Abstract
In West and Central Africa, virulent Newcastle disease virus (NDV) strains of the recently identified genotypes XIV, XVII, and XVIII are enzootic in poultry, representing a considerable threat to the sector. The increasing number of reports of virulent strains in wild birds at least in other parts of the world raised the question of a potential role of wild birds in the spread of virulent NDV in sub-Saharan Africa as well. We investigated 1,723 asymptomatic birds sampled at live-bird markets and sites important for wild-bird conservation in Nigeria and 19 sick or dead wild birds in Côte d'Ivoire for NDV class I and II. Typical avirulent wild-type genotype I strains were found in wild waterfowl in wetlands in northeastern Nigeria. They were unrelated to vaccine strains, and the involvement of inter- or intracontinental migratory birds in their circulation in the region is suggested. Phylogenetic analyses also revealed that genotype VI strains found in pigeons, including some putative new subgenotype VIh and VIi strains, were introduced on multiple separate occasions in Nigeria. A single virulent genotype XVIII strain was found in a dead wild bird in Côte d'Ivoire, probably as a result of spillover from sick poultry. In conclusion, screening of wild birds and pigeons for NDV revealed the presence a variety of virulent and avirulent strains in West Africa but did not provide strong evidence that wild birds play an important role in the spread of virulent strains in the region.
INTRODUCTION
Newcastle disease, caused by virulent Newcastle disease virus (NDV), an avian Paramyxovirus, is one of the most important diseases in poultry. Depending on the severity of the clinical symptoms observed in chickens, NDV are separated into asymptomatic enteric, lentogenic, mesogenic, viscerotropic velogenic, and neurotropic velogenic pathotypes (1). In addition, avirulent and virulent strains can be distinguished on the basis of the cleavage site sequence of their fusion (F) protein (1), one of the most important virulence factors.
Although all NDV strains belong to a single serotype, they are nevertheless genetically highly diverse. Based on the complete fusion gene sequences, NDV strains are divided into two classes, I and II. In the latter, at least 18 genotypes and multiple subgenotypes have been defined (2–4), but the diversity continues to increase as surveillance improves.
Virtually all domestic and wild bird species are susceptible to infection with NDV (5). Wild waterbirds seem to be the reservoir of avirulent strains, whereas poultry are the most likely reservoir of virulent viruses, but both hosts exchange viruses. In Israel and Mexico, when captive zoo birds became infected with virulent strains similar to those causing outbreaks in local poultry, spillover from infected domestic birds was suspected but never demonstrated (6, 7). However, free-living migratory species, such as waterfowls or white storks, may carry virulent NDV strains without obvious contact with poultry (8–10). Thus, it could be that wild birds are carriers of virulent strains but transmission routes of virulent NDV strains in particular are not yet fully understood. In West and Central Africa, virulent strains of the recently identified genotypes XIV, XVII, and XVIII are enzootic in poultry (4). Highly similar strains in poultry across West and Central Africa (4) and an increasing number of reports of virulent strains in wild birds on other continents (8–10) raise the question of the potential role of wild birds in the spread of virulent NDV in sub-Saharan Africa as well. Anti-NDV antibodies and NDV strains have been detected in wild bird species in South Africa (11), Burkina Faso (12), and Nigeria (13, 14), but genetic information was available only from doves and pigeons (genotype VI) in Nigeria (15, 16) and South Africa (17). In this study, we therefore investigated the presence of NDV in wild birds and pigeons in Nigeria and Côte d'Ivoire.
MATERIALS AND METHODS
Sample collection.
A total of 1,723 asymptomatic birds were sampled in six Nigerian states (Oyo, n = 830; Yobe, n = 668; Plateau, n = 154; Sokoto, n = 37; Lagos, n = 32; Nasarawa, n = 2) between 2006 and 2013 (Table 1; Fig. 1). Pooled oropharyngeal and cloacal swabs (n = 1345), cloacal swabs (n = 74), tracheal swabs (n = 12), and fresh feces (n = 292) were collected, and bird species were recorded. Birds were sampled at live-bird markets (n = 140) or in three sites important for wild bird conservation (n = 1,583), including the International Institute for Tropical Agriculture Forest Reserve (Oyo State), the Amurum woodlands (Plateau State), and the Hadejia-Nguru wetlands (Yobe State). Organs from 19 moribund or dead birds were collected in Lagunes province in Côte d'Ivoire between 2006 and 2008 (Table 1).
Table 1.
Number of birds (classified by order and family) sampled in six Nigerian states (2006 to 2013) and Côte d'Ivoire (2006 to 2008) tested for NDV
| Order | Family | No. of samples collected (no. positive) |
||||||
|---|---|---|---|---|---|---|---|---|
| Nigeria |
Côte d'Ivoire | |||||||
| Oyo | Yobe | Plateau | Lagos | Nasarawa | Sokoto | |||
| Accipitriformes | Accipitridae | 4 | 4 | |||||
| Anseriformes | Anatidae | 143 | 130 (5) | |||||
| Charadriiformes | Burhinidae | 3 | ||||||
| Charadriidae | 6 | 1 | ||||||
| Jacanidae | 8 | 32 | ||||||
| Rostratulidae | 12 | |||||||
| Scolopacidae | 18 | |||||||
| Ciconiiformes | Ciconiidae | 6 | ||||||
| Coliiformes | Coliidae | 9 | ||||||
| Columbiformes | Columbidae | 70 (8) | 161 | 4 | 32 (1) | 2 | 37 | 11 |
| Coraciiformes | Alcedinidae | 10 | 20 | 1 | ||||
| Coraciidae | 1 | |||||||
| Cuculiformes | Cuculidae | 1 | 1 | |||||
| Galliformes | Phasianidae | 1 | 1 | |||||
| Gruiformes | Rallidae | 5 | 8 | |||||
| Passeriformes | Acrocephalidae | 4 | 1 | |||||
| Alaudidae | 1 | |||||||
| Cisticolidae | 46 | 22 | ||||||
| Corvidae | 4 | |||||||
| Dicruridae | 2 | 1 | ||||||
| Emberizidae | 1 | |||||||
| Estrildidae | 23 | 14 | 38 | |||||
| Fringillidae | 37 | |||||||
| Hirundinidae | 9 | |||||||
| Macrosphenidae | 8 | 6 | ||||||
| Malconotidae | 7 | |||||||
| Monarchidae | 28 | |||||||
| Motacillidae | 14 | 1 | ||||||
| Muscicapidae | 14 | 6 | ||||||
| Nectariniidae | 82 | 8 | ||||||
| Nicatoridae | 5 | |||||||
| Passeridae | 56 | 2 | ||||||
| Pellorneidae | 9 | |||||||
| Phylloscoidae | 2 | |||||||
| Platysteiridae | 11 | 1 | ||||||
| Ploceidae | 34 | 123 | 12 | 1 (1) | ||||
| Pycnonotidae | 225 | 3 | ||||||
| Stenostiridae | 1 | |||||||
| Sylviidae | 2 | |||||||
| Turdidae | 6 | 2 | ||||||
| Viduidae | 21 | 1 | ||||||
| Zosteropidae | 4 | |||||||
| Unknown | 3 | |||||||
| Pelecaniformes | Ardeidae | 5 | 1 | |||||
| Piciformes | Indicatoridae | 1 | 2 | |||||
| Lybiidae | 2 | 11 | ||||||
| Picidae | 5 | 5 | ||||||
| Unknown | 66 | 1 | ||||||
| Total | 830 (8) | 668 (5) | 154 | 32 (1) | 2 | 37 | 19 (1) | |
Fig 1.
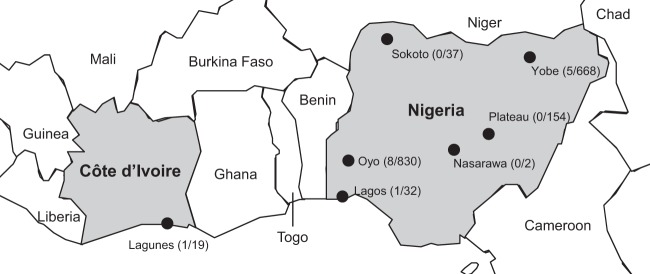
Collection sites in Nigeria and Côte d'Ivoire. Sampled provinces or states are indicated by circles. The number of positive samples and the total number of samples tested are in parentheses. (Base map copyright, Map Resources; reproduced with permission.)
Nucleic acid extraction, PCR, and sequencing.
All swabs and approximately 100 mg of fecal material were homogenized and stored in 500 μl of virus transport medium (phosphate-buffered saline with 2,000 U of penicillin/ml, 200 mg of streptomycin/ml, 2,000 U of polymyxin B/ml, 250 mg of gentamicin/ml, 60 mg of ofloxacin/ml, 200 mg of sulfamethoxazole/ml, and 2.5 mg of amphotericin B/ml). RNA was extracted from 140 μl of medium using a QIAamp viral RNA minikit (Qiagen, Venlo, The Netherlands) or from 50 μl using a MagMAX-96 AI/ND viral RNA isolation kit (Life Technologies, Merelbeke, Belgium) with KingFisher (Thermo Fisher, Waltham, MA). Organs were homogenized in 600 μl of lysis buffer of the RNeasy minikit (Qiagen) with stainless steel beads and TissueLyzer II (Qiagen). RNA extraction was then performed according to the manufacturer's instructions. NDV detection was performed by a multiplex real-time RT-PCR detecting both class I and class II strains (18). Full-length (n = 13) or partial (n = 2) F genes were amplified using several overlapping fragments in (semi)nested PCRs (primer sequences are available upon request). PCR product purification, sequencing, and contig assembly were performed as described before (4). The primers used for generating the PCR fragments were also utilized in the sequencing reaction.
Data analysis.
Genetic distances and between-group distances were calculated using the maximum composite likelihood model and 500 bootstrap replicates as implemented in MEGA v5.03 (19). Amino acid distances of deduced fusion protein sequences were calculated with the Poisson correction model (MEGA v5.03). Phylogenetic analyses including 1,164 complete F gene sequences available on GenBank and 13 sequences obtained in this study were performed with the maximum likelihood method (MEGA v5.03). Criteria to define new subgenotypes were based on those reported previously (3, 4), i.e., (i) the tree topology, (ii) bootstrap values of ≥60%, (iii) mean evolutionary distances between subgenotypes between 0.03 and 0.1, and (iv) a minimum of four sequences from at least two distinct outbreaks per subgenotype. Phylogenetic analyses on partial sequences (372 nucleotides), including 3,453 publicly available sequences and 15 sequences from this study, were performed with the neighbor-joining method with the Kimura 2-parameter model (MEGA v5.03). Representative strains were selected on the basis of these preliminary analyses. Final trees were calculated with the maximum likelihood method and the general time-reversible (GTR) substitution model with a gamma (G) and invariant (I) site heterogeneity model and are displayed in Fig. 2. The following strain nomenclature was used: host/country/strain number/year.
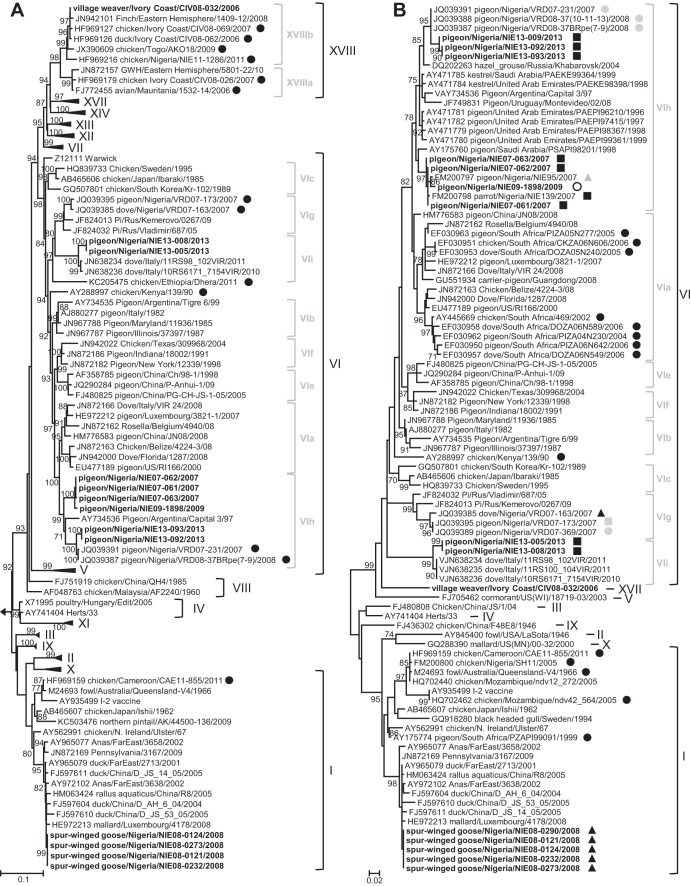
Fig 2.
Phylogenetic analysis of complete (1,662 nt) (A) or partial (372 nt) (B) F gene sequences. (A) Phylogenetic tree calculated with class I strain Goose/Alaska/415/1991 as the outgroup. Sequences generated in this study are in bold. Previously published sequences are indicated with their accession numbers. African strains are shown with black circles. (B) The state of origin of Nigerian sequences is indicated as follows: gray circles, Jigawa State; white circles, Lagos State; black squares, Oyo State; gray squares, Kano State; black triangles, Yobe State; gray triangles, Sokoto State. Only bootstrap values of ≥70% are shown. The scale corresponds to the number of base substitutions per site. GWH, green wood hoopoe.
Nucleotide sequence accession numbers.
Sequences were submitted to GenBank under accession numbers HG326600 to HG326609 and HG424625 to HG424629.
RESULTS AND DISCUSSION
Fifteen out of 1,742 birds tested positive for NDV. Based on partial or full F-gene sequences (Fig. 2A and B), five strains were assigned to genotype I, nine strains to genotype VI, and one to genotype XVIII, all in class II. No sample was positive for class I strains.
Genotype I.
Five highly similar sequences (genetic distance, 0 to 0.1%) from spur-winged geese (Plectropterus gambensis, order Anseriformes) sampled in the Hadejia-Nguru wetlands in Yobe State, Nigeria, during three consecutive days in April 2008 formed a cluster within genotype I (Fig. 2A), suggesting that they shared a very recent ancestor. Their cleavage site sequence, 112GKQGR↓L117 (the arrow represents the site of cleavage of the precursor protein, F0, into its F1 and F2 subunits), was typical of avirulent genotype I strains. All strains belonged to a cluster of strains found mainly in migratory wild waterfowl and domestic ducks in Asia, the United States, and Europe (Fig. 2A). They were not related to live vaccine strains derived from Ulster, Queensland/V4, or I-2, in contrast to other genotype I strains found in South Africa, Nigeria, Cameroon, and Mozambique (Fig. 2B). Their antigenic distances from genotype I and II vaccine strains ranged from 0.011 to 0.035 and 0.069 to 0.075, respectively (Table 2). No similar genotype I strains were detected in poultry in West and Central Africa even during more intensive sampling periods in 2006 to 2011 (4), suggesting that these strains may have been introduced more recently by intercontinental migratory birds. Indeed, in the wetlands in northeastern Nigeria, spur-winged geese often mix with overwintering Eurasian migratory birds (20). Equally, the paucity of wild bird screening for NDV in Africa makes it possible that such genotype I strains circulate in African wild birds and may have been introduced in the wetlands by birds migrating within the African continent. However, virus introduction by poultry also cannot be excluded, despite the ban on importation of poultry into Nigeria since 2002. The presence of free-roaming domestic ducks in the wetlands (21) offers opportunities for transmission of NDV from wild to domestic waterfowl and vice versa.
Table 2.
Estimates of evolutionary distances of deduced fusion proteins between 13 strains obtained in this study and common vaccine strains of genotypes I and IIa
| Strain | Distance (no. of amino acid substitutions per site) |
||||
|---|---|---|---|---|---|
| Genotype I |
Genotype II |
||||
| Queensland-V4 (M24693) | Ulster (M24694) | I-2 (AY935499) | LaSota (AY845400) | B1 (JN872150) | |
| Pigeon/Nigeria/NIE07-061/2007 | 0.101 | 0.087 | 0.099 | 0.115 | 0.109 |
| Pigeon/Nigeria/NIE07-062/2007 | 0.101 | 0.087 | 0.099 | 0.115 | 0.109 |
| Pigeon/Nigeria/NIE07-063/2007 | 0.103 | 0.089 | 0.101 | 0.117 | 0.111 |
| Pigeon/Nigeria/NIE09-1898/2009 | 0.101 | 0.087 | 0.097 | 0.115 | 0.109 |
| Pigeon/Nigeria/NIE13-005/2013 | 0.129 | 0.115 | 0.125 | 0.156 | 0.15 |
| Pigeon/Nigeria/NIE13-008/2013 | 0.129 | 0.115 | 0.125 | 0.156 | 0.15 |
| Pigeon/Nigeria/NIE13-092/2013 | 0.111 | 0.099 | 0.111 | 0.125 | 0.119 |
| Pigeon/Nigeria/NIE13-093/2013 | 0.111 | 0.099 | 0.111 | 0.125 | 0.119 |
| Spur-winged goose/Nigeria/NIE08-0121/2008 | 0.029 | 0.013 | 0.035 | 0.075 | 0.069 |
| Spur-winged goose/Nigeria/NIE08-0124/2008 | 0.027 | 0.011 | 0.033 | 0.073 | 0.071 |
| Spur-winged goose/Nigeria/NIE08-0232/2008 | 0.028 | 0.011 | 0.033 | 0.073 | 0.069 |
| Spur-winged goose/Nigeria/NIE08-0273/2008 | 0.028 | 0.011 | 0.033 | 0.071 | 0.069 |
| Village weaver/Ivory Coast/CIV08-032/2006 | 0.101 | 0.091 | 0.093 | 0.119 | 0.117 |
Analyses were conducted using the Poisson correction model in MEGA v5.03. All ambiguous positions were removed for each sequence pair. There were a total of 553 positions in the final data set.
Interestingly, 650 birds belonging to 44 other species were also sampled during the same period of time (March-April 2008) in the same region, but no other species tested positive for NDV. Also, spur-winged geese and white-faced whistling ducks (Dendrocygna viduata) were the only two species carrying avian influenza H5N2 viruses among 17 (22) and 44 (23) species investigated, suggesting that they may play a particular role in the epidemiology of both avian influenza and NDV in the region. These two species have similar behavior, i.e., they forage in shallow water, are both intracontinental migrants, and gather around water bodies (24) where they can intermix with migratory birds, which have been shown as risk factors for avian influenza reservoirs (25).
Genotype VI.
Nine sequences from pigeons sampled in Nigerian live-bird markets clustered in genotype VI (Fig. 2A and B). One cluster was formed by three very similar strains (genetic distances 0.1 to 0.2%) collected in Oyo State in 2007 and a fourth sequence from a pigeon sampled in Lagos State in 2009 (genetic distance of 0.7 to 0.8% from the three strains from 2007) (Fig. 2A). They shared the same predicted virulent cleavage site sequence, 112RRKKR↓F117. Phylogenetic analyses based on partial F gene revealed that these four strains also clustered with two other Nigerian strains from Oyo (Parrot/Nigeria/NIE139/2007) and Sokoto (Pigeon/Nigeria/NIE95/2007) states (Fig. 2B). Three strains (Oyo State, 2013) clustered together with pigeon strains from Jigawa State (Fig. 2A and B) and shared the same virulent cleavage site, 112RRRKR↓F117. The clustering of the Nigerian strains together with Pigeon/Argentina/Capital_3/97 as a distinct subgroup within genotype VI and their great genetic distance from other VI subgenotypes (0.07 to 0.104) (Table 3), greater than 0.03 (the cutoff for subgenotypes), suggested the definition of a new subgenotype, VIh. In addition, two more strains (Oyo State, 2013) clustered with recent Italian strains identified during an episode of high mortality in collared doves (26) and shared the same 112RRQKR↓F117 cleavage site motif. Similarly, their genetic distances from other subgenotypes within VI (0.094 to 0.123) (Table 3) would indicate an additional subgenotype, VIi. However, some of the genetic distances between subgenotype VIi and the other VI subgenotypes were even higher than the 0.1 cutoff value between genotypes, suggesting that subgenotype VIi may need to be further subdivided when more strains become available. The presence of similar strains (subgenotype VIh) (Fig. 2B) in several Nigerian states suggests an exchange between regions. However, none of them were related to other genotype VI strains from the more distant countries, such as South Africa (subgenotype VIa), Kenya, or Ethiopia (Fig. 2B), suggesting multiple introductions of genotype VI strains into Africa during the last 10 years and limited long-distance circulation.
Table 3.
Estimates of evolutionary distances over sequence pairs between subgenotypes of genotype VI
| Subgenotype | No. of strains | No. of base substitutions per site or standard error estimatea |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| VIa | VIb | VIc | VIe | VIf | VIg | VIh | VIi | ||
| VIa | 70 | [0.005] | [0.006] | [0.006] | [0.006] | [0.007] | [0.005] | [0.008] | |
| VIb | 10 | 0.067 | [0.005] | [0.006] | [0.005] | [0.006] | [0.006] | [0.008] | |
| VIc | 12 | 0.09 | 0.071 | [0.006] | [0.006] | [0.006] | [0.007] | [0.007] | |
| VIe | 12 | 0.077 | 0.064 | 0.085 | [0.005] | [0.008] | [0.006] | [0.008] | |
| VIf | 12 | 0.07 | 0.057 | 0.083 | 0.06 | [0.007] | [0.005] | [0.008] | |
| VIg | 4 | 0.092 | 0.08 | 0.088 | 0.097 | 0.089 | [0.008] | [0.008] | |
| VIh | 9 | 0.07 | 0.072 | 0.101 | 0.086 | 0.082 | 0.104 | [0.009] | |
| VIi | 5 | 0.112 | 0.101 | 0.106 | 0.11 | 0.107 | 0.094 | 0.123 | |
Numbers below the diagonal are numbers of base substitutions per site from averaging over all sequence pairs between groups (1,662 nucleotides). Numbers above the diagonal are standard error estimates (500 bootstrap replicates). Analyses were conducted using the maximum composite likelihood model in MEGA v5.03. Codon positions included were first + second + third + noncoding. All ambiguous positions were removed for each sequence pair.
All neutralizing epitopes on the F protein (D72, E74, A75, K78, A79, L343, 151ILRLKESIAATNEAVHEVTDG171, [27, 28]) were conserved between vaccine and genotype VI strains, except for two subgenotype VIh strains (Pigeon/Nigeria/NIE13-092/2013 and Pigeon/Nigeria/NIE13-093/2013) that shared a A79V substitution. Amino acid distances of genotype VI strains to genotype I or II vaccine strains ranged from 0.087 to 0.129 and 0.109 to 0.156, respectively (Table 2).
In some countries outside Africa, genotype VI strains from domestic pigeons spread to other species, such as feral pigeons and doves, where they became enzootic (29, 30), sometimes resulting in high mortality rates (26, 31). In our study, most NDV-positive pigeons were sampled in live-bird markets, indicating that they were most likely domestic pigeons reared for their meat. Nevertheless, 169 samples from nine wild dove species were negative for NDV, suggesting that genotype VI may not yet be enzootic in wild doves in the sampled region. Genotype VI was also not found in more than 3,000 domestic birds sampled in West and Central Africa (4). Nevertheless, these virulent genotype VI strains can also cause serious outbreaks in chickens (32, 33), for which they may be virulent, at least after circulation and adaptation (34, 35). At least two such outbreaks have been reported from South Africa (17) (subgenotype VIa) (Fig. 2B), where NDV surveillance is more intense. Although vaccination usually protects against clinical signs and mortality induced by virulent strains, not all farmers can afford the costs, and inappropriate vaccination may reduce efficacy. In addition, as a result of antigenic distances of these genotype VI strains from commonly used genotype I and II vaccines, vaccination may provide only incomplete protection (36, 37). Vaccines with at least a fusion protein sequence closer to the challenge strains would most probably provide a better immunity (38). Thus, the mingling of species in live-poultry markets increases the risk of transmission of genotype VI viruses to chickens, an additional risk factor for the poultry industry in Africa, already weakened by the circulation of other virulent NDV strains (4). Therefore, surveillance in domestic and wild Columbidae species is warranted to assess the risk associated with these virulent genotype VI strains.
Genotype XVIII.
One strain from a village weaver (Ploceus cucullatus, order Passeriformes) sampled in Côte d'Ivoire clustered within subgenotype XVIIIb together with chicken and duck strains from Côte d'Ivoire, Nigeria, and Togo (Fig. 2A). Genetic distances ranged from 0.4% for duck/Ivory Coast/CIV08-062/2006 to 3% for chicken/Nigeria/NIE11-1286/2011. Its cleavage site sequence, 112RRQKR↓F117, and the presence of a 6-nucleotide insertion in the intergenic region between the hemagglutinin-neuraminidase and polymerase genes (data not shown) were typical of virulent subgenotype XVIIIb isolates (4). This is compatible with the death of the bird, although another cause of death was not ruled out by a necropsy. This isolate represents so far the only case of the recently defined genotypes XIV, XVII, and XVIII in wild birds, except for two strains from a finch and a green wood hoopoe isolated from an import quarantine station in the United States (Fig. 2A) (39). In contrast to the spur-winged geese, village weavers were most probably infected by direct or indirect contact with sick poultry, since other cases of infection with genotype XVIIIb viruses were found in chickens in the same region in Côte d'Ivoire (4). Village weavers live in groups in urban or rural areas, feed on insects and seeds, and sometimes interact with free-roaming poultry during feeding. Similarly, cases of spillover of virulent NDV strains to wild birds were already described in China, where house sparrows (Passer domesticus) were infected with genotype VII strains similar to those causing outbreaks in poultry in the same area (40). The role of these synanthropic species in the epidemiology and spread of virulent NDV is unclear, since the virulence of the strains varies between species. However, morbidity and mortality of village weavers were likely to limit the spread of the virus. So far, in wild birds, virulent strains seem only enzootic in cormorants in North America (41) and in pigeons worldwide (42–44), but the two genotype XVIII cases detected in imported wild birds in the United States are puzzling. Therefore, monitoring infections in wild birds will be essential to better understand the host range of the virulent strains enzootic in West and Central Africa.
In our study, no class I strains were found in wild birds. Concern about these strains increased when some virulent viruses, derived from avirulent class I strains, caused outbreaks in Ireland in 1990 (45). They have never been found or systematically investigated in Africa, and there is no reason why they would not also occur on the African continent. Indeed, class I strains were identified in migratory birds in the United States (46) and Europe (47), and they could be introduced into wetlands of West Africa. Studies in live-bird markets in the United States showed that similar class I strains were found in wild waterfowl or shorebirds and poultry from live-bird markets (46). Ducks and chickens were also infected by class I strains in live-poultry markets in China and Hong Kong (48, 49). These two examples suggest that interactions with wild birds may result in virus transmission. Therefore, screening of poultry in markets in Africa could help to understand the epidemiology of class I in addition to wild bird sampling, which is much more difficult.
In summary, we have shown that several strains of avirulent and virulent NDV circulate in wild birds and pigeons in West Africa, in addition to the existing high genetic diversity of NDV strains found in poultry (4). Genotype VI strains were probably introduced on multiple separate occasions in Africa, and in Nigeria in particular. Typical avirulent wild-type strains were also found in wild birds. However, we did not find strong evidence that wild birds actively contribute to the spread of the enzootically circulating virulent genotype XIV, XVII, and XVIII strains. Further studies will be necessary to improve our understanding of the spread of virulent strains in West Africa and the role of wild birds.
ACKNOWLEDGMENTS
We thank A. Sausy, E. Charpentier and R. Brunnhöfer for their excellent technical help and J. Garba, H. Iyiola, I. Nwankwo, and J. Odeyemi for sample collection in Nigeria. We thank C. Afonso, Southeast Poultry Research Laboratory, USDA, for providing PCR controls.
We gratefully acknowledge the Ministry of Cooperation of Luxembourg, the Ministry of Health, the Ministry of Research and the Centre de Recherche Public de la Santé for their generous financial and moral support. C.J.S. was supported by an AFR fellowship (TR_PHD BFR08-095) from the Fonds National de la Recherche, Luxembourg. This study was also supported by contribution no. 70 from A.P. Leventis Ornithological Research Institute, Nigeria.
Footnotes
Published ahead of print 11 October 2013
REFERENCES
- 1.OIE 2012. Newcastle disease. In Manual of diagnostic tests and vaccines for terrestrial animals. OIE, Paris, France: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.14_NEWCASTLE_DIS.pdf [Google Scholar]
- 2.Courtney SC, Susta L, Gomez D, Hines NL, Pedersen JC, Brown CC, Miller PJ, Afonso CL. 2013. Highly divergent virulent isolates of Newcastle disease virus from the Dominican Republic are members of a new genotype that may have evolved unnoticed for over 2 decades. J. Clin. Microbiol. 51:508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diel DG, da Silva LH, Liu H, Wang Z, Miller PJ, Afonso CL. 2012. Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect. Genet. Evol. 12:1770–1779 [DOI] [PubMed] [Google Scholar]
- 4.Snoeck CJ, Owoade AA, Couacy-Hymann E, Alkali BR, Okwen MP, Adeyanju AT, Komoyo GF, Nakoune E, Le Faou A, Muller CP. 2013. High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. J. Clin. Microbiol. 51:2250–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander DJ, Senne DA. 2008. Newcastle disease, p 75–100 In Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE. (ed), Diseases of poultry. 12th ed. Blackwell Publishing, Ames, IA [Google Scholar]
- 6.Cardenas Garcia S, Navarro Lopez R, Morales R, Olvera MA, Marquez MA, Merino R, Miller PJ, Afonso CL. 2013. Molecular epidemiology of Newcastle disease in Mexico and the potential spillover of viruses from poultry into wild bird species. Appl. Environ. Microbiol. 79:4985–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddas R, Meir R, Perk S, Horowitz I, Lapin E, Rosenbluth E, Lublin A. 2013. Newcastle disease virus in little owls (Athene noctua) and African penguins (Spheniscus demersus) in an Israeli zoo. Transbound. Emerg. Dis. [Epub ahead of print.] 10.1111/tbed.12064 [DOI] [PubMed] [Google Scholar]
- 8.Kaleta EF, Kummerfeld N. 2012. Isolation of herpesvirus and Newcastle disease virus from White Storks (Ciconia ciconia) maintained at four rehabilitation centres in northern Germany during 1983 to 2001 and failure to detect antibodies against avian influenza A viruses of subtypes H5 and H7 in these birds. Avian Pathol. 41:383–389 [DOI] [PubMed] [Google Scholar]
- 9.Takakuwa H, Ito T, Takada A, Okazaki K, Kida H. 1998. Potentially virulent Newcastle disease viruses are maintained in migratory waterfowl populations. Jpn. J. Vet. Res. 45:207–215 [PubMed] [Google Scholar]
- 10.Yuan X, Wang Y, Li J, Yu K, Yang J, Xu H, Zhang Y, Ai H, Wang J. 2013. Surveillance and molecular characterization of Newcastle disease virus in seafowl from coastal areas of China in 2011. Virus Genes 46:377–382 [DOI] [PubMed] [Google Scholar]
- 11.Pfitzer S, Verwoerd DJ, Gerdes GH, Labuschagne AE, Erasmus A, Manvell RJ, Grund C. 2000. Newcastle disease and avian influenza A virus in wild waterfowl in South Africa. Avian Dis. 44:655–660 [PubMed] [Google Scholar]
- 12.Tarnagda Z, Yougbare I, Kam A, Tahita MC, Ouedraogo JB. 2011. Prevalence of infectious bronchitis and Newcastle disease virus among domestic and wild birds in H5N1 outbreaks areas. J. Infect. Dev. Ctries. 5:565–570 [DOI] [PubMed] [Google Scholar]
- 13.Oladele SB, Enam SJ, Okubanjo OO. 2012. Pathogenic haemoparasites and antibody to Newcastle disease virus from apparently healthy wild birds in Zaria, Nigeria. Vet. World. 5:13–18 [Google Scholar]
- 14.Ibu OJ, Okoye JOA, Adulugba EP, Chah KF, Shoyinka SVO, Salihu E, Chukwuedo AA, Baba SS. 2009. Prevalence of Newcastle disease viruses in wild and captive birds in central Nigeria. Int. J. Poultry Sci. 8:574–578 [Google Scholar]
- 15.Snoeck CJ, Ducatez MF, Owoade AA, Faleke OO, Alkali BR, Tahita MC, Tarnagda Z, Ouedraogo JB, Maikano I, Mbah PO, Kremer JR, Muller CP. 2009. Newcastle disease virus in West Africa: new virulent strains identified in non-commercial farms. Arch. Virol. 154:47–54 [DOI] [PubMed] [Google Scholar]
- 16.Van Borm S, Obishakin E, Joannis T, Lambrecht B, van den Berg T. 2012. Further evidence for the widespread co-circulation of lineages 4b and 7 velogenic Newcastle disease viruses in rural Nigeria. Avian Pathol. 41:377–382 [DOI] [PubMed] [Google Scholar]
- 17.Abolnik C. 2007. Molecular epidemiology of Newcastle disease and avian influenza in South Africa. Ph.D. thesis, University of Pretoria, Pretoria, South Africa [Google Scholar]
- 18.Kim LM, Suarez DL, Afonso CL. 2008. Detection of a broad range of class I and II Newcastle disease viruses using a multiplex real-time reverse transcription polymerase chain reaction assay. J. Vet. Diagn. Invest. 20:414–425 [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezealor AU. 2001. Nigeria, p 673–692 In Fishpool LDC, Evans MI. (ed), Important bird areas in Africa and associated islands: priority sites for conservation. Pisces Publications and BirdLife International, Newbury, United Kingdom [Google Scholar]
- 21.Cecchi G, Ilemobade A, Le Brun Y, Hogerwerf L, Slingenbergh J. 2008. Agro-ecological features of the introduction and spread of the highly pathogenic avian influenza (HPAI) H5N1 in northern Nigeria. Geospat. Health 3:7–16 [DOI] [PubMed] [Google Scholar]
- 22.Gaidet N, Cattoli G, Hammoumi S, Newman SH, Hagemeijer W, Takekawa JY, Cappelle J, Dodman T, Joannis T, Gil P, Monne I, Fusaro A, Capua I, Manu S, Micheloni P, Ottosson U, Mshelbwala JH, Lubroth J, Domenech J, Monicat F. 2008. Evidence of infection by H5N2 highly pathogenic avian influenza viruses in healthy wild waterfowl. PLoS Pathog. 4:e1000127. 10.1371/journal.ppat.1000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snoeck CJ, Adeyanju AT, De Landtsheer S, Ottosson U, Manu S, Hagemeijer W, Mundkur T, Muller CP. 2011. Reassortant low-pathogenic avian influenza H5N2 viruses in African wild birds. J. Gen. Virol. 92:1172–1183 [DOI] [PubMed] [Google Scholar]
- 24.Scott DA, Rose PM. 1996. Atlas of Anatidae populations in Africa and western Eurasia. Wetlands International, Wageningen, The Netherlands [Google Scholar]
- 25.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. 2006. Global patterns of influenza a virus in wild birds. Science 312:384–388 [DOI] [PubMed] [Google Scholar]
- 26.Bonfante F, Terregino C, Heidari A, Monne I, Salviato A, Taddei R, Raffini E, Capua I. 2012. Identification of APMV-1 associated with high mortality of collared doves (Streptoelia decaocto) in Italy. Vet. Rec. 171:327. [DOI] [PubMed] [Google Scholar]
- 27.Neyt C, Geliebter J, Slaoui M, Morales D, Meulemans G, Burny A. 1989. Mutations located on both F1 and F2 subunits of the Newcastle disease virus fusion protein confer resistance to neutralization with monoclonal antibodies. J. Virol. 63:952–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyoda T, Gotoh B, Sakaguchi T, Kida H, Nagai Y. 1988. Identification of amino acids relevant to three antigenic determinants on the fusion protein of Newcastle disease virus that are involved in fusion inhibition and neutralization. J. Virol. 62:4427–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terregino C, Cattoli G, Grossele B, Bertoli E, Tisato E, Capua I. 2003. Characterization of Newcastle disease virus isolates obtained from Eurasian collared doves (Streptopelia decaocto) in Italy. Avian Pathol. 32:63–68 [DOI] [PubMed] [Google Scholar]
- 30.Werner O, Romer-Oberdorfer A, Kollner B, Manvell RJ, Alexander DJ. 1999. Characterization of avian paramyxovirus type 1 strains isolated in Germany during 1992 to 1996. Avian Pathol. 28:79–88 [DOI] [PubMed] [Google Scholar]
- 31.Schuler KL, Green DE, Justice-Allen AE, Jaffe R, Cunningham M, Thomas NJ, Spalding MG, Ip HS. 2012. Expansion of an exotic species and concomitant disease outbreaks: pigeon paramyxovirus in free-ranging Eurasian collared doves. Ecohealth 9:163–170 [DOI] [PubMed] [Google Scholar]
- 32.Alexander DJ, Parsons G, Marshall R. 1984. Infection of fowls with Newcastle disease virus by food contaminated with pigeon faeces. Vet. Rec. 115:601–602 [DOI] [PubMed] [Google Scholar]
- 33.Irvine RM, Aldous EW, Manvell RJ, Cox WJ, Ceeraz V, Fuller CM, Wood AM, Milne JC, Wilson M, Hepple RG, Hurst A, Sharpe CE, Alexander DJ, Brown IH. 2009. Outbreak of Newcastle disease due to pigeon paramyxovirus type 1 in grey partridges (Perdix perdix) in Scotland in October 2006. Vet. Rec. 165:531–535 [DOI] [PubMed] [Google Scholar]
- 34.Dortmans JC, Rottier PJ, Koch G, Peeters BP. 2011. Passaging of a Newcastle disease virus pigeon variant in chickens results in selection of viruses with mutations in the polymerase complex enhancing virus replication and virulence. J. Gen. Virol. 92:336–345 [DOI] [PubMed] [Google Scholar]
- 35.Kommers GD, King DJ, Seal BS, Brown CC. 2003. Virulence of six heterogeneous-origin Newcastle disease virus isolates before and after sequential passages in domestic chickens. Avian Pathol. 32:81–93 [DOI] [PubMed] [Google Scholar]
- 36.Miller PJ, King DJ, Afonso CL, Suarez DL. 2007. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine 25:7238–7246 [DOI] [PubMed] [Google Scholar]
- 37.Samuel A, Nayak B, Paldurai A, Xiao S, Aplogan GL, Awoume KA, Webby RJ, Ducatez MF, Collins PL, Samal SK. 2013. Phylogenetic and pathotypic characterization of Newcastle disease viruses circulating in west Africa and efficacy of a current vaccine. J. Clin. Microbiol. 51:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SH, Wanasen N, Paldurai A, Xiao S, Collins PL, Samal SK. 2013. Newcastle disease virus fusion protein is the major contributor to protective immunity of genotype-matched vaccine. PLoS One 8:e74022. 10.1371/journal.pone.0074022 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Hines NL, Killian ML, Pedersen JC, Reising MM, Mosos NA, Mathieu-Benson C, Miller CL. 2012. An rRT-PCR assay to detect the matrix gene of a broad range of avian paramyxovirus serotype-1 strains. Avian Dis. 56:387–395 [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Dong J, Xie Z, Liu Q, Khan MI. 2010. Phylogenetic and pathogenic analysis of Newcastle disease virus isolated from house sparrow (Passer domesticus) living around poultry farm in southern China. Virus Genes 40:231–235 [DOI] [PubMed] [Google Scholar]
- 41.Diel DG, Miller PJ, Wolf PC, Mickley RM, Musante AR, Emanueli DC, Shively KJ, Pedersen K, Afonso CL. 2012. Characterization of Newcastle disease viruses isolated from cormorant and gull species in the United States in 2010. Avian Dis. 56:128–133 [DOI] [PubMed] [Google Scholar]
- 42.Aldous EW, Fuller CM, Mynn JK, Alexander DJ. 2004. A molecular epidemiological investigation of isolates of the variant avian paramyxovirus type 1 virus (PPMV-1) responsible for the 1978 to present panzootic in pigeons. Avian Pathol. 33:258–269 [DOI] [PubMed] [Google Scholar]
- 43.Kim LM, King DJ, Guzman H, Tesh RB, Travassos da Rosa AP, Bueno R, Jr, Dennett JA, Afonso CL. 2008. Biological and phylogenetic characterization of pigeon paramyxovirus serotype 1 circulating in wild North American pigeons and doves. J. Clin. Microbiol. 46:3303–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Wang Z, Son C, Wang Y, Yu B, Zheng D, Sun C, Wu Y. 2006. Characterization of pigeon-origin Newcastle disease virus isolated in China. Avian Dis. 50:636–640 [DOI] [PubMed] [Google Scholar]
- 45.Alexander DJ, Campbell G, Manvell RJ, Collins MS, Parsons G, McNulty MS. 1992. Characterisation of an antigenically unusual virus responsible for two outbreaks of Newcastle disease in the Republic of Ireland in 1990. Vet. Rec. 130:65–68 [DOI] [PubMed] [Google Scholar]
- 46.Kim LM, King DJ, Curry PE, Suarez DL, Swayne DE, Stallknecht DE, Slemons RD, Pedersen JC, Senne DA, Winker K, Afonso CL. 2007. Phylogenetic diversity among low-virulence Newcastle disease viruses from waterfowl and shorebirds and comparison of genotype distributions to those of poultry-origin isolates. J. Virol. 81:12641–12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindh E, Ek-Kommonen C, Vaananen VM, Alasaari J, Vaheri A, Vapalahti O, Huovilainen A. 2012. Molecular epidemiology of outbreak-associated and wild-waterfowl-derived Newcastle disease virus strains in Finland, including a novel class I genotype. J. Clin. Microbiol. 50:3664–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim LM, King DJ, Suarez DL, Wong CW, Afonso CL. 2007. Characterization of class I Newcastle disease virus isolates from Hong Kong live bird markets and detection using real-time reverse transcription-PCR. J. Clin. Microbiol. 45:1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Wang X, Wu S, Hu S, Peng Y, Xue F, Liu X. 2009. Surveillance for avirulent Newcastle disease viruses in domestic ducks (Anas platyrhynchos and Cairina moschata) at live bird markets in Eastern China and characterization of the viruses isolated. Avian Pathol. 38:377–391 [DOI] [PubMed] [Google Scholar]