. 94:641–654 [DOI] [PubMed] [Google Scholar]
- 8.Meroth CB, Walter J, Hertel C, Brandt MJ, Hammes WP. 2003. Monitoring the bacterial population dynamics in sourdough fermentation processes by using PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 69:475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheirlinck I, Van der Meulen R, Van Schoor A, Vancanneyt M, De Vuyst L, Vandamme P, Huys G. 2007. Influence of geographical origin and flour type on diversity of lactic acid bacteria in traditional Belgian sourdoughs. Appl. Environ. Microbiol. 73:6262–6269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minervini F, Di Cagno R, Lattanzi A, De Angelis M, Antonielli L, Cardinali G, Cappelle S, Gobbetti M. 2012. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: interactions between ingredients and microbial species diversity. Appl. Environ. Microbiol. 78:1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocha JM, Malcata FX. 2012. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microbiol. 31:72–88 [DOI] [PubMed] [Google Scholar]
- 12.Lattanzi A, Minervini F, Di Cagno R, Diviccaro A, Antonielli L, Cardinali G, Cappelle S, De Angelis M, Gobbetti M. 2013. The lactic acid bacteria and yeast microbiota of eighteen sourdoughs used for the manufacture of traditional Italian sweet leavened baked goods. Int. J. Food Microbiol. 163:71–79 [DOI] [PubMed] [Google Scholar]
- 13.Hammes WP, Brandt MJ, Francis KL, Rosenheim J, Seitter MFH, Vogelmann SA. 2005. Microbial ecology of cereal fermentations. Trends Food Sci. Technol. 16:4–11 [Google Scholar]
- 14.Vogel RF, Ehrmann MA. 2008. Sourdough fermentations, p 119–144 In Cocolin L, Ercolini D. (ed), Molecular techniques in the microbial ecology of fermented foods. Springer, Berlin, Germany [Google Scholar]
- 15.Minervini F, Lattanzi A, De Angelis M, Di Cagno R, Gobbetti M. 2012. Influence of artisan bakery- or laboratory-propagated sourdoughs on the diversity of lactic acid bacterium and yeast microbiotas. Appl. Environ. Microbiol. 78:5328–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobbetti M, De Angelis M, Corsetti A, Di Cagno R. 2005. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci. Technol. 16:57–69 [Google Scholar]
- 17.Gänzle MG, Vogel RF. 2003. Contribution of reutericyclin production to the stable persistence of Lactobacillus reuteri in an industrial sourdough fermentation. Int. J. Food Microbiol. 80:31–45 [DOI] [PubMed] [Google Scholar]
- 18.Hammes WP, Stolz P, Gänzle M. 1996. Metabolism of lactobacilli in traditional sourdoughs. Adv. Food Sci. 18:176–184 [Google Scholar]
- 19.Gänzle MG, Vermeulen N, Vogel RF. 2007. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 24:128–138 [DOI] [PubMed] [Google Scholar]
- 20.Onno B, Roussel P. 1994. Technologie et microbiologie de la panification au levain, p 293–320 In De Roissart H, Luquet FM. (ed), Bactèries lactiques, vol 11 Lorica, Uriage, France [Google Scholar]
- 21.Van der Meulen R, Scheirlinck I, Van Schoor A, Huys G, Vancanneyt M, Vandamme P, De Vuyst L. 2007. Population dynamics and metabolite target analysis during laboratory fermentations of wheat and spelt sourdoughs. Appl. Environ. Microbiol. 73:4741–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weckx S, Van der Meulen R, Maes D, Scheirlinck I, Huys G, Vandamme P, De Vuyst L. 2010. Lactic acid bacteria community dynamics and metabolite production of rye sourdough fermentations share characteristics of wheat and spelt sourdough fermentations. Food Microbiol. 27:1000–1008 [DOI] [PubMed] [Google Scholar]
- 23.Ercolini D. 2013. High-throughput sequencing and metagenomics: moving forward in the culture-independent analysis of food microbial ecology. Appl. Environ. Microbiol. 79:3148–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokulich N, Mills DA. 2012. Next-generation approaches to the microbial ecology of food fermentations. J. Biochem. Mol. Biol. 45:377–389 [DOI] [PubMed] [Google Scholar]
- 25.Ercolini D, De Filippis F, La Storia A, Iacono M. 2012. “Remake” by high-throughput sequencing of the microbiota involved in the production of water buffalo mozzarella cheese. Appl. Environ. Microbiol. 78:8142–8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeppa G, Conterna L, Gerbi V. 2001. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food Chem. 49:2722–2726 [DOI] [PubMed] [Google Scholar]
- 27.Cocolin L, Bisson LF, Mills DA. 2000. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett. 189:81–87 [DOI] [PubMed] [Google Scholar]
- 28.Di Maro E, Ercolini D, Coppola S. 2007. Yeast dynamics during spontaneous fermentation of the Catalanesca grape. Int. J. Food Microbiol. 111:201–210 [DOI] [PubMed] [Google Scholar]
- 29.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 30.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Filippis F, La Storia A, Villani F, Ercolini D. 2013. Exploring the sources of bacterial spoilers in beefsteaks by culture-independent high-throughput sequencing. PLoS One 8:e70222. 10.1371/journal.pone.0070222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378 [DOI] [PubMed] [Google Scholar]
- 33.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13:2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding GC, Piceno YM, Heuer H, Weinert N, Dohrmann AB, Carrillo A, Andersen GL, Castellanos T, Tebbe CC, Smalla K. 2013. Changes of soil bacterial diversity as a consequence of agricultural land use in a semi-arid ecosystem. Bacteroidetes. PLoS One 8:e59497. 10.1371/journal.pone.0059497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azmuda N, Rahman MZ, Madsen MS, Khan SI, Birkeland NK. 2012. Prevalence of a novel division-level bacterial lineage in Lake Dhanmondi, Dhaka, Bangladesh, as revealed by deep sequencing of 16S rRNA gene amplicons. Curr. Microbiol. 65:356–360 [DOI] [PubMed] [Google Scholar]
- 36.Benedek T, Vajna B, Táncsics A, Márialigeti K, Lányi S, Máthé I. 30 April 2013. Remarkable impact of PAHs and TPHs on the richness and diversity of bacterial species in surface soils exposed to long-term hydrocarbon pollution. World J. Microbiol. Biotechnol. 10.1007/s11274-013-1362-9.9 [DOI] [PubMed] [Google Scholar]
- 37.Corsetti A, Settanni L, Lopez CC, Felis GE, Mastrangelo M, Suzzi G. 2007. A taxonomic survey of lactic acid bacteria isolated from wheat (Triticum durum) kernels and non-conventional flours. Syst. Appl. Microbiol. 30:561–571 [DOI] [PubMed] [Google Scholar]
- 38.Scheirlinck I, Van der Meulen R, De Vuyst L, Vandamme P, Huys G. 2009. Molecular source tracking of predominant lactic acid bacteria in traditional Belgian sourdoughs and their production environments. J. Appl. Microbiol. 106:1081–1092 [DOI] [PubMed] [Google Scholar]
- 39.Vrancken G, De Vuyst L, Van der Meulen R, Huys G, Vandamme P, Daniel H-M. 2010. Yeast species composition differs between artisan bakery and spontaneous laboratory sourdoughs. FEMS Yeast Res. 10:471–481 [DOI] [PubMed] [Google Scholar]
- 40.Siragusa S, Di Cagno R, Ercolini D, Minervini F, Gobbetti M, De Angelis M. 2009. Taxonomic structure and monitoring of the dominant population of lactic acid bacteria during wheat flour sourdough type I propagation using Lactobacillus sanfranciscensis starters. Appl. Environ. Microbiol. 75:1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minervini F, De Angelis M, Di Cagno R, Pinto D, Siragusa S, Rizzello CG, Gobbetti M. 2010. Robustness of Lactobacillus plantarum starters during daily propagation of wheat flour sourdough type I. Food Microbiol. 27:897–908 [DOI] [PubMed] [Google Scholar]
- 42.Pepe O, Blaiotta G, Anastasio M, Moschetti G, Ercolini D, Villani F. 2004. Technological and molecular diversity of Lactobacillus plantarum strains isolated from naturally fermented sourdoughs. Syst. Appl. Microbiol. 27:443–453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.