Abstract
This large-scale study compared incubation temperatures (37°C versus 42°C) to study the detection of thermophilic Campylobacter species, including Campylobacter jejuni, C. coli, and C. lari, in various surface water samples and bird fecal droppings around Hamilton Harbor, Lake Ontario. The putative culture isolates obtained from incubation temperatures of 37 and 42°C were confirmed by Campylobacter genus- and species-specific triplex PCR assays targeting the 16S rRNA gene and the 16S-23S rRNA gene internal transcribed spacer (ITS) region. A total of 759 water, wastewater, and bird fecal dropping samples were tested. Positive amplification reactions for the genus Campylobacter were found for 454 (60%) samples incubated at 37°C, compared to 258 (34%) samples incubated at 42°C. C. jejuni (16%) and C. lari (12%) were detected significantly more frequently at the 42°C incubation temperature than at 37°C (8% and 5%, respectively). In contrast, significantly higher rates of C. coli (14%) and other Campylobacter spp. (36%) were detected at the 37°C incubation temperature than at 42°C (8% and 7%, respectively). These results were consistent across surface water, wastewater, and bird fecal dropping samples. At times, Campylobacter spp. were recovered and detected at 37°C (3% for C. jejuni, 10% for C. coli, and 3% for C. lari) when the same samples incubated at 42°C were negative. A significantly higher rate of other Campylobacter spp. was detected only at 37°C (32%) than only at 42°C (3%). These results indicate that incubation temperature can significantly influence the culturability and detection of thermophilic and other fastidious Campylobacter spp. and that a comprehensive characterization of the Campylobacter spp. in surface water, wastewaters, or bird fecal droppings will require incubation at both 37 and 42°C.
INTRODUCTION
Campylobacter species have been recognized as one of the leading causes of bacterial gastroenteritis in humans (1–3). The genus Campylobacter currently comprises 22 species and 8 subspecies (4), but from a public health perspective, Campylobacter jejuni, C. coli, and C. lari are the most frequently implicated species in human infections (5). These species were first isolated on a selective agar incubated microaerobically at 43°C (6, 7), and that led to the name thermophilic campylobacters. These three species can commonly occur in the gastrointestinal tracts of humans and other animals, including bovines, pigs, and birds. Sea gulls and several other wild birds can be an important source of Campylobacter spp., such as C. jejuni and C. lari (3, 8, 9). Campylobacters from the feces of birds or domestic and wild animals, municipal sewage discharges, or agricultural runoff can contaminate water (3), and water is an established vehicle for the transmission of these species to human and domestic animals, leading to outbreaks of waterborne disease.
Several Campylobacter isolation procedures have been developed for water, including an ISO standard method (10) using concentration of cells by membrane filtration or centrifugation, as well as enrichment regimens using different selective growth media. These procedures are used widely to isolate these species from food and water (11, 12). There are numerous challenges in recovery of Campylobacter spp. from water, such as the frequently small numbers of cells, low growth rate, intrinsic fastidious growth requirements, and presence of a significant proportion of organisms that may be injured or have difficulty in adapting to in vitro conditions. Incubation temperature can play a vital role in the culturability and detection of thermophilic Campylobacter spp. (13–15).
Traditionally, procedures to isolate these organisms from food, water, and feces have most commonly been conducted at a 42°C incubation temperature (16–18). Many investigations of Campylobacter spp. in water have used a 42°C incubation temperature (9, 19–25). However, other water studies have used a 37°C incubation temperature (26–28). Several studies have used the Cape Town method with an incubation temperature of 37°C for detection of Campylobacter from food and human feces, suggesting this method as better for detecting a wide range of Campylobacter spp. (14, 29–32). Although the Cape Town method has been considered a useful tool for the isolation of Campylobacter spp. from clinical samples (31, 32), other studies have reported it as insufficient for the recovery and detection of thermophilic Campylobacter spp. in aquatic environments, where species such as C. jejuni and C. lari could be present in small numbers (26, 27). Another approach in water studies has been to have a 2- to 4-h preenrichment step at 37°C before subsequent enrichment at 42°C (33–38). However, studies that used a preenrichment step of 37°C followed by enrichment at 42°C have often observed a low detection rate for C. coli, suggesting that a 42°C incubation temperature might not support the growth of stressed and injured cells of C. coli present in small numbers in water. Phillips (39) suggested that more than one incubation temperature might substantially improve the isolation of thermophilic Campylobacter spp. without diminishing the isolation of other fastidious Campylobacter spp.
To our knowledge, no large-scale study has previously compared Campylobacter detection rates at 37 and 42°C across diverse environmental matrices, such as surface water samples, municipal wastewater samples, and bird fecal droppings common to urban environments. It is important to assess the limitations of different incubation temperatures regarding the culturability and detection of thermophilic and other fastidious Campylobacter spp. from various environmental matrices. This study compared incubation temperatures of 37 and 42°C for detecting Campylobacter spp., including C. jejuni, C. coli, and C. lari, in water samples collected from two freshwater beaches and offshore harbor water, as well as municipal wastewater and bird fecal dropping samples from around Hamilton Harbor, Lake Ontario. We refer to campylobacters other than C. jejuni, C. coli, and C. lari as “other Campylobacter spp.”
MATERIALS AND METHODS
Collection of surface water and bird fecal dropping samples.
Surface water samples were collected from two freshwater beaches (Bayfront Park and Pier4 Park) in Hamilton Harbor, Lake Ontario, from 2007 to 2009. Sampling at the beaches was carried out along a single transect at each beach, at three depth zones, including sand pore water and ankle- and chest-depth waters. Sand pore water was collected by digging a hole in the wet foreshore sand about 1 meter inland from the water's edge and collecting the water that seeped into the hole. Offshore surface water samples were collected by boat from the middle of the harbor, near a wastewater treatment plant offshore outfall. Municipal wastewater samples were obtained from the final effluents of four municipal sewage treatment plants (STPs) that discharge into the harbor area and a combined sewer overflow (CSO) storage tank located at Bayfront and Pier4 Park beaches that occasionally overflowed during storm events. Fresh bird fecal dropping samples from ring-billed gulls (Larus delawarensis) and Canada geese (Branta canadensis) were collected on the beaches within 2 m of the waterline. All water, wastewater, and bird fecal samples were collected on a biweekly basis between April and December over 3 years (2007 to 2009). Water and wastewater samples were collected in 2-liter sterile bottles, whereas fecal samples were collected in sterile tubes containing 2 ml phosphate-buffered saline (1× PBS) solution. The water, wastewater, and fecal samples were returned on ice to the laboratory and processed on the same day of their collection.
Isolation and culture conditions.
Samples were processed following a protocol described by Khan et al. (12). Briefly, 1 liter of each water or wastewater sample was centrifuged at 14,000 × g for 20 min (Beckman, Indianapolis, IN), and the pellet was resuspended in 3 ml saline (0.85%) solution for concentration of Campylobacter cells. Bird fecal samples collected in PBS solution were homogenized by vortexing and analyzed to detect the presence/absence of thermophilic Campylobacter spp. To estimate the number of Campylobacter cells per liter, the resuspended pellet obtained from 1 liter of centrifuged water was analyzed by a minimum probable number (MPN) method using a 10-fold serial dilution approach for a semiquantitative analysis of Campylobacter occurrence. One-milliliter aliquots of each suspended pellet and fecal sample were inoculated into two sets of Bolton broth (Oxoid) tubes containing a selective antibiotic (cefoperazone, cycloheximide, trimethoprim, and vancomycin) supplement. The inoculum was serially diluted in the two sets of tubes, and one set each was incubated at 37 and 42°C under microaerophilic conditions (5% O2, 85% N2, and 10% CO2) for 48 h in an MCO-18 M multigas incubator (Sanyo, Tokyo, Japan). The semiquantitative enumeration was carried out by assessing turbidity and subculture confirmation. The cultures from each tube were further streaked with a sterile loop onto modified Karmali agar (MKA) (Oxoid) containing a selective supplement including antibacterial and antifungal (amphotericin B, cefoperazone, sodium pyruvate, and vancomycin) agents, and plates were incubated at 37 and 42°C under microaerophilic conditions for 24 to 48 h. The putative Campylobacter cultures were selected based on their growth characteristics and colony morphology, i.e., smooth, shiny, and convex with defined or flat edges, transparent or translucent, colorless to grayish or light cream (24). The plates that contained such colonies were further analyzed by DNA extraction and PCR assays.
DNA extraction and genus-specific PCR amplification.
DNA extractions from scraping of multiple isolates recovered from an MKA growth medium plate were carried out using a boiling protocol as previously described (40). Briefly, putative Campylobacter colonies were resuspended in 75 μl 1× TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). The suspended solution was boiled for 10 min and centrifuged. The supernatant containing DNA was transferred to a 1.5-ml sterile microcentrifuge tube and kept at −20°C for further analysis. For the confirmation of putative cultures to genus-level identification, a DNA-based PCR amplification assay was performed using Campylobacter genus-specific oligonucleotide primers (41). The 25-μl reaction mixture contained 50 to 70 ng of template DNA, 0.15 unit of Ex Taq DNA polymerase (TaKaRa, Shiga, Japan), 1× Ex Taq buffer with MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate (dNTP), and 50 ng each of forward (5′-GGA TGA CAC TTT TCG GAG C-3′) and reverse (5′-CAT TGT AGC ACG TGT GTC-3′) primers. The amplification was performed by an initial template denaturation step at 94°C for 3 min, followed by 30 cycles of amplification by repeating denaturation at 94°C for 60 s, annealing at 55°C for 60 s, and extension at 72°C for 60 s, with a final 5-min incubation at 72°C, using a Mastercycler gradient PCR system (Eppendorf, Hamburg, Germany). The PCR amplicons were electrophoresed in a 1% agarose gel matrix (Fisher Scientific, NJ) with 1× (Tris-acetate-EDTA) TAE buffer, using a 100-bp DNA size marker (PGC Scientific, MD). The gels were stained with ethidium bromide (0.5 μg/ml), visualized on a UV transilluminator, and photographed using an Ingenius Syngene Bioimaging gel documentation system.
Species-specific PCR amplification.
A species-specific PCR amplification was further performed to identify Campylobacter spp. by a triplex PCR assay using oligonucleotide primer pairs for three thermophilic Campylobacter spp. (C. jejuni, C. coli, and C. lari) as described by Khan and Edge (42). The triplex PCR amplification reaction was carried out in a Mastercycler Gradient PCR system (Eppendorf) with a 25-μl reaction mixture containing 50 to 70 ng of template DNA, 1.25 units of Ex Taq DNA polymerase (TaKaRa), 1× Ex Taq buffer with MgCl2, a 200 μM concentration of each dNTP, and 80 ng each of the forward and reverse primer pairs for each target Campylobacter spp., including C. jejuni (forward, ACT AAA TGA TTT AGT CTC A; and reverse, CTT AGA TTT ATT TTT ATC TTT AAC T), C. lari (forward, AAA TAT ATA CTT GCT TTA GAT T; and reverse, CAA TAA AAC CTT ACT ATC TC), and C. coli (forward, GAA GTA TCA ATC TTA AAA AGA TAA; and reverse, CTT ACT TTA GGT TTT AAG ACC). The final volume (25 μl) was adjusted with filtered, sterile distilled water. The PCR was performed using an initial template denaturation step of 94°C for 3 min followed by 30 cycles of amplification (denaturation at 94°C for 30 s, annealing at 46°C for 45 s, and extension at 72°C for 30 s) and ending with a 5-min extension at 72°C. Due to the expected small amplicon fragment size, the PCR amplicons were electrophoresed in a 2% agarose gel matrix, stained, and scanned as described in the preceding section.
Statistical analysis.
Statistical analyses were performed using Statistica 10.0 (StatSoft Inc.). McNemar chi-square contingency tests were applied to test for significant differences in the recovery and detection of thermophilic and other Campylobacter spp. between 37 and 42°C. Differences were considered significant if the P value was <0.05.
RESULTS
A total of 759 surface water, wastewater, and bird fecal dropping samples were collected around Hamilton Harbor between 2007 and 2009. These samples included 288 beach water samples, 89 offshore water samples, 220 wastewater samples, and 162 bird fecal dropping samples. All putative Campylobacter culture isolates observed at incubation temperatures of 37 and 42°C on MKA medium showed typical growth patterns and were consistently confirmed by the Campylobacter genus-specific 16S rRNA gene PCR assay, with an expected amplicon size of 816 bp. Species-specific detection of Campylobacter spp. in water and fecal samples was further confirmed using the triplex PCR assay, with expected amplicon sizes of 349, 279, and 72 bp for C. jejuni, C. lari, and C. coli, respectively.
Analysis of all 759 beach water, wastewater, offshore, and bird fecal samples indicated that Campylobacter was detected significantly more frequently at an incubation temperature of 37°C (60%) than at one of 42°C (34%) (P < 0.05). These findings were consistent across beach, wastewater, offshore water, and bird fecal dropping samples. Campylobacter recoveries from beach water (63%), wastewater (63%), offshore water (61%), and bird fecal (50%) samples were significantly higher (P < 0.05) at 37°C than at the 42°C incubation temperature (49%, 15%, 24%, and 39%, respectively) (Fig. 1).
Fig 1.
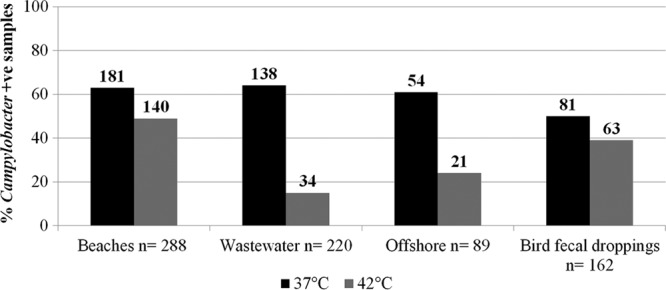
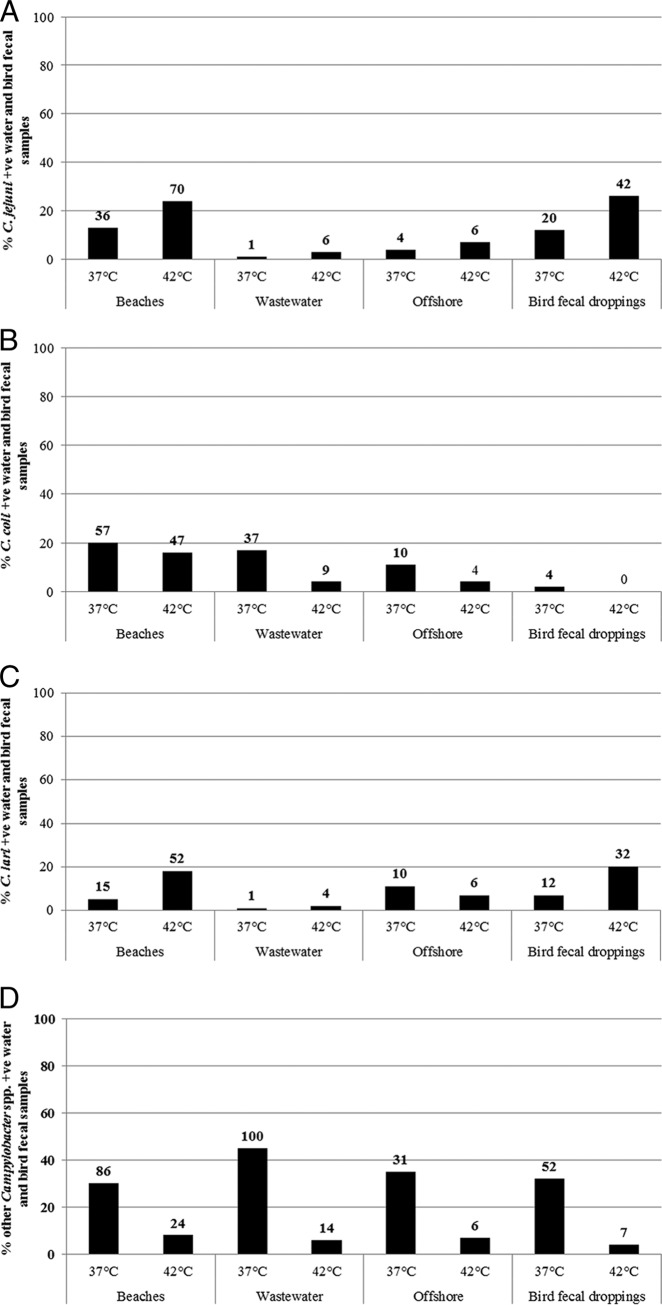
Percentages of beach water, wastewater, offshore water, and bird fecal dropping samples positive for campylobacters at two different incubation temperatures (n = total number of collected samples). The number of positive samples is presented above each bar.
A comparison of the two incubation temperatures revealed differences in the ability to recover and detect Campylobacter spp. across all 759 beach and offshore water, wastewater, and bird fecal samples (Table 1). Thermophilic Campylobacter spp., including C. jejuni (16%) and C. lari (12%), were detected significantly more often using the 42°C incubation temperature, whereas C. coli (14%) and other campylobacters (36%) were detected significantly more often using the 37°C incubation temperature (P < 0.05).
Table 1.
Numbers of beach water, wastewater, offshore water, and bird fecal dropping samples (n = 759) positive for thermophilic Campylobacter spp., using two different incubation temperature conditions
| Campylobacter(s) | No. (%) of samples |
|
|---|---|---|
| 37°C | 42°C | |
| Campylobacter spp. | 454 (60) | 258 (34) |
| C. jejuni | 61 (8) | 124 (16) |
| C. coli | 107 (14) | 60 (8) |
| C. lari | 40 (5) | 94 (12) |
| Other Campylobacter spp. | 270 (36) | 52 (7) |
These differences between incubation temperatures in the ability to recover and detect Campylobacter spp. were consistent across all beach water, offshore water, wastewater, and bird fecal samples (Fig. 2A to D). Based on the number of samples collected from each sampling location and type, C. jejuni and C. lari were always more commonly detected at 42°C than at 37°C, and they were more common in beach water samples (24 and 18%, respectively) and bird fecal droppings (26 and 20%, respectively) than in offshore (7 and 11%, respectively) and wastewater (3 and 1%, respectively) samples (Fig. 2A and C). On the other hand, C. coli and other Campylobacter spp. were always more commonly detected at 37°C than at 42°C, and they were more common in beach water (20 and 30%, respectively), wastewater (17 and 45%, respectively), and offshore (11 and 35%, respectively) samples than in bird fecal droppings (2 and 32%, respectively) (Fig. 2B and D). Interestingly, no C. coli was detected in bird fecal droppings at the 42°C incubation temperature.
Fig 2.
Percentages of recovery of C. jejuni (A), C. coli (B), C. lari (C), and other Campylobacter spp. (D) from various water and bird fecal dropping samples at two different incubation temperatures. The number of positive samples is presented above each bar.
Further analysis of the recovery and detection of multiple thermophilic Campylobacter spp., including C. jejuni, C. coli, and C. lari, in a single sample revealed an overall low frequency of co-occurrence of Campylobacter spp. in water and bird fecal samples. C. jejuni and C. lari were detected together more commonly at 42°C (6%) than at 37°C (2%) (Table 2). On the other hand, C. coli was rarely recovered together with other Campylobacter spp. (≤1%) at both incubation temperatures (Table 2). A higher frequency of the samples yielded only C. coli (13%) as opposed to only C. jejuni (5%) or C. lari (3%) at 37°C.
Table 2.
Recovery of multiple Campylobacter species from beach water, wastewater, offshore water, and bird fecal dropping samples (n = 759) at two different incubation temperatures
| Campylobacter spp. | No. (%) of samples |
|
|---|---|---|
| 37°C | 42°C | |
| C. jejuni only | 40 (5) | 63 (8) |
| C. coli only | 98 (13) | 46 (6) |
| C. lari only | 24 (3) | 37 (5) |
| C. jejuni and C. coli | 7 (1) | 5 (1) |
| C. jejuni and C. lari | 14 (2) | 48 (6) |
| C. coli and C. lari | 2 (<1) | 1 (<1) |
| C. jejuni, C. coli, and C. lari | 0 (0) | 8 (1) |
From 759 water, wastewater, and bird fecal dropping samples, a significantly higher frequency of campylobacters (35%; P < 0.05) was detected only at 37°C than only at 42°C (9%), whereas 25% of samples were positive for Campylobacter spp. at both incubation temperatures (Table 3). Significantly higher frequencies (P < 0.05) of C. jejuni (11%) and C. lari (10%) were detected only at 42°C than only at 37°C (3% for each species). In contrast, higher frequencies of C. coli (10%) and other Campylobacter spp. (32%) were detected only at 37°C than only at 42°C (Table 3).
Table 3.
Frequency of recovery of Campylobacter spp. from water and bird fecal dropping samples (n = 759) at 37 and/or 42°C
| Campylobacter(s) | No. (%) positive samples at: |
||
|---|---|---|---|
| Both temps | Only 37°C | Only 42°C | |
| Campylobacter spp. | 191 (25) | 263 (35) | 67 (9) |
| C. jejuni | 40 (5) | 21 (3) | 84 (11) |
| C. coli | 29 (4) | 78 (10) | 31 (4) |
| C. lari | 16 (2) | 24 (3) | 78 (10) |
| Other Campylobacter spp. | 26 (3) | 244 (32) | 26 (3) |
An analysis of the frequency of detection of Campylobacter at different sampling locations at 37°C and/or 42°C was performed, and the results showed that the highest frequencies of C. jejuni and C. lari were detected at 42°C for beach water (17% and 16%) and bird fecal dropping (16% and 15%) samples (Table 4). In contrast, C. coli and other Campylobacter spp. were detected most frequently at 37°C for wastewater (15% and 43%) (Table 4).
Table 4.
Frequency of recovery of thermophilic and other Campylobacter spp. from beach water, wastewater, and offshore water samples and bird fecal droppings at 37 and/or 42°Ca
| Campylobacter(s) | No. (%) positive samples |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beach water (n = 288) |
Wastewater (n = 220) |
Offshore water (n = 89) |
Bird fecal droppings (n = 162) |
|||||||||
| Both temps | Only 37°C | Only 42°C | Both temps | Only 37°C | Only 42°C | Both temps | Only 37°C | Only 42°C | Both temps | Only 37°C | Only 42°C | |
| Campylobacter spp. | 107 (37) | 73 (25) | 33 (11) | 22 (10) | 116 (52) | 11 (5) | 18 (20) | 37 (41) | 4 (4) | 44 (27) | 37 (23) | 19 (12) |
| C. jejuni | 22 (8) | 14 (5) | 48 (17) | 1 (≤1) | 0 (0) | 5 (2) | 1 (1) | 3 (3) | 5 (6) | 16 (10) | 4 (2) | 26 (16) |
| C. coli | 24 (8) | 33 (11) | 23 (8) | 4 (2) | 33 (15) | 5 (2) | 1 (1) | 9 (10) | 3 (3) | 0 (0) | 4 (2) | 0 (0) |
| C. lari | 5 (2) | 10 (3) | 47 (16) | 0 (0) | 1 (≤1) | 4 (2) | 4 (4) | 6 (7) | 2 (2) | 7 (4) | 5 (3) | 25 (15) |
| Other Campylobacter spp. | 13 (5) | 73 (25) | 11 (4) | 6 (3) | 94 (43) | 8 (4) | 1 (1) | 30 (34) | 5 (6) | 5 (3) | 47 (29) | 2 (1) |
n values are the total numbers of collected samples for the different sample types.
The concentration of Campylobacter cells (number of cells/liter) in water samples was estimated for both incubation temperatures by using an MPN method (Fig. 3). The highest Campylobacter cell concentrations (≥10,000 cells/liter) were commonly obtained from wastewater samples, where most of the samples with very high MPN values were Campylobacter negative at the 42°C incubation temperature but were found to contain C. coli or other Campylobacter spp. at 37°C. The lowest Campylobacter cell concentrations, ranging from 10 to 1,000 cells/liter, were commonly obtained from beach and offshore water samples that were positive for C. jejuni and C. lari at 42°C.
Fig 3.
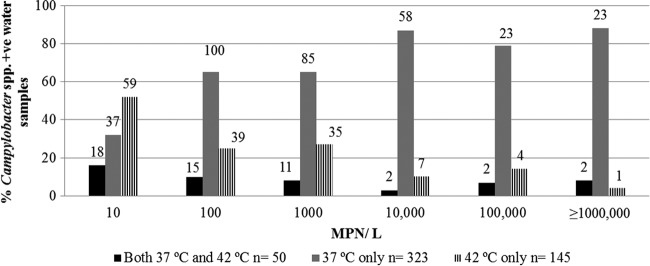
Effect of incubation temperature (37 and/or 42°C) on the percentage of Campylobacter sp.-positive beach water, wastewater, and offshore samples at various MPNs. The number of positive samples is presented above each bar.
DISCUSSION
Method comparative studies often require intensive and long-term sampling in order to capture the variation and to perform comprehensive comparative analyses as well as assessments of different parameters used in the study. This allows researchers to improve understanding and to recommend optimal parameters that can be used for future applications. Several comparison studies have been conducted for enhanced recovery and isolation of thermophilic Campylobacter spp., such as C. jejuni, from food, feces, and water by using different cell concentration approaches (filtration versus centrifugation), enrichment and growth media, and incubation durations (24 versus 48 h). However, samples processed for the isolation of thermophilic Campylobacter spp. have typically been incubated at 42°C (12, 43, 44). Preenrichment at 37°C for 2 to 4 h before exposure to selective agents and incubation at 42°C has also been reported as a recovery method that resulted in an increased isolation of Campylobacter spp. from natural waters (33–35, 37). Many previous studies on the occurrence of Campylobacter spp. in various waters, ranging from drinking water to river, lake, pond, urban, and agricultural watersheds, used a 42°C incubation temperature (9, 18–21, 23, 24, 45). These studies detected C. jejuni more commonly than other thermophilic species, such as C. coli. These results could reflect actual differences in species occurrence, or they could reflect the fact that a 42°C incubation temperature may not provide a comprehensive recovery of all campylobacters, including C. coli and other Campylobacter spp. Since C. coli has also been significantly implicated in human infections, it is important to consider the growth requirements of C. coli and other Campylobacter spp. at 37°C. Therefore, this large-scale 3-year study was designed with a goal to compare two incubation temperatures (37 versus 42°C) to determine if incubation temperature significantly influenced the detection of thermophilic Campylobacter spp. (C. jejuni, C. coli, and C. lari) that are often present in small numbers, with low growth rates, and with cells that may be stressed as well as sublethally injured. The comparative analysis was conducted on environmental samples collected from 597 water and wastewater samples and 162 bird fecal droppings, using an initial enrichment step with Bolton broth and incubation for 48 h at 37 and 42°C.
Campylobacter was detected significantly more frequently in water samples (including beach water, wastewater, and offshore water samples) and bird fecal droppings at 37°C than at the 42°C incubation temperature. In addition, the water samples collected from two different beaches showed a similar trend of significantly higher recovery of campylobacters at 37°C than at 42°C. Similar results were reported by Bolton et al. (46), where the majority of campylobacters showed better recovery and culturability at 37°C than at 42°C.
Interestingly, C. jejuni and C. lari were found to be detected more commonly at 42°C in our water and bird fecal dropping samples than at the 37°C incubation temperature. In contrast, C. coli and other Campylobacter spp. were significantly more commonly detected at 37°C than at 42°C. Studies of C. jejuni and C. fetus subsp. jejuni in human fecal samples, using incubation temperatures of 35 or 37 and 42°C and performed by Gee et al. (47) and Janssen and Helstad (48), found that 42°C resulted in recovery of more isolates of C. jejuni and C. fetus subsp. jejuni. Conversely, Bolton et al. (46) showed that C. fetus subsp. fetus grew better at 37°C than at 42°C. In our Hamilton Harbor study, the most notable difference between the two incubation temperatures was that more “other Campylobacter spp.” were recovered at 37°C than at 42°C. Other Campylobacter spp. were recovered from only 52 (7%) water and bird fecal dropping samples at 42°C, compared to 270 (36%) isolates that were recovered and detected at the 37°C incubation temperature. Although our environmental samples may have amplified the problem of recovery due to sublethal injury, cell stress, and small numbers of campylobacters, it is possible that these results are also applicable to clinical samples. For example, the occurrence of Campylobacter spp. such as C. coli may be underestimated in stool samples by using only a 42°C incubation temperature for recovering thermophilic Campylobacter spp.
It was observed that using a 37°C incubation temperature led to detection in water samples of large numbers of campylobacters (MPNs of ≥10,000 to 1,000,000 cells/liter) in 104 samples which were negative for campylobacters at 42°C. Most of the water samples with very high MPNs were found to contain C. coli or other Campylobacter spp. A 37°C incubation temperature may facilitate detection of cells of C. coli and other Campylobacter spp. in water samples by allowing growth of stressed and sublethally injured cells. It is also possible that some of the putative campylobacters detected at 37°C are as yet undescribed and potentially novel species within the Campylobacter genus or may also be closely related Campylobacter-like species from genera such as Arcobacter (24). However, Humphrey (33, 34) reported that preenrichment at 37°C may also increase isolation rates of C. jejuni from food and water, and we found that C. jejuni (3%) and C. lari (3%) cells could also be recovered at 37°C when the same samples were negative at 42°C. These results suggest that 37°C may facilitate the recovery and culturability of classical thermophilic Campylobacter spp., such as C. coli, C. jejuni, and C. lari, and also enhance the isolation of a wider range of other fastidious Campylobacter spp. that could not grow at 42°C.
Similarly, the growth of other fastidious Campylobacter spp. that may not be recovered at 37°C can be facilitated and recovered at a 42°C incubation temperature. The study suggests that 37°C could have advantages over 42°C in providing an optimum environment for enhancing culturability of C. coli and the diverse Campylobacter spp. present in water, wastewater, and bird fecal dropping samples. This appears to be particularly the case for investigating the occurrence of Campylobacter in municipal wastewaters, where an incubation temperature of 42°C may not detect many C. coli isolates. From a human health perspective, the objective of many previous studies was focused mainly on detecting clinically important species such as C. jejuni, and therefore, the 42°C incubation temperature has been used widely for the recovery and isolation of thermophilic Campylobacter spp. However, the presence of C. coli and multiple types of Campylobacter spp. in human infections has been recognized and is seen as a significant epidemiological problem, since diagnostic laboratories may only isolate and characterize a single colony. This has led to some studies suggesting that to obtain a wide range of Campylobacter spp., it may be necessary to enrich water, food, and fecal samples at incubation temperatures of both 37 and 42°C (15, 49).
In conclusion, this large-scale long-term study shows that incubation temperatures of both 37 and 42°C influence the ability to recover thermophilic and other Campylobacter spp. from water, wastewater, and bird fecal droppings, especially if unusual fastidious campylobacters are believed to be a significant problem and possible threat to human health. It is recommended that for a comprehensive characterization of the Campylobacter spp. in surface waters, wastewaters, or bird fecal droppings, incubation at both 37 and 42°C will likely be needed. Application of molecular methods would also be recommended to provide a more comprehensive characterization of campylobacters, including those that may occur in a viable but nonculturable condition. Further research on subtyping of thermophilic Campylobacter sp. isolates recovered from 37 and 42°C is needed in order to compare the strains recovered from water samples at these two temperatures. This subtyping might help in assessing strain diversity and in identifying the source of contamination of campylobacters in freshwater and bird fecal droppings.
ACKNOWLEDGMENTS
This study was funded by Environment Canada's Great Lakes Action Plan program.
We give many thanks to the City of Hamilton and the Region of Halton for providing CSO tank and STP final effluent samples. We also thank the many co-op students and technical operations (NWRI) staff for providing assistance in boat and beach sampling and in processing and analyzing data.
Footnotes
Published ahead of print 27 September 2013
REFERENCES
- 1.Park RWA, Griffiths PL, Moreno GS. 1991. Sources and survival of campylobacters: relevance to enteritis and the food industry. J. Appl. Bacteriol. 70(Suppl):97S–106S [PubMed] [Google Scholar]
- 2.Frost JA. 2001. Current epidemiological issues in human campylobacteriosis. J. Appl. Microbiol. 90(Suppl):85S–95S [DOI] [PubMed] [Google Scholar]
- 3.Jones K. 2001. Campylobacters in water, sewage and the environment. J. Appl. Microbiol. 90:68S–79S [DOI] [PubMed] [Google Scholar]
- 4.Debruyne L, Stephen LWO, Brandt ED, Vandamme P. 2009. Novel Campylobacter lari-like bacteria from humans and molluscs: description of Campylobacter peloridis sp. nov., Campylobacter lari subsp. concheus subsp. nov. and Campylobacter lari subsp. lari subsp. nov. Int. J. Syst. Evol. Microbiol. 59:1126–1132 [DOI] [PubMed] [Google Scholar]
- 5.Butzler JP. 2004. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10:868–876 [DOI] [PubMed] [Google Scholar]
- 6.Skirrow MB. 1977. Campylobacter enteritis: a “new” disease. Br. Med. J. 2:9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skirrow MB, Benjamin J. 1980. ‘1001′ campylobacters: cultural characteristics of intestinal campylobacters from man and animals. J. Hyg. 85:427–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lévesque B, Brousseau P, Bernier F, Dewailly É, Joly J. 2000. Study of the bacterial content of ring-billed gull droppings in relation to recreational water quality. Water Res. 34:1089–1096 [Google Scholar]
- 9.Van Dyke MI, Morton VK, McLellan NL, Huck PM. 2010. The occurrence of Campylobacter in river water and waterfowl within a watershed in southern Ontario, Canada. J. Appl. Microbiol. 109:1053–1066 [DOI] [PubMed] [Google Scholar]
- 10.International Organization for Standardization 2005. ISO 17995. Water quality: detection and enumeration of thermotolerant Campylobacter species. International Organization for Standardization, Geneva, Switzerland [Google Scholar]
- 11.Corry JE, Post DE, Colin P, Laisney MJ. 1995. Culture media for the isolation of campylobacters. Int. J. Food Microbiol. 26:43–76 [DOI] [PubMed] [Google Scholar]
- 12.Khan IUH, Gannon VPJ, Loughborough A, Jokinen CC, Phillips R, Kent R, Koning W, Lapen DR, Medeiros DT, Miller JJ, Neumann NF, Robertson WJ, Schreier H, Topp E, van Bochove E, Edge TA. 2009. A methods comparison for the isolation and detection of thermophilic Campylobacter in agricultural watersheds. J. Microbiol. Methods 79:307–313 [DOI] [PubMed] [Google Scholar]
- 13.Rollins DM, Colwell RR. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DM, Sutcliffe EM, Curry A. 1991. Recovery of viable but non-culturable Campylobacter jejuni. J. Gen. Microbiol. 137:2477–2482 [DOI] [PubMed] [Google Scholar]
- 15.Scates P, Moran L, Madden RH. 2003. Effect of incubation temperature on isolation of Campylobacter jejuni genotypes from foodstuffs enriched in Preston broth. Appl. Environ. Microbiol. 69:4658–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goossens H, Vlaes L, Galand I, Van den Borre C, Butzler JP. 1989. Semisolid blood-free selective-motility medium for the isolation of campylobacters from stool specimens. J. Clin. Microbiol. 27:1077–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baggerman WI, Koster T. 1992. A comparison of enrichment and membrane filtration methods for the isolation of Campylobacter from fresh and frozen foods. Food Microbiol. 9:87–94 [Google Scholar]
- 18.Savill MG, Hudson JA, Ball A, Klena JD, Scholes P, Whyte RJ, McCormick RE, Jankovic D. 2001. Enumeration of Campylobacter in New Zealand recreational and drinking waters. J. Appl. Microbiol. 91:38–46 [DOI] [PubMed] [Google Scholar]
- 19.Carter AM, Pacha RE, Clark GW, Williams EA. 1987. Seasonal occurrence of Campylobacter spp. in surface waters and their correlation with standard indicator bacteria. Appl. Environ. Microbiol. 53:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martikainen PJ, Korhonen LK, Kosunen TU. 1990. Occurrence of thermophilic campylobacters in rural and urban surface waters in central Finland. Water Res. 91:91–96 [Google Scholar]
- 21.Brennhovd O, Kapperud G, Langeland G. 1992. Survey of thermotolerant Campylobacter spp. and Yersinia spp. in three surface water sources in Norway. Int. J. Food Microbiol. 15:327–338 [DOI] [PubMed] [Google Scholar]
- 22.Walters SP, Gannon VP, Field KG. 2007. Detection of Bacteroidales fecal indicator and the zoonotic pathogens E. coli O157:H7, Salmonella, and Campylobacter in river water. Environ. Sci. Technol. 41:1856–1862 [DOI] [PubMed] [Google Scholar]
- 23.Jokinen C, Schreier H, Mauro W, Taboada E, Isaac-Renton JL, Topp E, Edge T, Thomas JE, Gannon VPJ. 2010. The occurrence and sources of Campylobacter spp., Salmonella enterica and Escherichia coli O157:H7 in the Salmon River, British Columbia, Canada. J. Water Health 8:374–386 [DOI] [PubMed] [Google Scholar]
- 24.Khan IUH, Hill S, Nowak E, Palmer ME, Jarjanazi H, Lee D-Y, Mueller M, Schop R, Weir S, Abbey AM, Winter J, Edge TA. 2013. Investigation of the prevalence of thermophilic Campylobacter species at Lake Simcoe recreational beaches. Inland Waters 3:93–104 [Google Scholar]
- 25.Ugboma AN, Salihu MD, Magaji AA, Abubakar MB. 2013. Prevalence of Campylobacter species in ground water in Sokoto, Sokoto State, Nigeria. Vet. World 6:285–287 [Google Scholar]
- 26.Diergaardt SM, Venter SN, Chalmers M, Theron J, Brözel VS. 2003. Evaluation of the Cape Town Protocol for the isolation of campylobacters from environmental waters. Water SA 29:225–229 [Google Scholar]
- 27.Diergaardt SM, Venter SN, Spreeth A, Theron J, Brözel VS. 2004. The occurrence of campylobacters in water sources in South Africa. Water Res. 38:2589–2595 [DOI] [PubMed] [Google Scholar]
- 28.Hu TL, Kuo PC. 2011. Isolation of Campylobacter spp. in surface waters in Taiwan. J. Microbiol. Immunol. Infect. 44:15–20 [DOI] [PubMed] [Google Scholar]
- 29.Steele TW, McDermott SN. 1984. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 16:263–265 [DOI] [PubMed] [Google Scholar]
- 30.Koenraad PMFJ, Rombouts FM, Notermans SHW. 1997. Epidemiological aspects of thermophilic Campylobacter in water-related environments: a review. Water Environ. Res. 69:52–63 [Google Scholar]
- 31.Le Roux E, Lastovica AJ. 1998. The Cape Town protocol: how to isolate the most campylobacters for your dollar, pound, franc, yen, etc., p 31–33 In Lastovica AJ, Newell D, Lastovica EE. (ed), Proceedings of the 9th International Workshop on Campylobacter, Helicobacter and related organisms. Institute of Child Health, Cape Town, South Africa [Google Scholar]
- 32.Engberg J, On SLW, Harrington CS, Gerner-Smidt P. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphrey TJ. 1986. Techniques for the optimum recovery of cold injured Campylobacter jejuni from milk or water. J. Appl. Bacteriol. 61:125–132 [DOI] [PubMed] [Google Scholar]
- 34.Humphrey TJ. 1989. An appraisal of the efficacy of pre-enrichment for the isolation of Campylobacter jejuni from water and food. J. Appl. Bacteriol. 66:119–126 [DOI] [PubMed] [Google Scholar]
- 35.Koenraad PMFJ, Hazeleger WC, Laan van der Beumer TRR, Rombouts FM. 1994. Survey of Campylobacter spp. in sewage plants in The Netherlands. Food Microbiol. 11:65–73 [Google Scholar]
- 36.Lévesque S, St-Pierre K, Frost E, Michaud S. 2005. Determination of the optimal culture conditions for detecting Campylobacter spp. in environmental water, abstr A49 13th Int. Workshop Campylobacter, Helicobacter Related Organisms, Gold Coast, Queensland, Australia [Google Scholar]
- 37.St-Pierre K, Lévesque S, Frost E, Carrier N, Arbeit RD, Michaud S. 2009. Thermotolerant coliforms are not a good surrogate for Campylobacter spp. in environmental water. Appl. Environ. Microbiol. 75:6736–6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lévesque S, St-Pierre K, Frost E, Arbeit RD, Michaud S. 2011. Determination of the optimal culture conditions for detecting thermophilic campylobacters in environmental water. J. Microbiol. Methods 86:82–88 [DOI] [PubMed] [Google Scholar]
- 39.Phillips CA. 1995. Incidence, epidemiology and prevention of foodborne Campylobacter species. Trends Food Sci. Technol. 6:83–87 [Google Scholar]
- 40.Hamelin K, Bruant G, El-Shaarawi A, Hill S, Edge TA, Fairbrother J, Harel J, Maynard C, Masson L, Brousseau R. 2007. Occurrence of virulence and antimicrobial resistance genes in Escherichia coli isolates from different aquatic ecosystems within the St. Clair River and Detroit River areas. Appl. Environ. Microbiol. 73:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linton D, Owen RJ, Stanley J. 1996. Rapid identification by PCR of the genus Campylobacter and of five Campylobacter species enteropathogenic for man and animals. Res. Microbiol. 147:707–718 [DOI] [PubMed] [Google Scholar]
- 42.Khan IUH, Edge TA. 2007. Development of a novel triplex PCR assay for the detection and differentiation of thermophilic species of Campylobacter using 16S-23S rDNA internal transcribed spacer (ITS) region. J. Appl. Microbiol. 103:2561–2569 [DOI] [PubMed] [Google Scholar]
- 43.Karmali MA, Simor AE, Roscoe M, Fleming PC, Smith SS, Lane J. 1986. Evaluation of a blood-free, charcoal-based, selective medium for the isolation of Campylobacter organisms from feces. J. Clin. Microbiol. 23:456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korhonen LK, Martikainen PJ. 1990. Comparison of some enrichment broths and growth media for the isolation of thermophilic campylobacters from surface water samples. J. Appl. Microbiol. 68:593–599 [DOI] [PubMed] [Google Scholar]
- 45.Bolton FJ, Coates D, Hutchinson DN, Godfree AF. 1987. A study of thermophilic campylobacters in river system. J. Appl. Bacteriol. 62:167–176 [DOI] [PubMed] [Google Scholar]
- 46.Bolton FJ, Hutchinson DN, Parker G. 1988. Reassessment of selective agars and filtration techniques for isolation of Campylobacter species from faeces. Eur. J. Clin. Microbiol. Infect. Dis. 7:155–160 [DOI] [PubMed] [Google Scholar]
- 47.Gee B, Nye KJ, Fallon D, Messer S, Howe S, Warren RE, Andrews N. 2002. Effect of incubation temperature on the isolation of thermophilic species of Campylobacter from faeces. Commun. Dis. Public Health 5:282–284 [PubMed] [Google Scholar]
- 48.Janssen D, Helstad AG. 1982. Isolation of Campylobacter fetus subsp. jejuni from human fecal specimens by incubation at 35 and 42°C. J. Clin. Microbiol. 16:398–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson JF, Frost JA, Kramer JM, Thwaites RT, Bolton FJ, Wareing DRA, Gordon JA. 2001. Coinfection with Campylobacter species: an epidemiological problem? J. Appl. Microbiol. 91:206–211 [DOI] [PubMed] [Google Scholar]